Abstract
The causative agent of coronavirus disease‐2019 (COVID‐19), severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), enters the host cells via an angiotensin‐converting enzyme 2 (ACE2)‐mediated endocytosis‐dependent manner. Because ACE2 is highly expressed in the heart, SARS‐CoV‐2 can severely infect heart tissue and arteries, causing acute and chronic damage to the cardiovascular system. Therefore, special attention should be paid to finding appropriate agents to protect this vital system during COVID‐19 treatment. Papaverine is a unique vasodilator alkaloid that is clinically used in the treatment of vasospasm. Interestingly, this compound has potent and direct effects on a wide range of viruses, and could also prevent viral exploitation mechanisms of the host cell facilities by inhibiting some cellular signaling pathways such as p38 MAPK. This pathway was recently introduced as a promising target for the treatment of COVID‐19. Papaverine also has anti‐inflammatory effects which is useful in combating the hyper‐inflammatory phase of the COVID‐19. Unlike some medications that have severe dosage‐restrictions in the treatment of COVID‐19 due to cardiac side effects, papaverine is recommended for use in many heart disorders. The ability of papaverine to treat COVID‐19 has become more promising when the results of some extensive screenings showed the strong ability of this compound to inhibit the cytopathic effects of SARS‐CoV‐2 with EC50 of 1.1 μM. Having several therapeutic effects along with desired safety profile raises this hypothesis that papaverine could be a promising compound for the suppression of SARS‐CoV‐2 and prevention of ischemia/vasoconstriction‐related complications in COVID‐19 disease, especially in patients with underlying cardiovascular diseases (CVDs).
Keywords: cardiovascular diseases (CVDs), COVID‐19, p38 MAPK, Papaverine, SARS‐CoV‐2
1.
As is clear, coronavirus disease‐2019 (COVID‐19) can cause severe cardiovascular damages increasing the risk of death in patients with underlying cardiovascular disease (CVD) (Nishiga et al., 2020). Previous observations revealed that other famous beta‐coronaviruses Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome coronavirus (SARS‐CoV) cause remarkable cardiac complications such as acute myocarditis (Arabi et al., 2015; Oudit et al., 2009). This disorder is also significantly observed in patients with COVID‐19 especially those with CVD that is detectable by increasing the level of some cardiac injury biomarkers such as elevated highly sensitive troponin I (hs‐cTnI) and creatine kinase (CK) (Guzik et al., 2020). Therefore, finding new treatments to prevent SARS‐CoV‐2 damage to the cardiovascular system is very important.
The angiotensin‐converting enzyme 2 (ACE2) receptor plays a basic role in the infectivity of SARS‐CoV‐2, and also has a key role in the cardiovascular system and blood circulation (Gurwitz, 2020a). The virus enters the host cells via an ACE2‐mediated endocytosis‐dependent manner (Hoffmann et al., 2020). Because ACE2 is highly expressed in the lungs, acute respiratory distress syndrome (ARDS) is the most prominent feature of COVID‐19 infection. ACE2 is also greatly expressed in the cardiovascular system, and has a critical role in the regulation of blood pressure through catalyzing the hydrolysis of vasoconstrictor angiotensin II (Ang II) into vasodilator angiotensin 1–7 (Ang 1–7) (Keidar et al., 2007). According to clinical observations, severe inflammatory processes and vascular contractions in cardiac patients with COVID‐19 pose a high risk of death (Tomasoni et al., 2020). The mechanism of hyper‐inflammation and fatal tissue damages caused by SARS‐CoV‐2 is not yet fully understood. Because SARS‐CoV‐2 depends on the use of host cell facilities for survival and replication, understanding host–cellular functions used by the virus would be essential. Given the advances in this area, scientists suggest that the Renin‐Angiotensin System (RAS) and the p38 mitogen‐activated protein kinase (p38 MAPK) pathway are promising targets for the treatment of COVID‐19 (Dean et al., 2021; Grimes & Grimes, 2020a; Pucci et al., 2021; Sohag et al., 2020). Accordingly, the use of modulators of such cellular pathways is attracting great attention for the treatment of COVID‐19 (Valipour et al., 2021).
The destructive effects of SARS‐CoV‐2 on endothelial and vascular function are so significant that COVID‐19 has been termed a vascular disease (Siddiqi et al., 2021). As stated in previous studies, the RAS signaling pathway plays a key role in cardiovascular fluid balance and blood pressure, and its normal activity plays an important role in the treatment and survival of COVID‐19 patients (Huang et al., 2020). Ang II is a potent vasoconstrictor present in this system whose activity increases peripheral vascular resistance and thus increases blood pressure. This enzyme is produced by the interaction of ACE residing in the vascular endothelium with the Ang I. By converting Ang II to Ang 1–7, Mas receptor is stimulated and provided conditions eventually lead to vasodilation, as well as suppression of inflammation, atrophy, and fibrosis (Ferrario & Strawn, 2006).
The p38 MAPK pathway is a critical cellular signaling that regulates the release of macrophage‐ and fibroblast‐derived proteins, and proinflammatory cytokines. Some studies suggest that the inflammatory effects of Ang II are mediated by the activation of the p38 MAPK pathway, therefore, some scientists consider this pathway as a promising target for the treatment of COVID‐19 (Grimes & Grimes, 2020). Also, there are many studies that highlight the role of abnormal activation of the p38 MAPK pathway in the development of heart damages. By summarizing these studies, some reviews reported that hyper‐activation of this pathway leads to cardiac‐related pathogenic conditions such as disruption of cardiac fibroblast function that eventually leads to serious cellular heart damages (Fisk et al., 2014; Turner & Blythe, 2019; Wang et al., 2016).
Mechanistic evaluations suggest that there is a remarkable correlation between RAS disruption and disproportionate activity of the p38 MAPK pathway in SARS‐CoV‐2 infectivity. When SARS‐CoV‐2 binds to ACE2 during cell entry and alters its normal activity, the routine conversion pathway of Ang II to Ang 1–7 is disrupted. Disruption of this pathway causes Ang II to over‐affect the AT1 receptor, leading to severe inflammation and vasoconstriction following disproportionate activation of the p38 MAPK pathway (Park et al., 2007; Simões e et al., 2013). Also, other previous studies have confirmed that SARS‐CoV (the closest relative of SARS‐CoV‐2) regulates the p38 MAPK pathway for replication and pathogenesis (Kopecky‐Bromberg et al., 2006; Marchant et al., 2010).
Because p38 MAPK‐inhibitors can potentially counteract the pathogenicity of SARS‐CoV‐2 in several ways and also do not have a direct adverse effect on the cardiovascular system, they appear to be promising therapeutic agents for the treatment of COVID‐19. By that logic, some clinical trials today are evaluating the role of the p38 MAPK inhibitors in the treatment of COVID‐19 patients (ClinicalTrials. gov Identifier: NCT04511819).
Papaverine is known as a vasodilator and direct‐acting smooth muscle relaxant. In clinics, this alkaloid is used for the treatment of vasospasm as an effective vasodilator, especially those involving cerebral and heart disorders. This drug relaxes the smooth muscles of the coronary arteries well, and has a direct effect on the heart muscle reducing conduction and prolonging the refractory period (Wilson & White, 1986). The broad‐spectrum antiviral effect is another property of this compound that has recently become more highlighted through showing potent inhibitory effect on the SARS‐CoV‐2 cytopathicity with EC50 = 1.1 μM (discussed below) (Ellinger et al., 2021). Previous studies have shown that papaverine can also exert its antiviral effects by modulating host cells signaling pathways such as MAPK (Aggarwal et al., 2020a; García‐Cárceles et al., 2021). Furthermore, the results of a new study indicated that the potent anti‐ischemic and anti‐inflammatory effects of papaverine are significantly related to the regulation of the p38 MAPK pathway and modulation of neurovascular inflammation, and this compound well regulated both Th17‐derived cytokine generation and the IL‐17 signaling pathway by acting on p38 MAPK and IL‐6 (Guan et al., 2020).
As shown schematically in Figure 1, papaverine can fight the SARS‐CoV‐2 pathogenicity in several ways due to its outstanding therapeutic effects which are discussed below.
Figure 1.
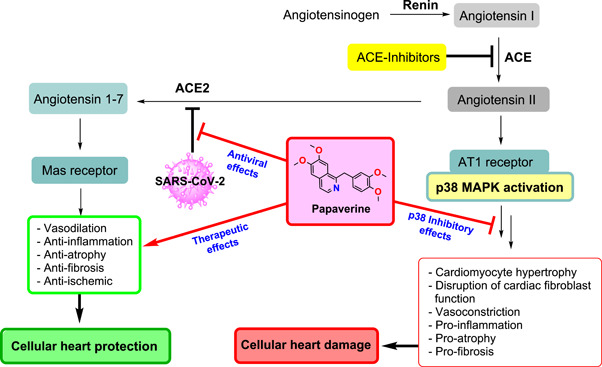
Possible pathogenesis of SARS‐CoV‐2, and multifunctional therapeutic effects of the papaverine against SARS‐CoV‐2 infection.
In addition to having prominent vasodilatory activities, one of the most important therapeutic effects of papaverine is its broad‐spectrum antiviral activity that has been reported in previous studies against a wide range of viruses. A recent study also reported that papaverine can effectively inhibit various strains of influenza and paramyxoviruses (Aggarwal et al., 2020). Importantly, papaverine has remarkable inhibitory effects against coronaviruses which was confirmed by several independent studies. A few months before the advent of SARS‐CoV‐2, Shen et al. performed a high‐throughput screening (HTS) to find effective anti‐coronavirus agents. Results of this study showed that papaverine has a broad‐spectrum anti‐coronavirus activity against HCoV‐NL63 (EC50 = 7.32 µM, CC50 = 11.71 µM), HCoV‐OC43 (EC50 = 1.61 µM, CC50 = 12.11 µM), MERS‐CoV (EC50 = 9.45 µM, CC50 = 11.98 µM), and MHV‐A59 (EC50 = 11.46 µM, CC50 = 12.44 µM) (Shen et al., 2019).
SARS‐CoV‐2 is a highly cytocidal virus that causes severe morphological changes in cardiac cells (as well as changes in cell physiology and subsequent biosynthetic events) that ultimately cause the destruction of host cells and tissues (Perez‐Bermejo et al., 2021; Zhu et al., 2020). These virus‐induced cellular lesions are called cytopathic effects (CPE). Bioactive compounds that can inhibit the CPE of viruses have valuable potential for clinical applications (Byrd & Hruby, 2006). This effect can occur through the effects of CPE inhibitors through both direct effects on viral targets and regulation of host‐dependent factors. In a recent study, Elinger et al. performed a high‐content screening on a library of 5632 compounds using the microscopy method for their ability to inhibit virus‐induced structural damages in host cells resulting from SARS‐CoV‐2 infection in the Caco‐2 cells. The results of this study also confirmed the strong ability of papaverine to reduce CPE of SARS‐CoV‐2 with EC50 = 1.1 μM, and even its analogs ethaverine and drotaverine with remarkable EC50 of 2.15 μM and 6.07 μM, respectively (Ellinger et al., 2021).
In summary, SARS‐CoV‐2 causes damage to the myocardium, and patients with cardiovascular disease are more vulnerable to COVID‐19. Although cardiac side effects severely limit the use of some approved drugs with strong anti‐SARS‐CoV‐2 activity in the treatment of COVID‐19 (such as chloroquine and emetine), papaverine is probably the only anti‐SARS‐CoV‐2 compound recommended for use in ischemia/vasoconstriction‐related complications and many heart disorders such as angina pectoris, peripheral vascular disease, peripheral and pulmonary embolism, and vascular spasm associated with acute myocardial infarction. This therapeutic feature highlights the capability of papaverine to be used in the medication regimen of COVID‐19 patients with underlying heart disorders. Of course, it is important to note that papaverine is contraindicated in a few heart patients, such as patients with the complete atrioventricular block (Chauhan et al., 1992).
Drug repurposing is a cost‐effective strategy that can provide new treatment options to COVID‐19 patients without the need for costly and time‐consuming safety‐related clinical trials (Gurwitz, 2020b; Phadke & Saunik, 2020). As mentioned above, papaverine could be useful in the treatment of COVID‐19 patients in several ways. First, this drug has p38 MAPK inhibitory activity, so it can maintain normal heart activity by reducing vasoconstriction and inflammation caused by the disproportionate activity of this pathway. Having vasodilatory activity, is another beneficial effect of papaverine for COVID‐19 patients to improve their heart function. Interestingly, papaverine has also represented strong inhibitory effects against SARS‐CoV‐2 cytopathicity. In addition, this compound has a good safety profile and does not cause severe side effects in the body.
Due to the beneficial effects of papaverine, this commentary suggests that this interesting drug can be considered as a new option in the medication regimen for the treatment of COVID‐19 patients, especially for those with underlying CVD.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Valipour, M. , Irannejad, H. , & Emami, S. (2022). Papaverine, a promising therapeutic agent for the treatment of COVID‐19 patients with underlying cardiovascular diseases (CVDs). Drug Development Research, 83, 1246–1250. 10.1002/ddr.21961
Contributor Information
Hamid Irannejad, Email: irannejadhamid@gmail.com.
Saeed Emami, Email: sdemami12@gmail.com, Email: semami@mazums.ac.ir.
DATA AVAILABILITY STATEMENT
None declared.
REFERENCES
- Aggarwal, M. , Leser, G. P. , & Lamb, R. A. (2020). Repurposing papaverine as an antiviral agent against influenza viruses and paramyxoviruses. Journal of Virology, 94(6), e01888–01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi, Y. M. , Harthi, A. , Hussein, J. , Bouchama, A. , Johani, S. , Hajeer, A. H. , Saeed, B. T. , Wahbi, A. , Saedy, A. , AlDabbagh, T. , Okaili, R. , Sadat, M. , & Balkhy, H. (2015). Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection, 43(4), 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, C. M. , & Hruby, D. E. (2006). Viral proteinases: Targets of opportunity. Drug Development Research, 67(6), 501–510. [Google Scholar]
- Chauhan, D. , Mullins, P. , Thuraisingham, S. , & Schofield, P. (1992). Intracoronary papaverine and complete atrioventricular block. BMJ: British Medical Journal, 305(6858), 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, A. Q. , Bozza, W. P. , Twomey, J. D. , Luo, S. , Nalli, A. , & Zhang, B. (2021). The fight against COVID‐19: Striking a balance in the renin–angiotensin system. Drug Discovery Today, 26(10), 2214–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger, B. , Bojkova, D. , Zaliani, A. , Cinatl, J. , Claussen, C. , Westhaus, S. , Keminer, O. , Reinshagen, J. , Kuzikov, M. , Wolf, M. , Geisslinger, G. , Gribbon, P. , & Ciesek, S. (2021). A SARS‐CoV‐2 cytopathicity dataset generated by high‐content screening of a large drug repurposing collection. Scientific Data, 8(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario, C. M. , & Strawn, W. B. (2006). Role of the renin‐angiotensin‐aldosterone system and proinflammatory mediators in cardiovascular disease. The American Journal of Cardiology, 98(1), 121–128. [DOI] [PubMed] [Google Scholar]
- Fisk, M. , Gajendragadkar, P. R. , Mäki‐Petäjä, K. M. , Wilkinson, I. B. , & Cheriyan, J. (2014). Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. American Journal of Cardiovascular Drugs, 14(3), 155–165. [DOI] [PubMed] [Google Scholar]
- García‐Cárceles, J. , Caballero, E. , Gil, C. , & Martínez, A. (2021). Kinase inhibitors as underexplored antiviral agents. Journal of Medicinal Chemistry, 27, 935–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, J. M. , & Grimes, K. V. (2020). p38 MAPK inhibition: A promising therapeutic approach for COVID‐19. Journal of Molecular and Cellular Cardiology, 144, 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, S. , Liu, Q. , Gu, H. , Zhang, Y.‐y , Wei, P.‐l , Qi, Y.‐f , Liu, J. , & Wang, Z. (2020). Pluripotent anti‐inflammatory immunomodulatory effects of papaverine against cerebral ischemic‐reperfusion injury. Journal of Pharmacological Sciences, 144(2), 69–75. [DOI] [PubMed] [Google Scholar]
- Gurwitz, D. (2020a). Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Development Research, 81(5), 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz, D. (2020b). Repurposing current therapeutics for treating COVID‐19: A vital role of prescription records data mining. Drug Development Research, 81(7), 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik, T. J. , Mohiddin, S. A. , Dimarco, A. , Patel, V. , Savvatis, K. , Marelli‐Berg, F. M. , Madhur, M. S. , Tomaszewski, M. , Maffia, P. , D'Acquisto, F. , Nicklin, S. A. , Marian, A. J. , Nosalski, R. , Murray, E. C. , Guzik, B. , Berry, C. , Touyz, R. M. , Kreutz, R. , Wang, D. W. , … McInnes, I. B. (2020). COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 116(10), 1666–1687. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , & Nitsche, A. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 18(2), 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z. , Cao, J. , Yao, Y. , Jin, X. , Luo, Z. , Xue, Y. , Zhu, C. , Song, Y. , Wang, Y. , Zou, Y. , Qian, J. , Yu, K. , Gong, H. , & Ge, J. (2020). The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Annals of Translational Medicine, 8(7), 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidar, S. , Kaplan, M. , & Gamliel‐Lazarovich, A. (2007). ACE2 of the heart: from angiotensin I to angiotensin (1–7). Cardiovascular Research, 73(3), 463–469. [DOI] [PubMed] [Google Scholar]
- Kopecky‐Bromberg, S. A. , Martinez‐Sobrido, L. , & Palese, P. (2006). 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen‐activated protein kinase. Journal of Virology, 80(2), 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, D. , Singhera, G. K. , Utokaparch, S. , Hackett, T. L. , Boyd, J. H. , Luo, Z. , Si, X. , Dorscheid, D. R. , McManus, B. M. , & Hegele, R. G. (2010). Toll‐like receptor 4‐mediated activation of p38 mitogen‐activated protein kinase is a determinant of respiratory virus entry and tropism. Journal of Virology, 84(21), 11359–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiga, M. , Wang, D. W. , Han, Y. , Lewis, D. B. , & Wu, J. C. (2020). COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nature Reviews Cardiology, 17(9), 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit, G. , Kassiri, Z. , Jiang, C. , Liu, P. , Poutanen, S. , Penninger, J. , & Butany, J. (2009). SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation, 39(7), 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.‐K. , Fischer, R. , Dechend, R. , Shagdarsuren, E. , Gapeljuk, A. , Wellner, M. , Meiners, S. , Gratze, P. , Al‐Saadi, N. , Feldt, S. , Fiebeler, A. , Madwed, J. B. , Schirdewan, A. , Haller, H. , Luft, F. C. , & Muller, D. N. (2007). p38 mitogen‐activated protein kinase inhibition ameliorates angiotensin II–induced target organ damage. Hypertension, 49(3), 481–489. [DOI] [PubMed] [Google Scholar]
- Perez‐Bermejo, J. A. , Kang, S. , Rockwood, S. J. , Simoneau, C. R. , Joy, D. A. , Silva, A. C. , Ramadoss, G. N. , Flanigan, W. R. , Fozouni, P. , Li, H. , Chen, P. Y. , Nakamura, K. , Whitman, J. D. , Hanson, P. J. , McManus, B. M. , Ott, M. , Conklin, B. R. , & McDevitt, T. C. (2021). SARS‐CoV‐2 infection of human iPSC‐derived cardiac cells reflects cytopathic features in hearts of patients with COVID‐19. Science Translational Medicine, 13(590), eabf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke, M. , & Saunik, S. (2020). COVID‐19 treatment by repurposing drugs until the vaccine is in sight. Drug Development Research, 81(5), 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci, F. , Annoni, F. , Santos, R. A. S. d , Taccone, F. S. , & Rooman, M. (2021). Quantifying renin‐angiotensin‐system alterations in COVID‐19. Cells, 10(10), 2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, L. , Niu, J. , Wang, C. , Huang, B. , Wang, W. , Zhu, N. , Deng, Y. , Wang, H. , Ye, F. , Cen, S. , & Tan, W. (2019). High‐throughput screening and identification of potent broad‐spectrum inhibitors of coronaviruses. Journal of Virology, 93(12), e00023–00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi, H. K. , Libby, P. , & Ridker, P. M. (2021). COVID‐19–A vascular disease. Trends in Cardiovascular Medicine, 31(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões e, S. , Silveira, A. , Ferreira, K. , & Teixeira, A. M. (2013). ACE2, angiotensin‐(1‐7) and M as receptor axis in inflammation and fibrosis. British Journikal of Pharmacology, 169(3), 477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohag, A. A. M. , Hannan, M. A. , Rahman, S. , Hossain, M. , Hasan, M. , Khan, M. K. , Khatun, A. , Dash, R. , & Uddin, M. J. (2020). Revisiting potential druggable targets against SARS‐CoV‐2 and repurposing therapeutics under preclinical study and clinical trials: A comprehensive review. Drug Development Research, 81(8), 919–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni, D. , Italia, L. , Adamo, M. , Inciardi, R. M. , Lombardi, C. M. , Solomon, S. D. , & Metra, M. (2020). COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. European Journal of Heart Failure, 22(6), 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, N. A. , & Blythe, N. M. (2019). Cardiac fibroblast p38 MAPK: a critical regulator of myocardial remodeling. Journal of Cardiovascular Development and Disease, 6(3), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valipour, M. , Zarghi, A. , Ebrahimzadeh, M. A. , & Irannejad, H. (2021). Therapeutic potential of chelerythrine as a multi‐purpose adjuvant for the treatment of COVID‐19. Cell Cycle, 20(22), 2321–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Ding, L. , Ji, H. , Xu, Z. , Liu, Q. , & Zheng, Y. (2016). The role of p38 MAPK in the development of diabetic cardiomyopathy. International Journal of Molecular Sciences, 17(7), 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R. F. , & White, C. W. (1986). Intracoronary papaverine: An ideal coronary vasodilator for studies of the coronary circulation in conscious humans. Circulation, 73(3), 444–451. [DOI] [PubMed] [Google Scholar]
- Zhu, N. , Wang, W. , Liu, Z. , Liang, C. , Wang, W. , Ye, F. , Huang, B. , Zhao, L. , Wang, H. , Zhou, W. , Deng, Y. , Mao, L. , Su, C. , Qiang, G. , Jiang, T. , Zhao, J. , Wu, G. , Song, J. , & Tan, W. (2020). Morphogenesis and cytopathic effect of SARS‐CoV‐2 infection in human airway epithelial cells. Nature Communications, 11(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None declared.


