Abstract
The eubacterial 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (EC 1.1.1.34) was purified 3,000-fold from Streptomyces sp. strain CL190 to apparent homogeneity with an overall yield of 2.1%. The purification procedure consisted of (NH4)2SO4 precipitation, heat treatment and anion exchange, hydrophobic interaction, and affinity chromatographies. The molecular mass of the enzyme was estimated to be 41 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and 100 to 105 kDa by gel filtration chromatography, suggesting that the enzyme is most likely to be a dimer. The enzyme showed a pH optimum of around 7.2, with apparent Km values of 62 μM for NADPH and 7.7 μM for HMG-CoA. A gene from CL190 responsible for HMG-CoA reductase was cloned by the colony hybridization method with an oligonucleotide probe synthesized on the basis of the N-terminal sequence of the purified enzyme. The amino acid sequence of the CL190 HMG-CoA reductase revealed several limited motifs which were highly conserved and common to the eucaryotic and archaebacterial enzymes. These sequence conservations suggest a strong evolutionary pressure to maintain amino acid residues at specific positions, indicating that the conserved motifs might play important roles in the structural conformation and/or catalytic properties of the enzyme.
A biosynthetic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (EC 1.1.1.34) utilizes two NADPH equivalents to reductively deacylate the thioester group of HMG-CoA to the primary alcohol of mevalonate as follows: HMG-CoA + 2NADPH + 2H+ → mevalonate + CoASH + 2NADP+.
An extensive body of information concerning HMG-CoA reductase exists in eucaryotes. This enzyme performs the rate-limiting step in the biosynthesis of terpenoids and is regulated by a variety of steps, including transcription, translation, and enzyme degradation and phosphorylation (9, 21). The eucaryotic enzyme is a transmembrane glycoprotein located in the endoplasmic reticulum (32). Modeling studies of the deduced primary sequence of HMG-CoA reductase from a variety of organisms suggest that it consists of eight membrane-spanning regions. The N-terminal region has been shown to be involved in the regulated degradation of HMG-CoA reductase. The C-terminal domain projects into the cytosol and contains the catalytic site (31).
In archaebacteria, HMG-CoA reductases from Haloferax volcanii (5, 28) and Sulfolobus solfataricus (7), which belong to divergent kingdoms of the Euryarchaeota and the Crenarchaeota (49), respectively, have been cloned and sequenced. Each gene has been expressed in Escherichia coli, and these recombinant enzymes have been purified and characterized.
In eubacteria, however, no biosynthetic HMG-CoA reductase gene has been cloned. Only a biodegradative HMG-CoA reductase (EC 1.1.1.88), mevalonate + CoASH + 2NAD+ ⇄ HMG-CoA + 2NADH + 2H+, from Pseudomonas mevalonii, which can grow on mevalonate as the sole carbon source, has been extensively characterized (4, 20, 48). In contrast to the biosynthetic HMG-CoA reductase, this enzyme utilizes NAD(H) rather than NADP(H) as an oxidoreductant. The reaction is reversible and favors mevalonate production (39). Thus, the enzyme has served as a model for elucidating the active site structure and the catalytic mechanism of biosynthetic HMG-CoA reductases, and some functional motifs have been recently proposed from its three-dimensional structure analysis (29). However, the P. mevalonii HMG-CoA reductase had less than 25% sequence identity with eucaryotic HMG-CoA reductase and lacked several regions conserved in both eucaryotic and archaebacterial HMG-CoA reductases. Therefore, a comprehensive understanding of functional motifs of the biosynthetic HMG-CoA reductase is still lacking.
In order to elucidate the catalytic domains of the biosynthetic HMG-CoA reductase, it is important to obtain the enzyme from an additional kingdom, Eubacteria. In some eubacteria, such as Myxococcus fulvus, Lactobacillus plantarum, and Staphylococcus carnosus, involvement of the enzymes concerning the mevalonate pathway has been demonstrated by an incorporation experiment with [14C]acetyl-CoA, [14C]HMG-CoA, or [14C]mevalonate (22). Furthermore, several terpenoid and hemiterpenoid metabolites, such as naphterpin (43, 44), furaquinocin (19), napyradiomycin (45), and terpentecin (23), produced by the genus Streptomyces, have been proved to be synthesized through the ubiquitous mevalonate pathway by incorporation experiments with 13C-labeled acetate. However, no biosynthetic HMG-CoA reductase has been purified from these eubacteria. Thus, we decided to clone the HMG-CoA reductase gene from Streptomyces sp. strain CL190, which produces naphterpin (44), because we were able to detect the enzyme activity with this organism.
Considering codon usage specific to Streptomyces sp. strain CL190, we first designed several sets of PCR primers based on the highly conserved amino acid sequences between the eucaryotic and archaebacterial HMG-CoA reductases to isolate the HMG-CoA reductase gene from CL190. Contrary to our expectation, however, we were unable to obtain a specific DNA fragment by PCR with these primers. These findings indicated that the amino acid sequence of Streptomyces HMG-CoA reductase was somewhat different from eucaryotic and archaebacterial amino acid sequences. Thus, purification of the CL190 HMG-CoA reductase followed by the determination of its N-terminal amino acid sequence was essential for cloning the corresponding gene.
In this study, we report the purification, characterization, molecular cloning, and sequence of HMG-CoA reductase from Streptomyces sp. strain CL190. Moreover, the corresponding gene, named hmgr, was overexpressed in E. coli, and its recombinant HMG-CoA reductase was purified and characterized.
MATERIALS AND METHODS
Materials.
Materials from commercial sources included R,S-[3-14C]HMG-CoA and [γ-32P]ATP (American Radiolabeled Chemicals, St. Louis, Mo.); R,S-[2-14C]mevalonolactone (Amersham Corp.); phenylmethylsulfonyl fluoride (PMSF) (Sigma); Butyl Toyopearl 650M and Toyopearl HW65 (TOSOH, Tokyo, Japan); DEAE Sephacel, Blue Sepharose CL-6B, Mono Q HR5/5, Superdex 200, epoxy-activated Sepharose 6B, and a high- and low-molecular-weight electrophoresis calibration kit (Pharmacia Biotech, Uppsala, Sweden); Coomassie brilliant blue R-250 (Fluka, Buchs, Switzerland); and polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, Calif.). Pravastatin was a gift from Sankyo Co. Ltd. A CoA affinity column was prepared by using epoxy-activated Sepharose 6B as described by the manufacturer. All other reagents were of the highest analytical grade available.
Conditions for growth.
Streptomyces sp. strain CL190 was cultured as previously described (43).
Buffered solutions.
Buffer A, used throughout purification, contained 25 mM potassium phosphate buffer (pH 7.2), 1 mM EDTA, 1 mM dithiothreitol, and 10% glycerol.
Buffer B contained buffer A plus 400 mM KCl.
Buffer C, used for heat treatment, contained 25 mM potassium phosphate buffer (pH 7.2), 1 mM EDTA, 1 mM dithiothreitol, 1 M KCl, and 33% glycerol.
Buffer D contained buffer B plus 30% saturation (NH4)2SO4.
Buffer E, used for Blue Sepharose CL-6B column chromatography, contained 50 mM potassium phosphate buffer (pH 7.2), 30 mM EDTA, 1 mM dithiothreitol, 200 mM KCl, and 100 mM sucrose.
Buffer F, used for Ni-nitrilotriacetic acid agarose column chromatography, contained 50 mM potassium phosphate buffer (pH 7.4), 200 mM KCl, and 10% glycerol.
Sodium dodecyl sulfate (SDS) sample buffer contained 1% SDS, 1% β-mercaptoethanol, 10% (vol/vol) glycerol, and 0.01% bromphenol blue in 60 mM Tris-HCl (pH 6.8).
Radiometric assay of HMG-CoA reductase activity.
The radiometric assay was conducted by the method described by Bach et al. (3) with some modifications. The assay system consisted of 25 mM potassium phosphate (pH 7.2), 50 mM KCl, 1 mM EDTA, 5 mM dithiothreitol, 10 mM glucose 6-phosphate, 14 mU of yeast glucose 6-phosphate dehydrogenase, 0.6 mM NADPH, 14 μM R,S-[3-14C]HMG-CoA, and 10% (vol/vol) glycerol in a final volume of 50 μl. The reaction was initiated by adding HMG-CoA to the complete assay mixture. The reaction was terminated by adding 5 μl of HCl, and the mixture was allowed to lactonize for an additional 30 min. Mevalonolactone was extracted with 100 μl of ethyl acetate, and an aliquot of the supernatant was subjected to thin-layer chromatography on a silica gel. The plate, developed with a mixture of toluene, acetone, and triethylamine (80:40:1), was exposed to an imaging plate (Fujifilm), in which the intensity of photostimulated luminescence was proportional to the adsorbed radiation energy on the imaging plate (2). The amount of mevalonolactone was determined by the estimation of photostimulated luminescence of the corresponding spot with a BAS-1500 (Fujifilm). One pU of HMG-CoA reductase activity was defined as the amount of enzyme that formed 1 pmol of mevalonolactone per min at 30°C.
Spectrophotometric assay of HMG-CoA reductase activity.
The spectrophotometric assay was conducted by the method described by Kleinsek et al. (26), with a slight modification. The assay system consisted of 25 mM potassium phosphate (pH 7.2), 50 mM KCl, 1 mM EDTA, 5 mM dithiothreitol, 0.3 mM NADPH, and 0.3 mM R,S-HMG-CoA in a final volume of 1 ml. The reaction was initiated by adding HMG-CoA to the complete assay mixture. The HMG-CoA-dependent oxidation of NADPH was monitored in a Shimadzu UV-160 spectrophotometer equipped with a cell holder adjusted at 30°C. One microunit of HMG-CoA reductase activity is defined as the amount of the enzyme that caused oxidation of 1 μmol of NADPH per min.
Purification of HMG-CoA reductase from Streptomyces sp. strain CL190.
Unless otherwise stated, all the purification procedures were done in a cold room maintained at 4°C, and centrifugation was done at 10,000 × g for 30 min.
The mycelial cake (500 g) collected by centrifugation from the whole fermentation broth (10 liters) was washed by resuspending it in 20 mM potassium phosphate buffer (pH 7.2) and frozen at −30°C. Freezing the cells had no adverse effect on subsequent recovery of the enzyme. Frozen mycelia were thawed overnight, suspended in buffer A (3 ml per mycelial weight) containing 1 mM PMSF, and ruptured by ultrasonication. The cell lysate was centrifuged, and the supernatant was retained as a crude extract.
The crude extract (1.8 liters) was applied to a DEAE cellulose column (5 by 20 cm) previously equilibrated with buffer A containing 1 mM PMSF. After the column extract was washed with 8 column volumes of the same buffer described above, elution was carried out with buffer B at a flow rate of 1 ml/min. Active fractions were combined to give a DEAE fraction (103 ml).
This sample was adjusted to 45% saturation in (NH4)2SO4 by adding solid (NH4)2SO4. The mixture was cooled on ice for an hour and centrifuged. The precipitate was dissolved and dialyzed against buffer C and centrifuged to give an ammonium sulfate fraction (13 ml).
To stabilize HMG-CoA reductase for heat treatment, NADPH was added to the ammonium sulfate fraction to give a final concentration of 2 mM (46). Then the enzyme solution was maintained at 60°C for 10 min, placed on ice, and centrifuged to remove denatured protein. The precipitate was washed with 2 volumes of buffer B. Following centrifugation, the initial supernatant liquid and wash were combined to give a heat fraction (29 ml).
This sample was dialyzed against buffer D and then applied to a Butyl-Toyopearl 650M column (2 by 30 cm) previously equilibrated with the same buffer. After the column was washed with 10 column volumes of buffer D, elution was carried out at 1 ml/min by mixing buffer D with a 300-min linear gradient of buffer B. The active fractions were combined to give a Butyl Toyopearl fraction (51 ml).
This sample was dialyzed against buffer E and applied to a Blue Sepharose CL-6B column (2 by 11 cm) previously equilibrated with buffer E. After washing with 5 column volumes of buffer E, elution was carried out at 1 ml/min by mixing buffer E with a 100-min linear gradient of 2.5 M KCl in buffer E. The active fractions were combined to give a Blue Sepharose CL-6B fraction (51 ml).
This sample was concentrated in a dialysis sac against polyethylene glycol 20,000, dialyzed against buffer A, and then applied to a Mono Q column (0.5 by 10 cm) (fast protein liquid chromatography system) previously equilibrated with buffer A. The column was washed with buffer A for 5 min, and then a linear KCl concentration gradient from 0 to 1 M was established over the next 60 min at a flow rate of 1 ml per min. The active fractions were combined to give a Mono Q fraction (2 ml).
This sample was dialyzed against buffer A and then applied to a CoA affinity column (0.6 by 2 cm) previously equilibrated with buffer A. Undesired proteins were removed from the column by washing with buffer A. The enzyme was then eluted at a flow rate of 0.1 ml/min with buffer A containing 250 mM KCl. The most active fraction (1 ml) was pooled and stored at 4°C.
Protein determination.
The protein concentration was measured by the method of Bradford (8), with bovine serum albumin as the standard.
Determination of molecular mass.
The molecular mass of the native HMG-CoA reductase was estimated by gel filtration on a Toyopearl HW65 (2 by 90 cm) and a Superdex 200 (1.6 by 60 cm) column which were equilibrated in buffer B. The column was eluted at a flow rate of 0.3 ml/min, and fractions of 2 ml were collected. Molecular mass was estimated by comparing the elution of HMG-CoA reductase with those of the standard proteins: ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), and bovine serum albumin (66 kDa).
pH-dependent activity of HMG-CoA reductase.
The radiometric assay was conducted as described above. Assay solutions consisted of 25 mM potassium phosphate at pH 6.0 to 7.6, 75 mM Tris-HCl at pH 7.6 to 9.0, 100 mM HEPES-KOH at pH 6.8 to 8.2, and 50 mM Gly-KOH at pH 8.5 to 9.5. Contained in the buffers used were 1 mM EDTA, 1 mM dithiothreitol, 50 mM KCl, and 10% glycerol.
Electrophoresis.
SDS-polyacrylamide gel electrophoresis (PAGE) and native PAGE were performed in slab gels of 8 to 25% polyacrylamide gradient gel with PhastSystem (Pharmacia). Protein was visualized by Coomassie brilliant blue R-250 staining.
Amino-terminal sequence determination.
Purified HMG-CoA reductase was applied to SDS-PAGE. Electrophoretic transfer to polyvinylidene difluoride membrane after SDS-PAGE was performed by the method of Towbin et al. (47). Amino acid sequencing was performed on a protein sequencer 492A (Applied Biosystems). The first 30 residues of the amino-terminal sequence were determined to be TETHAIAGVPMRWVGPLRISGNVAETETQV.
Cloning and DNA sequencing of the hmgr gene from Streptomyces sp. strain CL190 genome.
On the basis of the partial N-terminal sequence HAIAGVPMRW of HMG-CoA reductase from CL190, an oligonucleotide probe, 5′-CACGCSATCGCSGGGGTSCCSATGCGSTGG-3′, was synthesized (Amersham and Pharmacia) and used for Southern hybridization blotting with total DNA of CL190 and for colony hybridization with a genomic library of CL190. DNA manipulations and transformation in E. coli were done as described by Sambrook et al. (40). Chromosomal DNA extracted from Streptomyces sp. strain CL190 was partially digested with Sau3AI and size fractionated (2 to 4 kbp) by agarose gel electrophoresis. The 2- to 4-kbp DNA fragments were ligated to BamHI- and phosphatase-treated pUC118 (Takara). The resulting plasmids were used as a genomic library of Streptomyces sp. strain CL190.
DNA sequence analysis.
DNA sequence was determined by the dideoxy chain termination method (41) with an automated sequencer (model 4000L; Li-Cor) and the protocol of the supplier. A homology search with protein databases was performed by the FASTA program (30, 38). Amino acid sequences aligned by the GENETYX program (Software Development) were then edited visually to align consensus motifs.
Construction of the plasmid for overexpression in E. coli of the hmgr gene.
On the basis of the entire nucleotide sequence of HMG-CoA reductase from Streptomyces sp. strain CL190, two oligonucleotide primers, 5′-GGGGGATCCAGCCGCTCGGTTCTCGTCACC-3′ (5′ of the hmgr gene) and 5′-CCCAAGCTTGTGACCCATTCCCAGTCCGCC-3′ (3′ of the hmgr gene), including BamHI and HindIII restriction sites (underlined), were synthesized (Pharmacia) and used together with total DNA from Streptomyces sp. strain CL190 to amplify the hmgr gene. With Taq DNA polymerase (Boehringer) and the protocol of the supplier, a 1,074-bp fragment was amplified. The PCR fragment was cleaved with BamHI and HindIII and cloned into pUC118 (Takara). Strain JM109 (Takara) was used as a recipient in the transformation. Clones were analyzed for the correct insert by DNA sequencing as described above. A correct fragment was cloned into the multicloning site of the expression vector pQE30 (Qiagen) to give pQHR30. It was designed to have an affinity tag consisting of just six consecutive histidine residues in the N-terminal region of the recombinant enzyme. Ni-nitrilotriacetic acid resin has a strong affinity for protein which has such histidine residues.
Expression and purification of the recombinant HMG-CoA reductase.
E. coli M15 containing pREP4 [neo lacI] (Qiagen) was used as a host for expression of the hmgr gene. M15 (pREP4, pQHR30) was cultured at 37°C in 100 ml of Luria-Bertani medium containing 25 μg of kanamycin (Nacalai) per ml and 200 μg of ampicillin (Sigma) per ml for 5 h with 2 mM isopropyl-β-d-thiogalactoside (IPTG) added when an optical density at 660 nm of 0.8 was attained. Cells were harvested by centrifugation and resuspended in buffer F containing 1 mM PMSF. After brief sonication, the lysate was centrifuged at 10,000 × g for 20 min, and the supernatant was retained. The crude extract was applied to a Ni-nitrilotriacetic acid agarose column (1.3 by 2 cm) (Qiagen) previously equilibrated with buffer F containing 1 mM PMSF at a flow rate of 0.5 ml/min. Passing fractions (5 ml) were concentrated in a dialysis sac against polyethylene glycol 20,000, dialyzed against buffer A, and then applied to a Mono Q column (0.5 by 10 cm) previously equilibrated with buffer A. Elution was performed as described for the purification of the Streptomyces sp. strain CL190 enzyme.
Nucleotide sequence accession number.
The nucleotide sequence of the hmgr gene has been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB013457.
RESULTS
Purification of HMG-CoA reductase.
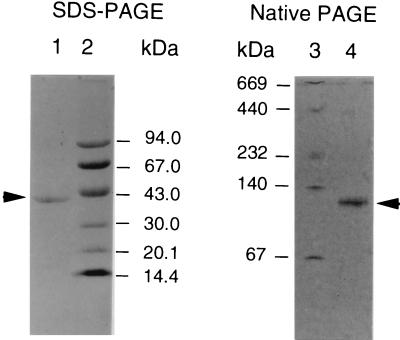
A summary of the procedure used to purify HMG-CoA reductase from Streptomyces sp. strain CL190 is presented in Table 1. Interestingly, after the first three steps, consisting of DEAE Sephacel column chromatography, ammonium sulfate precipitation, and heat treatment, no decrease of the total HMG-CoA reductase activity was observed, probably due to the removal of inhibitory compounds present in enzyme solution. These steps had relatively little influence on the purity of HMG-CoA reductase. On the other hand, Butyl Toyopearl and Blue Sepharose chromatographies resulted in an increase in the purification fold from 9.6 to 510, although recoveries decreased to only 21% compared with the activity present in the Blue Sepharose pool. After Mono Q and CoA affinity chromatographies, the enzyme was purified 3,000-fold from the initial step, with a yield of 2.1% (Table 1). The purified HMG-CoA reductase showed a single band by SDS-PAGE and native PAGE (Fig. 1).
TABLE 1.
Purification of HMG-CoA reductase
| Step | Total protein (mg) | Total activity (U) | Sp act (U/mg of protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 5,500 | 2,900 | 0.53 | 100 | 1 |
| DEAE Sephacel | 2,200 | 2,900 | 1.3 | 100 | 2.5 |
| Ammonium sulfate (0–45%) | 900 | 2,700 | 3.0 | 93 | 5.7 |
| Heat treatment (60°C, 10 min) | 570 | 2,900 | 5.1 | 100 | 9.6 |
| Butyl Toyopearl 650M | 44 | 1,400 | 32 | 48 | 60 |
| Blue Sepharose CL-6B | 2.3 | 620 | 270 | 21 | 510 |
| Mono Q | 0.50 | 250 | 500 | 8.6 | 940 |
| CoA affinity column | 0.040 | 62 | 1,600 | 2.1 | 3,000 |
FIG. 1.
Electrophoresis of the purified HMG-CoA reductase. Purified HMG-CoA reductase was analyzed by SDS–8 to 25% PAGE (left) and native 8 to 25% PAGE (right). Lanes: 1, SDS-treated enzyme (0.8 μg); 2 and 3, molecular mass standard; 4, native enzyme (1.0 μg). Proteins were stained with Coomassie brilliant blue R-250.
Determination of molecular mass of the HMG-CoA reductase from CL190.
The apparent molecular mass of the HMG-CoA reductase was estimated to be 105 kDa by Toyopearl HW 65 and 100 kDa by Superdex 200 gel filtration. SDS-PAGE showed a subunit molecular mass of 41 kDa. Native PAGE gave a single protein band with a mobility corresponding to 120 kDa (Fig. 1). Native PAGE depends on both charge and size. Thus, based on the gel filtration results, CL190 HMG-CoA reductase is most likely to form a dimer.
Optimum pH.
HMG-CoA reductase was most active in phosphate buffer. The optimum activity of the enzyme occurred at around pH 7.2.
Reaction temperature and heat stability.
The effect of temperature on the activity of the enzyme was investigated over the range of 15 to 60°C. Maximum activity was observed at around 35 to 60°C. The activation energy was estimated to be 200 kJ per mol by an Arrhenius plot whose curve was straight over the range of 20 to 30°C (data not shown). This value was higher than those reported for Sulfolobus solfataricus (7) and Raphanus sativus (3) (Table 2). The purified enzyme was heat stable at 55°C, but heating at 70°C led to 55% loss of the activity.
TABLE 2.
Comparison of the enzymatic properties of Streptomyces sp. strain CL190 HMG-CoA reductase with those of other biosynthetic HMG-CoA reductases
| Sample source | Vmax (μU/mg of protein) |
Km (μM)
|
Ki (nM) | Km/Ki | pH | Activation energy (kJ/mol) | Reference or source | |
|---|---|---|---|---|---|---|---|---|
| NADPH | HMG-CoA | |||||||
| Eucaryotes | ||||||||
| Yeast | 89 | 2.4 | 6.5 | 24d | ||||
| Rat | 4.0 | 0.64,a 1.4b | 6,300,a 2,900b | 1g | ||||
| 30 | 17 | 6.3–7.3 | 25h | |||||
| 30 | 0.5 | 34d | ||||||
| Syrian hamster | 37 | 35 | 4.3 | 5.5a | 780 | 6.2–6.8 | 18f | |
| 62 | 8.2 | 6.8 | 17f | |||||
| Human | ||||||||
| 58 kDa | 2.0 | 0.2a | 10,000 | 7.2 | 33f | |||
| 52 kDa | 2.5 | 0.3a | 8,300 | |||||
| Raphanus sativus | 27 | 1.5 | 7.5 | 92 | 3d | |||
| Archaebacteria | ||||||||
| Haloferax volcanii | 34 | 66 | 60 | 15a | 4,000 | 7.3 | 5f | |
| Halobacterium halobium | 20 | 20a | 1,000 | 10g | ||||
| Sulfolobus solfataricus | 17 | 23 | 17 | 5.5 | 47 | 7f | ||
| Eubacterium | ||||||||
| Streptomyces sp. strain CL190 | ||||||||
| 62 | 7.7 | 5.5c | 1,400 | 7.2 | 200 | This studyd | ||
| 52 | 7.3 | This studye | ||||||
| 3.5 | 20 | 2.7 | This studyf | |||||
Lovastatin was used as the inhibitor.
ML-236B was used as the inhibitor.
Pravastatin was used as the inhibitor.
Radiometric assay (purified enzyme).
Radiometric assay (recombinant enzyme).
Spectrophotometric assay (recombinant enzyme).
Radiometric assay (partially purified enzyme).
Spectrophotometric assay (purified enzyme).
Kinetic parameters of the HMG-CoA reductase from CL190.
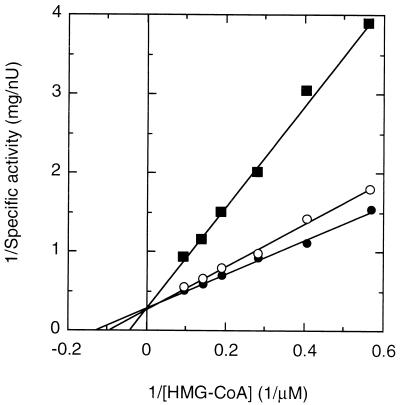
Km values calculated from double-reciprocal plots were 62 μM for NADPH and 7.7 μM for HMG-CoA, and Vmax was 3.3 nU per mg of protein at 30°C. No activity was detectable when NADH was substituted for NADPH. Pravastatin (1, 10), which competitively inhibits HMG-CoA reductase activity, inhibited the HMG-CoA reductase from CL190. From Fig. 2, summarizing pravastatin’s mode of inhibition, a Ki value of 5.5 nM was calculated for pravastatin. Thus, CL190’s Km (HMG-CoA)/Ki (pravastatin) ratio of 1,400 was similar to the Km (HMG-CoA)/Ki (mevinolin) ratio for Halobacterium halobium (10) and rat liver HMG-CoA reductase (1) (Table 2).
FIG. 2.
Double-reciprocal plots for inhibition by pravastatin. HMG-CoA reductase activity of the purified CL190 enzyme was assayed in the presence of 0 μM (●), 1 μM (○), or 10 μM (■) pravastatin as described in Materials and Methods, except that the assay was initiated by adding NADPH. Each point represents the average of two determinations.
Comparison of amino acid sequences of the HMG-CoA reductases from various origins.
The hmgr gene of CL190 encodes 353 amino acid residues with a predicted molecular mass of 37,723 Da, which is the smallest value that has been reported so far. The deduced amino acid sequence of the hmgr gene showed significant sequence similarity to the catalytic domains of HMG-CoA reductase from eucaryote and to archaebacterial HMG-CoA reductases in the databases of the DDBJ (Fig. 3).
FIG. 3.
Comparison of amino acid sequences of HMG-CoA reductase. A multiple alignment of the amino acid sequences was determined by using the GENETYX program. Identical amino acids among nine proteins are marked by asterisks and sharps. Dashes indicate gaps introduced for optimization of the alignment. Indicated numbers refer to amino acid positions. CL, Streptomyces sp. strain CL190; Hs, Homo sapiens (SWISS-PROT, P04035); Rn, Rattus norvegicus (SWISS-PROT, P51639); Dm, Drosophila melanogaster (SWISS-PROT, P14773); At, A. thaliana (SWISS-PROT, P14891); Sc, Saccharomyces cerevisiae (SWISS-PROT, P12683); Mj, Methanococcus jannaschii (SWISS-PROT, Q58116); Ss, Sulfolobus solfataricus (DAD, U95360); Hv, Haloferax volcanii (SWISS-PROT, Q59468). I, II, III, and IV indicate proposed binding sites for HMG-CoA (I and II) and NAD(P) (III and IV) (29). Sharps indicate amino acids that have been proposed to function in catalysis (6, 13, 14, 18, 48). Motifs A to G indicate regions in which the sequences were highly conserved. The dotted line over the sequences after motif G indicates putative kinase recognition sequences that are conserved in higher eucaryotes.
Purification and enzymatic properties of the recombinant enzyme.
We assumed that the recombinant HMG-CoA reductase from CL190 had a strong affinity for Ni-nitrilotriacetic acid resin. However, the enzyme activity was found only in the passing fraction. The proteins eluted from the column with buffer F containing 200 mM imidazole showed no HMG-CoA reductase activity. Steric hindrance around the N-terminal region may weaken the binding between a 6-His tag and a Ni-nitrilotriacetic acid resin. Thus, the passing fraction was further purified by Mono Q column chromatography. The purified enzyme afforded a homogeneous protein band by SDS-PAGE with a molecular mass of 41 kDa. To compare the properties of the recombinant enzyme with those of the CL190 enzyme, we carried out the HMG-CoA reductase assay by a radiometric method. The optimal activity of the HMG-CoA reductase expressed in E. coli occurred between pH 7.0 and 7.4 in potassium phosphate buffer, with a Vmax of 3.1 nU per mg of protein. The Km values calculated from double-reciprocal plots were 52 μM for NADPH and 7.3 μM for HMG-CoA. The enzyme showed no activity when NADH was substituted for NADPH. Thus, it was proven that the recombinant enzyme had the same nature as the CL190 enzyme.
Comparison of kinetic parameters.
To compare the kinetic parameters from the recombinant enzyme with those from well-characterized eucaryotic or archaebacterial HMG-CoA reductases, we conducted the HMG-CoA reductase assay by the spectrophotometric method. The Km (20 μM) of the recombinant enzyme for NADPH parallels the Km values for archaebacterial HMG-CoA reductases. The Km (2.7 μM) of the recombinant enzyme for HMG-CoA was close to those for eucaryotic HMG-CoA reductases. However, the Vmax (3.5 μU/mg of protein) of the recombinant enzyme was lower than those of the enzymes from eucaryotes and archaebacteria by a factor of 5 to 10 (Table 2).
DISCUSSION
We successfully purified the biosynthetic HMG-CoA reductase from Streptomyces sp. strain CL190 by a procedure consisting of (NH4)2SO4 precipitation, heat treatment and anion exchange, hydrophobic interaction, and affinity chromatographies. The final products appeared to be homogeneous as judged by SDS-PAGE.
The purified enzyme showed enzymatic properties similar to those of other biosynthetic HMG-CoA reductases as summarized in Table 2. A significant difference from other HMG-CoA reductases was observed in the low Vmax value of the CL190 enzyme. We speculate that this may result, at least in part, from the presence of a negatively charged amino acid (Asp) at position 345, located only six residues after the predicted catalytic His339 in the amino acid sequence of CL190, because in both Syrian hamster and P. mevalonii the mutant enzymes introduced with Asp at the corresponding position exhibited only 10% of the parent enzymatic activity (15, 36).
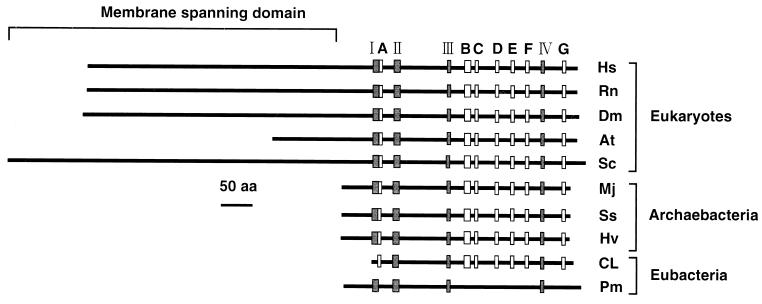
The hmgr gene encodes a 353-residue HMG-CoA reductase with a predicted molecular mass of 37,723 Da. It should be noted that the subunit molecular mass of the enzyme was smaller than any other eucaryotic and archaebacterial enzymes reported so far. The membrane anchor domain, which is not essential for catalytic activity, consists of as many as eight transmembrane helices in mammals (27, 34), whereas it is truncated in plant HMG-CoA reductases and absent from the known noneucaryote HMG-CoA reductases (Fig. 4). The N-terminal sequence of the CL190 enzyme also lacks the multiple hydrophobic segments. Furthermore, it has been reported that phosphorylation-mediated regulation of HMG-CoA reductase activity by AMP-activated protein kinase involves a single serine, Ser871, of rat (11) and hamster enzymes (37, 42) or Ser577 of isoform 1 of Arabidopsis thaliana HMG-CoA reductase (12) (Fig. 3). However, amino acid residues 339 to 349 of CL190 HMG-CoA reductase indicate the absence of a suitable target Ser for the kinase and putative kinase recognition sequence (15, 16). On the other hand, the entire amino acid sequence of the CL190 enzyme showed significant sequence similarity (37 to 40%) to the catalytic domains of the HMG-CoA reductases from the eucaryotes and archaebacteria (Fig. 3). Furthermore, three amino acid residues (E, D, and H), which have been implicated by mutagenesis and kinetic analysis as functioning in catalysis by H. volcanii (6), Syrian hamster (13, 18), and P. mevalonii (14, 48) HMG-CoA reductases, were also conserved in the CL190 enzyme (Fig. 3).
FIG. 4.
Structures and conserved motifs of the HMG-CoA reductase proteins. Location of conserved motifs I to IV and A to G are indicated by shaded and open boxes, respectively. Pm indicates the HMG-CoA reductase from P. mevalonii. Other abbreviations used are as in Fig. 3.
As shown in Fig. 3, the HMG-CoA binding motif of an E83 loop (motif II) and NAD(P) binding motifs of DAMGXN (motif III) and GX2G2XT (motif IV), which had been proposed on the basis of the crystal structure analysis of the HMG-CoA reductase from P. mevalonii (29), were found in the amino acid sequence deduced from the CL190 hmgr gene. However, an EX3GX4P motif (motif I), which had been reported for the HMG-CoA binding site (29), was not conserved in the amino acid sequence of the CL190 enzyme. In addition, the amino acid sequence of the CL190 HMG-CoA reductase revealed several additional limited motifs (A to G) which were highly conserved and common to other biosynthetic HMG-CoA reductases (Fig. 3). However, these motifs are not found in the amino acid sequence of the P. mevalonii HMG-CoA reductase (Fig. 4). These sequence conservations in the biosynthetic HMG-CoA reductases suggest strong evolutionary pressure to maintain these amino acid residues at specific positions, thus indicating that seven motifs (A to G) might play important roles in the structural conformation and/or catalytic properties of the enzyme. Crystal structure analysis of the HMG-CoA reductase from CL190 is indispensable for a thorough understanding of the functional motifs. Since the CL190 enzyme showed high heat stability, its three-dimensional structure is suitable for analysis by X-ray crystallography.
ACKNOWLEDGMENTS
S. Takahashi and T. Kuzuyama contributed equally to this work.
This work was supported in part by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture, Japan (09760114 to T.K.), by a Grant-in-Aid for Scientific Research (B), the Ministry of Education, Science, Sports and Culture, Japan (no. 10460047), to H.S., and by a Research for the Future Program (RFTF) grant from the Japanese Society for the Promotion of Science (JSPS), JSPS-RFTF96I00301, to H.S.
REFERENCES
- 1.Alberts A W, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amemiya Y, Miyahara J. Imaging plate illuminates many fields. Nature. 1988;336:89–90. doi: 10.1038/336089a0. [DOI] [PubMed] [Google Scholar]
- 3.Bach T J, Rogers D H, Rudney H. Detergent-solubilization, purification, and characterization of membrane-bound 3-hydroxy-3-methylglutaryl-coenzyme A reductase from radish seedlings. Eur J Biochem. 1986;154:103–111. doi: 10.1111/j.1432-1033.1986.tb09364.x. [DOI] [PubMed] [Google Scholar]
- 4.Beach M J, Rodwell V W. Cloning, sequencing, and overexpression of mvaA, which encodes Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Bacteriol. 1989;171:2994–3001. doi: 10.1128/jb.171.6.2994-3001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff K M, Rodwell V W. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase from Haloferax volcanii: purification, characterization, and expression in Escherichia coli. J Bacteriol. 1996;178:19–23. doi: 10.1128/jb.178.1.19-23.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff K M, Rodwell V W. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase of Haloferax volcanii: role of histidine 398 and attenuation of activity by introduction of a negative charge at position 404. Protein Sci. 1997;6:1–6. doi: 10.1002/pro.5560060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochar D A, Brown J R, Doolittle W F, Klenk H-P, Lam W L, Schenk M E, Stauffacher C V, Rodwell V W. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase of Sulfolobus solfataricus: DNA sequence, phylogeny, expression in Escherichia coli of the hmgA gene, and purification and kinetic characterization of the gene product. J Bacteriol. 1997;179:3632–3638. doi: 10.1128/jb.179.11.3632-3638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown M S, Goldstein J L. Multivalent feedback regulation of HMG-CoA reductase, a control mechanism coodinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- 10.Cabrera J A, Bolds J, Shields P E, Havel C M, Watson J A. Isoprenoid synthesis in Halobacterium halobium. J Biol Chem. 1986;261:3578–3583. [PubMed] [Google Scholar]
- 11.Clarke P R, Hardie D G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale S, Arro M, Becerra B, Morrice N G, Boronat A, Hardie D G, Ferrer A. Bacterial expression of the catalytic domain of 3-hydroxy-3-methylglutaryl-CoA reductase (isoform HMGR1) from Arabidopsis thaliana, and its inactivation by phosphorylation at Ser577 by Brassica oleracea 3-hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem. 1995;233:506–513. doi: 10.1111/j.1432-1033.1995.506_2.x. [DOI] [PubMed] [Google Scholar]
- 13.Darnay B G, Rodwell V W. His865 is the catalytically important histidyl residue of syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:8429–8435. [PubMed] [Google Scholar]
- 14.Darnay B G, Wang Y, Rodwell V W. Identification of the catalytically important histidine of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1992;267:15064–15070. [PubMed] [Google Scholar]
- 15.Friesen J A, Rodwell V W. Protein engineering of the HMG-CoA reductase of Pseudomonas mevalonii. Construction of mutant enzymes whose activity is regulated by phosphorylation and dephosphorylation. Biochemistry. 1997;36:2173–2177. doi: 10.1021/bi962254w. [DOI] [PubMed] [Google Scholar]
- 16.Friesen J A, Rodwell V W. Identification of elements critical for phosphorylation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by adenosine monophosphate-activated protein kinase: protein engineering of the naturally nonphosphorylatable 3-hydroxy-3-methylglutaryl coenzyme A reductase from Pseudomonas mevalonii. Biochemistry. 1997;36:1157–1162. doi: 10.1021/bi962104l. [DOI] [PubMed] [Google Scholar]
- 17.Frimpong K, Darnay B G, Rodwell V W. Syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase expressed in Escherichia coli: production of homogeneous protein. Protein Expr Purif. 1993;4:337–344. doi: 10.1006/prep.1993.1044. [DOI] [PubMed] [Google Scholar]
- 18.Frimpong K, Rodwell V W. Catalysis by syrian hamster 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proposed roles of histidine 865, glutamate 558, and aspartate 766. J Biol Chem. 1994;269:11478–11483. [PubMed] [Google Scholar]
- 19.Funayama S, Ishibashi M, Komiyama K, Omura S. Biosynthesis of furaquinocins A and B. J Org Chem. 1990;55:1132–1133. [Google Scholar]
- 20.Gill J F, Jr, Beach M J, Rodwell V W. Mevalonate utilization in Pseudomonas sp. M. J Biol Chem. 1985;260:9393–9398. [PubMed] [Google Scholar]
- 21.Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 22.Horbach S, Sahm H, Welle R. Isoprenoid biosynthesis in bacteria: two different pathways? FEMS Microbiol Lett. 1993;111:135–140. doi: 10.1111/j.1574-6968.1993.tb06375.x. [DOI] [PubMed] [Google Scholar]
- 23.Isshiki K, Tamamura T, Sawa T, Naganawa H, Takeuchi T, Umezawa H. Biosynthetic studies of terpentecin. J Antibiot. 1986;39:1634–1635. doi: 10.7164/antibiotics.39.1634. [DOI] [PubMed] [Google Scholar]
- 24.Kirtley M E, Rudney H. Some properties and mechanism of action of the β-hydroxy-β-methylglutaryl coenzyme A reductase of yeast. Biochemistry. 1967;6:230–238. doi: 10.1021/bi00853a036. [DOI] [PubMed] [Google Scholar]
- 25.Kleinsek D A, Porter J W. An alternate method of purification and properties of rat liver β-hydroxy-β-methylglutaryl coenzyme A reductase. J Biol Chem. 1979;254:7591–7599. [PubMed] [Google Scholar]
- 26.Kleinsek D A, Ranganathan S, Porter J W. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Proc Natl Acad Sci USA. 1977;74:1431–1435. doi: 10.1073/pnas.74.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristin T C, Simoni R D. The role of the membrane domain in the regulated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1992;267:4236–4246. [PubMed] [Google Scholar]
- 28.Lam W L, Doolittle W F. Mevinolin-resistant mutations identify a promoter and the gene for a eukaryote-like 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the Archaebacterium Haloferax volcanii. J Biol Chem. 1992;267:5829–5834. [PubMed] [Google Scholar]
- 29.Lawrence C M, Rodwell V W, Stauffacher C V. Crystal structure of Pseudomonas mevalonii HMG-CoA reductase at 3.0 angstrom resolution. Science. 1995;268:1758–1762. doi: 10.1126/science.7792601. [DOI] [PubMed] [Google Scholar]
- 30.Lipman D J, Person W R. Rapid and sensitive similarity protein searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 31.Liscum L, Finer-Moore J, Stroud R M, Luskey K L, Brown M S, Goldstein J L. Domain structure of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J Biol Chem. 1985;260:522–530. [PubMed] [Google Scholar]
- 32.Liscum L, Cummings R D, Anderson G W, DeMartino G N, Goldstein J L, Brown M S. 3-Hydroxy-3-methylglutaryl-CoA reductase: a transmembrane glycoprotein of the endoplasmic reticulum with N-linked “high-mannose” oligosaccharides. Proc Natl Acad Sci USA. 1983;80:7165–7169. doi: 10.1073/pnas.80.23.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer R J, Debouck C, Metcalf B W. Purification and properties of the catalytic domain of 3-hydroxy-3-methylglutaryl-CoA reductase expressed in Escherichia coli. Arch Biochem Biophys. 1988;267:110–118. doi: 10.1016/0003-9861(88)90014-8. [DOI] [PubMed] [Google Scholar]
- 34.Ness G C, Spindler C D, Moffler M H. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Arch Biochem Biophys. 1979;197:493–499. doi: 10.1016/0003-9861(79)90272-8. [DOI] [PubMed] [Google Scholar]
- 35.Olender E H, Simoni R D. The intracellular targeting and membrane topology of 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem. 1992;267:4223–4235. [PubMed] [Google Scholar]
- 36.Omkumar R V, Darnay B G, Rodwell V W. Modulation of syrian hamster 3-hydroxy-3-methylglutaryl-CoA reductase activity by phosphorylation. J Biol Chem. 1994;269:6810–6814. [PubMed] [Google Scholar]
- 37.Omkumar R V, Rodwell V W. Phosphorylation of Ser871 impairs the function of His865 of syrian hamster 3-hydroxy-3-methylglutaryl-CoA reductase. J Biol Chem. 1994;269:16862–16866. [PubMed] [Google Scholar]
- 38.Person W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodwell V W, Nordstrom J L, Mitschelin J J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 41.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato R, Goldstein J L, Brown M S. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc Natl Acad Sci USA. 1993;90:9261–9265. doi: 10.1073/pnas.90.20.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seto H, Watanabe H, Furihata K. Simultaneous operation of the mevalonate and nonmevalonate pathways in the biosynthesis of isopentenyl diphosphate in Streptomyces aeriouvifer. Tetrahedron Lett. 1996;12:4411–4412. [Google Scholar]
- 44.Shin-ya K, Furihata K, Hayakawa Y, Seto H. Biosynthetic studies of naphterpin, a terpenoid metabolite of Streptomyces. Tetrahedron Lett. 1990;31:6025–6026. [Google Scholar]
- 45.Shiomi K, Iinuma H, Naganawa H, Isshiki K, Takeuchi T, Umezawa H. Biosynthesis of napyradiomycins. J Antibiot. 1987;40:1740–1745. doi: 10.7164/antibiotics.40.1740. [DOI] [PubMed] [Google Scholar]
- 46.Tormanen C D, Redd W L, Srikantaiah M V, Scallen T J. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Biochem Biophys Res Commun. 1976;68:754–762. doi: 10.1016/0006-291x(76)91209-2. [DOI] [PubMed] [Google Scholar]
- 47.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Darnay B G, Rodwell V W. Identification of the principal catalytically important acidic residue of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1990;265:21634–21641. [PubMed] [Google Scholar]
- 49.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]