ABSTRACT
Elective nodal irradiation (ENI) and involved field radiotherapy (IFRT) are definitive radiotherapeutic approaches used to treat patients with limited-disease small cell lung cancer (LD-SCLC). However, no solid consensus exists on their optimal target volume. The current study aimed to assess the clinical outcomes of patients with LD-SCLC who received definitive ENI or IFRT. A retrospective single-institution study of patients who received definitive radiotherapy between 2008 and 2020 was performed. All patients underwent whole-body positron emission tomography/computed tomography before three-dimensional conformal radiotherapy. Among the 37 patients analyzed, 22 and 15 received ENI and IFRT, respectively. The thoracic radiotherapy dose was mostly either 60 Gy in 30 fractions delivered in 2-Gy fractions once daily or 45 Gy in 30 fractions delivered in 1.5-Gy fractions twice daily. The median follow-up period was 21.4 months. A total of 12 patients (32%) experienced locoregional relapse: 10 within and 2 outside the irradiation fields. One patient in the IFRT group experienced isolated nodal failure. Differences in locoregional relapse-free, progression-free, and overall survival rates between ENI and IFRT were not significant. Overall, IFRT did not promote a significant increase in locoregional recurrence compared to ENI. Our findings suggested the utility of IFRT in standard clinical practice and support its use for patients with LD-SCLC.
Key Words: small cell lung cancer, limited disease, chemoradiotherapy, involved field radiotherapy, elective nodal irradiation
INTRODUCTION
Small cell lung cancer (SCLC) accounts for 15%–25% of all lung cancer cases, with 25%–40% exhibiting limited diseases.1 Moreover, previous reports have shown that SCLC carries a poor prognosis, with a median overall survival (OS) of only 19–27 months and 2-year OS of 37%–55%, even for limited diseases.2-6 Thoracic surgery has evolved drastically in recent years,7 however, early concurrent chemoradiation remains the standard treatment for limited-disease SCLC (LD-SCLC) given its low degree of differentiation, shorter doubling time, and high sensitivity to chemotherapy and radiotherapy. Although etoposide- and cisplatin-based regimens chemotherapy continue to be the standard of care,8,9 no solid consensus on irradiation dose or radiotherapy target volume has been established. Historically, treatment volumes included all gross disease present at the time of initial diagnosis, as well as adjacent uninvolved nodal regions elective nodal irradiation (ENI). Recent reports, on the other hand, have suggested that omitting ENI might be appropriate.2,10-12 However, available literature on involved field radiotherapy (IFRT) strategies for LD-SCLC have been much more limited compared to that for locally advanced non-small cell lung cancer (NSCLC). Thus, the current study aimed to assess the clinical outcomes in patients with LD-SCLC who received definitive radiotherapy with either ENI or IFRT.
MATERIALS AND METHODS
Patients
Records of consecutive patients with histopathologically confirmed LD-SCLC who received definitive radiotherapy at our institution between January 2008 and April 2020 were retrospectively reviewed. Clinical stages were assessed using contrast-enhanced computed tomography (CT) from the neck to the abdomen, whole-body 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT), and magnetic resonance imaging (MRI) of the brain. Complete blood count, biochemical tests, and electrocardiography were also performed to assess fitness for chemotherapy. Disease stage was based on the latest TNM classification upon diagnosis (UICC 8th edition). Limited disease was defined as a tumor confined to one hemi thorax, including regional lymph nodes, ipsilateral hilar, bilateral mediastinal, and bilateral supraclavicular nodes, without pleural effusion. Patients with a history of lung resection or thoracic radiation were excluded. All patients with early-stage disease were medically ineligible to undergo surgical resection or refused to undergo it. Treatment was decided by a tumor board that included surgeons, endoscopists, medical oncologists, and radiation oncologists. The study was approved by the institutional review board of Kyoto Prefectural University of Medicine (approval number: ERB-C-1934). Written informed consent was obtained from each patient to treatment and data collection.
Radiation treatment planning
Patients received either ENI or IFRT based on physician treatment philosophy. Physicians were consistent with treatment planning and treated all their patients with either ENI or IFRT. Although patients were not randomly assigned to either group, there was no predetermined selection process for assigning patients to the various physicians. Radiotherapy was delivered using a three-dimensional conformal radiotherapy (3D-CRT) technique typically employing 6–10 MV photons. All patients were CT-simulated, after which planning was done using the Monaco treatment planning system with dose correction for tissue heterogeneity. All plans were normalized to the isocenter or calculation point. For IFRT treatment planning, gross tumor volume (GTV) included the primary tumor and any suspected lymph nodes with a diameter at least 1 cm in the short axis in CT or positive by FDG-PET. The clinical target volume (CTV) was obtained by expanding the GTV using a margin of 5–8 mm. The ipsilateral lung hilum was electively included in the CTV. After adjusting for anatomical boundaries, the planning target volume (PTV) was determined from the CTV by a radiation oncologist who used an automatic margining tool that typically added a standard margin of 5–10 mm. For ENI treatment planning, all mediastinal lymph node stations were included in the initial treatment field as the CTV. Supraclavicular fields were treated when gross involvement of the supraclavicular nodes was observed. A subsequent cone-down included the primary tumor and, if feasible, any grossly involved lymph nodes. Thus, clinically uninvolved mediastinal lymph nodes received prophylactic radiation treatment with ENI but not with IFRT. The median PTV for the IFRT and ENI group were 179.6 mL (range, 20.4-755.3 mL) and 367.7 mL (range, 88.45–997.7 mL), respectively (p = 0.06). The dose constraints for critical structures were maximum spinal cord dose ≤ 45Gy, lung mean dose ≤ 20 Gy, lung volume that receives at least 20 Gy ≤ 35%, and heart volume that receives at least 40 Gy <50%. Although no special constraints were used for esophagus, special attention was paid to minimize the maximum doses applied to this structure.
Treatment schedules
The thoracic radiotherapy dose was mostly either 60 Gy in 30 fractions delivered in 2-Gy fractions once daily or 45 Gy in 30 fractions delivered in 1.5-Gy fractions twice daily. After 2016, patients with stage 1 disease were treated with stereotactic radiation therapy, and two received this treatment (48 Gy in 4 fractions once daily, n = 1; 60 Gy in 10 fractions once daily, n = 1). The median total dose delivered was 45 Gy in 30 fractions (range, 44–66 Gy). Twice-daily treatments were delivered with a minimum inter-fraction interval of at least a 6-hour. Our institutional policy indicates that early radiotherapy be delivered concurrently with platinum-based chemotherapy; however, due to patterns of referral or patient factors, not all patients were treated in this fashion. One patient received sequential rather than concurrent chemoradiotherapy, while another received radiotherapy alone. Although chemotherapy generally consisted of etoposide and cisplatin, usually given for 4 cycles every 3 weeks, an etoposide and carboplatin regimen was optionally used depending on the patient’s general condition and age. Patients with a complete or near-complete response after the completion of chemoradiotherapy and had a favorable clinical condition were offered prophylactic cranial irradiation (PCI; 25 Gy in 10 fractions).
Follow-up and outcome evaluation
Patients were followed up at regular intervals, usually every 3–4 months for the first 2 years after treatment, followed by every 6 months over 3–5 years. Follow-up examinations included basic laboratory tests, liver and renal function tests, chest CT, brain MRI, and PET/CT when needed. Locoregional relapse was defined as progression located inside the original primary tumor or in the hilar, mediastinal, and/or supraclavicular lymph nodes, irrespective of distant failure. Locoregional relapses located within the PTV were classified as in-field failures, whereas those occurring outside the PTV but inside the hilar, mediastinal, or/and supraclavicular area were classified as out-of-field failures. Toxicities were scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Endpoints included locoregional relapse-free (LRRF), progression-free survival (PFS), and OS. PFS was defined as the date at which any recurrence was the first observation or death from any cause occurred. All endpoints were calculated from the first day of radiotherapy to the date of an event or censoring. Corresponding survival curves were estimated using the Kaplan–Meier method. Univariate analyses estimated differences between groups through the log-rank test. Comparisons between groups of patients were assessed using Chi-square test or Mann–Whitney U test. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Australia). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatics.13 The level of significance was conventionally set at p < 0.05 for all analyses.
RESULTS
Patient characteristics
Between January 2008 and April 2020, 37 patients with LD-SCLC were treated in our hospital, among whom 22 and 15 were irradiated with ENI and IFRT, respectively. Patients in both treatment groups had similar age, sex, baseline Eastern Cooperative Oncology Group performance status (PS), clinical stage, and TN stage, chemotherapy regimen, cycles of received chemotherapy, and pretreatment levels of serum lactate dehydrogenase. Patients’ characteristics are summarized in Table 1. All but one patient completed the planned radiotherapy (97%). One patient’s treatment was stopped after 44 Gy in 22 fractions because of prolonged recovery of grade 4 neutropenia and grade 3 esophagitis. Among the included patients, 28 (76%) and 4 (11%) started radiotherapy during their first and second cycles of chemotherapy, respectively. Given that some patients eligible for PCI after thoracic radiotherapy did not follow the doctor’s advice or refused to receive it, four patients ultimately received PCI.
Table 1.
Patient characteristics
| Characteristics | ENI (n = 22) | IFRT (n = 15) | p value |
| Age | |||
| Median (range), years | 68 (25–83) | 73 (59–81) | |
| ≤70 | 14 | 6 | 0.19 |
| >70 | 8 | 9 | |
| Sex, n | |||
| Male | 16 | 14 | 0.2 |
| Female | 6 | 1 | |
| Performance status, n | |||
| 0 | 19 | 9 | 0.12 |
| 1–2 | 3 | 6 | |
| Clinical stage | |||
| I | 3 | 3 | 0.72 |
| II | 5 | 4 | |
| III | 14 | 8 | |
| T Stage, n | |||
| 1–2 | 14 | 9 | 0.99 |
| 3–4 | 8 | 6 | |
| N Stage, n | |||
| 0 | 4 | 6 | 0.26 |
| 1–3 | 18 | 9 | |
| Chemotherapy regimen, n | |||
| Cisplatin-Etoposide | 15 | 10 | 0.99 |
| Others | 7 | 5 | |
| Cycles of chemotherapy, n | |||
| 0–3 | 4 | 7 | 0.08 |
| 4 | 18 | 8 | |
| Fractionation scheme | |||
| Once daily | 7 | 6* | 0.73 |
| Twice daily | 15 | 9 | |
| Pre-treatment serum LDH, n | |||
| <222 U/L | 12 | 8 | 0.99 |
| ≥222 U/L | 10 | 7 |
ENI: elective nodal irradiation
IFRT: involved field irradiation
LDH: lactate dehydrogenase
* Including 2 cases treated with stereotactic radiotherapy.
Patterns of failure
Twenty-three patients (62%) experienced locoregional and/or distant failure, most of whom were treated with chemotherapy (18/23). The most common initial failure pattern was distant metastasis (15/23). In our patient cohort, 12 patients (32%) had a locoregional relapse; in 6 cases this was the first failure event and in 2 cases distant metastases have been diagnosed simultaneously. The remaining four patients relapsed distantly first. Out-of-field recurrence was observed in two patients in the IFRT group, one of whom experienced isolated nodular failure (INF) that represented a single out-of-field nodal failure in the absence of other recurrence sites.
Treatment outcomes
The median follow-up period was 21.4 months (range, 6.2–114.6). At the last follow-up, 23 patients (62%) had died. Among these patients, 21 died of SCLC and 2 died of other causes (acute aortic dissection, n = 1; oropharyngeal cancer, n = 1). The 2-year OS, PFS, LRRF rates were 54%, 35%, and 61%, respectively (Fig 1). Table 2 presents the results of univariate analysis for PFS, LRRF, and OS. Accordingly, the clinical stage was correlated with both OS and PFS, while the age factor was correlated only with PFS. No significant differences in LRRF, PFS, and OS were observed between the ENI and IFRT group (Fig 2). The use of salvage chemotherapy (SCT) after the identification of recurrence was determined by the physicians according to each patient’s general condition, clinical course, and function of organs. Eighteen patients received amrubicin-based SCT and 5 patients received the best supportive care (BSC). SCT patients had a significantly better median OS (8.4 months versus 4.3 months, p<0.001) compared with BSC patients.
Fig. 1.
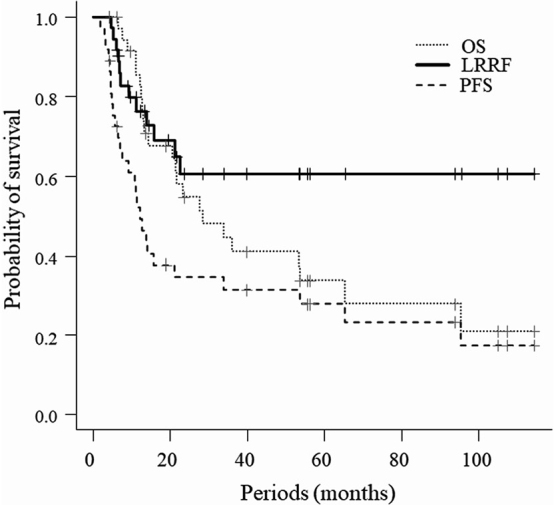
Overall survival, progression-free survival, and locoregional relapse-free rate for patients with limited-disease small cell lung cancer treated with radiotherapy
OS: overall survival
LRRF: locoregional relapse free
PFS: progression-free survival
Table 2.
Univariate analysis for PFS, LRRF, and OS rates
| PFS rate (%) | LRRF rate (%) | OS rate (%) | ||||||||||
| Factors | n | 1 year | 2 years | p value | 1 year | 2 years | p value | 1 year | 2 years | p value | ||
| Age | ||||||||||||
| ≤70 | 20 | 69 | 50 | 0.016 | 87 | 73 | 0.2 | 94 | 61 | 0.1 | ||
| >70 | 17 | 37 | 20 | 67 | 48 | 78 | 50 | |||||
| Stage | ||||||||||||
| I | 6 | 80 | 80 | 0.01 | 100 | 100 | 0.09 | 80 | 80 | 0.02 | ||
| II | 9 | 52 | 26 | 64 | 32 | 88 | 44 | |||||
| III | 22 | 38 | 18 | 76 | 60 | 80 | 44 | |||||
| PS | ||||||||||||
| 0 | 28 | 45 | 26 | 0.1 | 76 | 55 | 0.59 | 81 | 52 | 0.32 | ||
| 1–2 | 9 | 63 | 63 | 75 | 75 | 87 | 63 | |||||
| LDH (U/L) | ||||||||||||
| <222 | 20 | 58 | 43 | 0.42 | 79 | 65 | 0.55 | 81 | 54 | 0.34 | ||
| ≥222 | 17 | 44 | 25 | 74 | 56 | 89 | 55 | |||||
| CT regimen | ||||||||||||
| PE | 25 | 48 | 36 | 0.43 | 75 | 68 | 0.42 | 84 | 54 | 0.5 | ||
| Others | 12 | 61 | 31 | 68 | 46 | 90 | 57 | |||||
| Cycles of CT | ||||||||||||
| 0–3 | 11 | 51 | 34 | 0.71 | 74 | 81 | 0.78 | 92 | 61 | 0.31 | ||
| 4 | 26 | 55 | 36 | 59 | 65 | 73 | 41 | |||||
| FR scheme | ||||||||||||
| Once daily | 13 | 58 | 49 | 0.17 | 74 | 64 | 0.87 | 75 | 75 | 0.21 | ||
| Twice daily | 24 | 49 | 27 | 78 | 58 | 91 | 46 | |||||
| RT field | ||||||||||||
| IFRT | 15 | 53 | 40 | 0.72 | 72 | 62 | 0.82 | 87 | 47 | 0.77 | ||
| ENI | 12 | 51 | 36 | 80 | 60 | 85 | 62 | |||||
PFS: progression-free survival
LRRF: locoregional relapse free
OS: overall survival
PS: performance status
LDH: lactate dehydrogenase
CT: chemotherapy
PE: cisplatin-etoposide
FR: fractionation
RT: radiotherapy
IFRT: involved field irradiation
ENI: elective nodal irradiation
Fig. 2.
Kaplan-Meier curves for patients treated with involved field radiotherapy (IFRT) versus elective nodal irradiation (ENI)
Fig. 2A: Locoregional relapse free
Fig. 2B: Overall survival
Fig. 2C: Progression-free survival
Toxicity
In the ENI group, all patients experienced grade ≥3 acute hematological adverse events (AEs); leukopenia of grades 3 and 4 was seen in 6 (27%) and 12 patients (55%), anemia in 4 (18%) and 1 (5%) patient, and thrombocytopenia in 2 (9%) and 2 patients (9%), respectively. On the other hand, for non-hematological acute AEs, radiation esophagitis of grades 2 and 3 was seen in 11 (50%) and 4 patients (18%), respectively. Pneumonitis of grade 2 was seen in 3 patients (14%). In the IFRT group, regarding the hematological AEs throughout the treatment period, leukopenia of grades 3 and 4 occurred in 2 (27%) and 9 patients (55 %), anemia in 1 (7%) and 1 (7%) patient, and thrombocytopenia in 2 (13%) and 2 patients (13%), respectively. Acute radiation esophagitis of grades 2 and pneumonitis of grades 2 was seen in 5 (33%) and 2 patients (13%), respectively. Rates of acute radiation esophagitis (grade ≥2) were higher in the ENI group than in the IFRT group (p = 0.014). Grade ≥3 non-hematological AEs were not seen in the IFRT group. Regarding late AEs, in the ENI group, there were two AEs of grades 3, including pneumonitis in one patient (at 5 months after chemoradiation) and pericardial/pleural effusion in one patient (at 18 months after chemoradiation). In the IFRT group, no patients experienced grade ≥ 3 late AEs. Table 3 shows treatment-related toxicity of grade 2 or higher.
Table 3.
Treatment-related toxicity
| ENI (n = 22) | IFRT (n = 15) | p value | ||||||
| G2 | G3 | G4 | G2 | G3 | G4 | |||
| Worst grade of hematological parameters during chemoradiotherapy | ||||||||
| Decreased leucocytes | 1 (5%) | 6 (27%) | 12 (55%) | 0 | 2 (13%) | 9 (60%) | 0.99 | |
| Decreased hemoglobin | 8 (36%) | 4 (18%) | 1 (5%) | 3 (20%) | 1 (7%) | 1 (7%) | 0.11 | |
| Decreased platelets | 7 (32%) | 2 (9%) | 2 (9%) | 2 (13%) | 2 (13%) | 2 (13%) | 0.84 | |
| Non-hematologic acute toxicity | ||||||||
| Esophagitis, dysphagia | 11 (50%) | 4 (18%) | 0 | 5 (33%) | 0 | 0 | 0.014 | |
| Pneumonitis | 3 (14%) | 0 | 0 | 2 (13%) | 0 | 0 | >0.99 | |
| Nausea | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 0.44 | |
| Non-hematologic late toxicity | ||||||||
| Lung | 3 (14%) | 1 (5%) | 0 | 2 (13%) | 0 | 0 | 0.68 | |
| Pericardial/pleural effusion | 0 | 1 (5%) | 0 | 1 (7%) | 0 | 0 | 0.84 | |
G: grade
IFRT: involved field irradiation
ENI: elective nodal irradiation
DISCUSSION
The present study aimed to assess the clinical outcomes in patients with LD-SCLC who received definitive radiotherapy with either ENI or IFRT. There have been no studies directly comparing the treatment outcomes of ENI and IFRT incorporating the PET staging/target definition. Our findings showed 1- and 2-year LRRF rates of 72% and 62% in the IFRT group and 80% and 60% in the ENI group, respectively, with no significant differences having been observed between both treatment groups. Moreover, no significant differences in OS and PFS were observed between the treatment groups. Results regarding the OS and locoregional control in our patient cohort are in line with those presented in previously published studies.2-6
Modern thoracic radiotherapy has tended toward using more conformal and smaller fields.14,15 Although IFRT has been proven to be considerably adequate in NSCLC,16-23 no final consensus exists on omitting ENI in LD-SCLC. A phase II study that omitted ENI in patients with LD-SCLC based solely on CT scans resulted in a high proportion (3/27, crude rate of 11%) of INF despite its small sample size.24 Another phase II trial by Baas et al that evaluated recurrence patterns in 36 patients in whom ENI was omitted based on CT scan observed INF in 2 patients (5.5%).25 Moreover, Xia et al showed an INF rate of 4.6% in a retrospective IFRT based on the CT scan study of 108 patients.5 PET/CT has been shown to improve the staging accuracy of patients with LD-SCLC and could potentially clearly identify involved nodal sites.26-28 Several more modern series, both retrospective and prospective, have suggested that omitting ENI resulted in lower rates of INF, particularly when incorporating PET staging/target definition.2,10-12 van Loon et al evaluated the impact of PET scan usage on elective nodal irradiation in patients with LD-SCLC, with their results subsequently showing a low INF rate of 3%.2,10 Shirvani et al showed that only 2% of patients experienced INF when PET/CT-guided omission of ENI,11 with Reymen et al reporting similar outcomes for PET-based omission of ENI.12 In our patient cohort staged using PET/CT, only one case of INF occurred. Omitting ENI, while still unresolved, may be a reasonable choice, particularly when PET is incorporated into the staging and target definition.
Thoracic radiotherapy with a reduced irradiation field for LD-SCLC has been reported to decrease esophageal and lung toxicity and improve the patient’s quality of life.29 Our results show that acute esophageal toxicity can be reduced with IFRT compared to ENI. An online survey in 2016 to determine the patterns of practice in the United States regarding ENI in the treatment of LD-SCLC revealed that nearly two thirds of respondents did not recommend ENI.30 This suggests a shift in practice over the past decade, with a 2007 survey reporting that only 18% did not recommend any form of ENI for LD-SCLC.31 Moreover, current prospective clinical trials (eg, CALGB 30610/RTOG 0538 and the EORTC 08072 trial) have omitted ENI, seemingly suggesting the emergence of a new era in which ENI is avoided in an effort to reduce toxicity.
Some limitations of the current study include its single-institution and retrospective nature. Moreover, only a small number of patients had been included, while the selection of radiotherapeutic options had not been randomized. Furthermore, the radiation oncologists made a clinical decision on the choice of the irradiation field, but there may be a bias among physicians to not select IFRT especially for patients with mediastinal lymph node metastases on PET/CT. As such, larger studies with standardized, uniform procedures should be performed to validate our results.
In conclusion, our findings suggested the utility of IFRT in standard clinical practice and support its use for patients with LD-SCLC.
ACKNOWLEDGMENTS
The authors wish to thank Enago (www.enago.jp) for the English language review.
CONFLICT OF INTEREST STATEMENTS
The authors declare that they have no conflicts of interest regarding this study.
Abbreviations
- BSC
best supportive care
- CT
computed tomography
- CTV
clinical target volume
- ENI
elective nodal irradiation
- FDG
18F-fluorodeoxyglucose
- GTV
gross tumor volume
- IFRT
involved field radiotherapy
- IMRT
intensity modulated radiotherapy
- INF
isolated nodular failure
- LD-SCLC
limited-disease small cell lung cancer
- LDH
lactate dehydrogenase
- LRRF
locoregional relapse-free
- MRI
magnetic resonance imaging
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PCI
prophylactic cranial irradiation
- PET
positron emission tomography
- PET/CT
positron emission tomography/computed tomography
- PFS
progression-free survival
- PS
Eastern Cooperative Oncology Group performance status
- PTV
planning target volume
- SCLC
small cell lung cancer
- SCT
salvage chemotherapy
- 3D-CRT
three-dimensional conformal radiotherapy
REFERENCES
- 1.Youlden DR, Cramb SM, Baade PD. The International epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3(8):819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed]
- 2.van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of 18FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77(2):329–336. doi: 10.1016/j.ijrobp.2009.04.075. [DOI] [PubMed]
- 3.Simone CB, Bogart JA, Cabrera AR, et al. Radiation therapy for small cell lung cancer: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2020;10(3):158–173. doi: 10.1016/j.prro.2020.02.009. [DOI] [PMC free article] [PubMed]
- 4.Giuliani ME, Lindsay PE, Sun A, et al. Locoregional failures following thoracic irradiation in patients with limited-stage small cell lung carcinoma. Radiother Oncol. 2012;102(2):263–267. doi: 10.1016/j.radonc.2011.12.009. [DOI] [PubMed]
- 5.Xia B, Chen GY, Cai XW, et al. Is involved-field radiotherapy based on CT safe for patients with limited-stage small-cell lung cancer? Radiother Oncol. 2012;102(2):258–262. doi: 10.1016/j.radonc.2011.10.003. [DOI] [PubMed]
- 6.Bütof R, Gumina C, Valentini C, et al. Sites of recurrent disease and prognostic factors in SCLC patients treated with radiochemotherapy. Clin Transl Radiat Oncol. 2017;7:36–42. doi: 10.1016/j.ctro.2017.09.010. [DOI] [PMC free article] [PubMed]
- 7.Chen-Yoshikawa TF, Fukui T, Nakamura S, et al. Current trends in thoracic surgery. Nagoya J Med Sci. 2020;82(2):161–174. doi: 10.18999/nagjms.82.2.161. [DOI] [PMC free article] [PubMed]
- 8.Jänne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer. 2002;95(7):1528–1538. doi: 10.1002/cncr.10841. [DOI] [PubMed]
- 9.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366(9494):1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed]
- 10.van Loon J, Offermann C, Bosmans G, et al. 18FDG-PET based radiation planning of mediastinal lymph nodes in limited disease small cell lung cancer changes radiotherapy fields: a planning study. Radiother Oncol. 2008;87(1):49–54. doi: 10.1016/j.radonc.2008.02.019. [DOI] [PubMed]
- 11.Shirvani SM, Komaki R, Heymach JV, Fossella FV, Chang JY. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):e91–e97. doi: 10.1016/j.ijrobp.2010.12.072. [DOI] [PMC free article] [PubMed]
- 12.Reymen B, Van Loon J, van Baardwijk A, et al. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85(5):1319–1324. doi: 10.1016/j.ijrobp.2012.10.003. [DOI] [PubMed]
- 13.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed]
- 14.Levy A, Faivre-Finn C. Radiotherapy tumor volume for limited-stage small cell lung cancer: less is more. Ann Transl Med. 2020;8(17):1114. doi: 10.21037/atm.2020.04.45. [DOI] [PMC free article] [PubMed]
- 15.Qiu YF, Liu ZG, Yang WJ, et al. Research progress in the treatment of small cell lung cancer. J Cancer. 2017;8(1):29–38. doi: 10.7150/jca.16822. [DOI] [PMC free article] [PubMed]
- 16.Bradley J, Bae K, Choi N, et al. A phase II comparative study of gross tumor volume definition with or without PET/CT usion in dosimetric planning for Non–small-cell lung cancer (NSCLC): primary analysis of radiation therapy oncology group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 2012;82(1):435–441.e1. doi: 10.1016/j.ijrobp.2010.09.033. [DOI] [PMC free article] [PubMed]
- 17.Belderbos JS, Kepka L, Spring Kong FM, Martel MK, Videtic GM, Jeremic B. Report from the international atomic energy agency (IAEA) consultants’ meeting on elective nodal irradiation in lung cancer: non–small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2008;72(2):335–342. doi: 10.1016/j.ijrobp.2008.04.081. [DOI] [PubMed]
- 18.Sanuki-Fujimoto N, Sumi M, Ito Y, et al. Relation between elective nodal failure and irradiated volume in non-small-cell lung cancer (NSCLC) treated with radiotherapy using conventional fields and doses. Radiother Oncol. 2009;91(3):433–437. doi: 10.1016/j.radonc.2008.12.013. [DOI] [PubMed]
- 19.Sulman EP, Komaki R, Klopp AH, Cox JD, Chang JY. Exclusion of elective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol. 2009;4(1):5. doi: 10.1186/1748-717X-4-5. [DOI] [PMC free article] [PubMed]
- 20.Topkan E, Ozdemir Y, Guler OC, et al. Comparison of involved field radiotherapy versus elective nodal irradiation in stage IIIB/C non-small-cell lung carcinoma patients treated with concurrent chemoradiotherapy: a propensity score matching study. J Oncol. 2020;2020:7083149. doi: 10.1155/2020/7083149. [DOI] [PMC free article] [PubMed]
- 21.Fernandes AT, Shen J, Finlay J, et al. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): a comparative analysis of toxicities and clinical outcomes. Radiother Oncol. 2010;95(2):178–184. doi: 10.1016/j.radonc.2010.02.007. [DOI] [PubMed]
- 22.Chen M, Bao Y, Ma HL, et al. Involved-field radiotherapy versus elective nodal irradiation in combination with concurrent chemotherapy for locally advanced non-small cell lung cancer: a prospective randomized study. Biomed Res Int. 2013;2013:371819. doi: 10.1155/2013/371819. [DOI] [PMC free article] [PubMed]
- 23.Li R, Yu L, Lin S, et al. Involved field radiotherapy (IFRT) versus elective nodal irradiation (ENI) for locally advanced non-small cell lung cancer: a meta-analysis of incidence of elective nodal failure (ENF). Radiat Oncol. 2016;11(1):124. doi: 10.1186/s13014-016-0698-3. [DOI] [PMC free article] [PubMed]
- 24.De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: A phase II trial. Radiother Oncol. 2006;80(3):307–312. doi: 10.1016/j.radonc.2006.07.029. [DOI] [PubMed]
- 25.Baas P, Belderbos JSA, Senan S, et al. Concurrent chemotherapy (carboplatin, paclitaxel, etoposide) and involved-field radiotherapy in limited stage small cell lung cancer: a Dutch multicenter phase II study. Br J Cancer. 2006;94(5):625–630. doi: 10.1038/sj.bjc.6602979. [DOI] [PMC free article] [PubMed]
- 26.Brink I, Schumacher T, Mix M, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nuc Med Mol Imaging. 2004;31(12):1614–1620. doi: 10.1007/s00259-004-1606-x. [DOI] [PubMed]
- 27.Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol. 2004;22(16):3248–3254. doi: 10.1200/JCO.2004.11.089. [DOI] [PubMed]
- 28.Bütof R, Troost EGC. The role of functional imaging in lung cancer. Clin Transl Imaging. 2018;6(6):441–447. doi: 10.1007/s40336-018-0300-0. [DOI]
- 29.Cai S, Shi A, Yu R, Zhu G. Feasibility of omitting clinical target volume for limited-disease small cell lung cancer treated with chemotherapy and intensity-modulated radiotherapy. Radiat Oncol. 2014;9(1):17. doi: 10.1186/1748-717X-9-17. [DOI] [PMC free article] [PubMed]
- 30.Farrell MJ, Yahya JB, Degnin C, et al. Elective nodal irradiation for limited-stage small-cell lung cancer: survey of US radiation oncologists on practice patterns. Clin Lung Cancer. 2020;21(5):443–449.e4. doi: 10.1016/j.cllc.2020.02.020. [DOI] [PubMed]
- 31.Kong F, Gaspar LE, Komaki R, et al. Patterns of practice in radiation dose prescription and treatment planning for patients with lung cancer among members of American Society of Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2007;69(3 Supplement):S483. doi: 10.1016/j.ijrobp.2007.07.1686. [DOI]



