ABSTRACT
This study determined prognostic factors by comparing clinico-bacterial factors based on significant elevated serum procalcitonin levels in patients with suspected bloodstream infection (BSI). We retrospectively analyzed the medical records of 1,052 patients (age ≥16 years) with fever (temperature ≥38°C) and serum procalcitonin levels of ≥2.0 ng/mL, and blood culture results. The optimal cutoff value of the significant elevation of procalcitonin was determined using the minimum P-value approach. Clinico-bacterial factors were analyzed per the procalcitonin levels, and significant independent factors for short-term survival were investigated in 445 patients with BSI. Patients with suspected BSI were aged, on average, 72.3 ± 15.1 years, and the incidence of positive blood culture was 42.3%; and the 14-day survival was 83.4%. Procalcitonin ≥100 ng/mL was the most significant predictor for survival. Multivariate analysis in patients with suspected BSI showed that estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 and procalcitonin ≥100 ng/mL were significant independent unfavorable prognostic factors. Microorganisms were similar between patients with procalcitonin level 2–99 ng/mL (n=359) and those with ≥100 ng/mL (n=86). Multivariate analysis in patients with BSI showed that eGFR <30 mL/min/1.73 m2, procalcitonin ≥100 ng/mL, and primary infectious foci were significant independent prognostic factors. Patients with foci in the gastrointestinal tract and respiratory system had unfavorable 14-day survival. In conclusions, eGFR <30 mL/min/1.73 m2 and procalcitonin ≥100 ng/mL were significant independent unfavorable prognostic factors for suspected BSI. Primary infectious foci (gastrointestinal tract and respiratory system) were associated with unfavorable short-term survival in patients with positive blood culture.
Key Words: procalcitonin, bloodstream infection, survival, renal function, blood culture
INTRODUCTION
Since Assicot et al first observed marked increases in the circulating procalcitonin levels in patients with sepsis and other clinically significant bacterial infections,1 procalcitonin levels have been measured for various purposes, including (1) identification or exclusion of sepsis or bloodstream infection (BSI), (2) severity assessment and follow-up of patients with systemic inflammation caused by microbial infection, and (3) patient-adapted antibiotic therapy.2-7 Jones et al reported that procalcitonin using thresholds of 0.5 or 0.4 ng/mL had sensitivity and specificity of 76% and 70%, respectively, for identifying bacteremia.8
Elevated procalcitonin levels can indicate severe bacterial infections including sepsis, pneumonia, meningitis, pancreatitis, urinary tract infection, and infective surgical complications and predict poor outcomes.3,4,9-15 The clinical outcomes in bacterial infections such cases may depend on the numerical values of procalcitonin levels. Elevated procalcitonin levels have been frequently observed in patients with suspected BSI. We previously investigated short-term prognostic factors in patients with fever and elevated serum procalcitonin (>2.0 ng/mL), reported that C-reactive protein ≧ 22.57 mg/dL, serum albumin <2.8 g/dL, blood urea nitrogen ≧ 32 mg/dL, and red cell distribution width ≧ 15.3 were significant independent prognostic factors for 30-day survival.16 However, only few studies have investigated the relationship between procalcitonin levels and short-term outcomes in the general clinical setting. Thus, we investigated the procalcitonin value dependency on survival, and aimed to determine the significant elevated levels of procalcitonin in patients with suspected BSI. We compared the clinico-bacterial factors according to the significant elevated levels of procalcitonin and identified prognostic factors in patients with BSI.
MATERIALS AND METHODS
Study population and medical records
Our institute is one of the major referral hospitals with more than 800 beds and 31 clinical departments. In 2010, our infection control team developed a screening system for patients with severe bacterial infections to help clinicians promptly determine diagnosis and treatment. This system was used to screen patients according to the following criteria: patients who were ≥16 years old, had fever (temperature ≥38°C), and serum procalcitonin levels ≥2.0 ng/mL.2,16 This screening system identified 1,284 patients in 7 years between September 2010 and August 2017. Among them, 1052 who had blood cultures obtained within 24 hours at procalcitonin measurement were included in the study.
Laboratory tests
Blood samples were collected from each patient to determine the serum procalcitonin and creatinine levels. The estimated glomerular filtration rate (eGFR) was calculated using the following equation as recommended by the Japanese Society of Nephrology17:
eGFR (mL/min/1.73 m2) = 194 × Serum creatinine –1.094 × Age –0.287 × 0.739 (for women)
Blood samples for biochemical tests and blood cultures were collected within 24 hours. Plasma procalcitonin levels were determined using the Cobas e411 electrochemiluminescence immunoassay analyzer (Roche Diagnostics Japan, Tokyo, Japan). The reportable range of this assay (analytic measurement range and clinical reportable range) was between 0.02 and 100 ng/mL. All assays were performed in a single laboratory. Serum creatinine concentrations were assessed using a JCA-BM2250 analyzer (Japan Electron Optics, Tokyo).
Blood culture
Blood samples were collected in SA/SN or FA/FN bottles (SYSMEX bioMérieux, Tokyo, Japan) from September 2010 to August 2013, in FAPlus/FNPlus bottles (SYSMEX bioMérieux, Tokyo, Japan) from September 2013 to January 2017, and in Plus Aerobic/F or Anaerobic Lytic/10 bottles (Becton, Dickinson and Company, Tokyo, Japan) from February 2017 to August 2017. Blood culture bottles were incubated under aerobic and anaerobic conditions in an automated BacT/ALERT 3D system (SYSMEX bioMérieux, Tokyo, Japan) from September 2010 to January 2017, and in a BD BACTEC FX system (Becton, Dickinson and Company, Tokyo, Japan) from February 2017 to August 2017, either until a positive result was obtained or for up to 7 days. Microorganisms from positive blood cultures were further identified using standard laboratory methods,18,19 manually using the MicroScan WalkAway system (Siemens Healthcare Diagnostics Japan, Tokyo), VITEK MS, VITEK MS, or VITEK 2 (SYSMEX bioMérieux, Tokyo, Japan).
Primary infectious foci causing bacteremia were determined by the results of bacterial cultures from various samples, including urine, sputa, intra-abdominal fluid, bile, and stool and by the clinical course and findings.
Outcome measures
We determined the significant elevated levels of procalcitonin in patients with suspected BSI using a minimum P-value approach for the comparison of 14-day survival. The study population was classified into eleven cohorts according to the procalcitonin levels: 2–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, 70–80, 80–90, 90–100, and ≥100 ng/mL. The incidence of positive blood culture and the 14-day survival were investigated. Next, the 14-day survival was compared between patients with procalcitonin 2–10 vs those with ≥10 ng/mL, 2–20 vs ≥20 ng/mL, 2–30 vs ≥30 ng/mL, 2–40 vs ≥40 ng/mL, 2–50 vs ≥50 ng/mL, 2–60 vs ≥60 ng/mL, 2–70 vs ≥70 ng/mL, 2–80 vs ≥80 ng/mL, 2–90 vs ≥90 ng/mL, and 2–100 vs ≥100 ng/mL, respectively.
Clinical factors (age, sex, department in which the patients were treated, eGFR, incidence of positive blood cultures, and 14-day survival) were analyzed based on the significant elevated level of procalcitonin. Additionally, short-term prognostic factors were investigated in patients with suspected BSI. For patients with BSI, bacteriological factors (microorganisms from blood cultures and primary infectious foci) were evaluated based on the significant elevated level of procalcitonin. In addition, short-term prognostic factors were investigated among the clinico-bacteriological factors.
The study protocol was approved by the ethics committee of our hospital (2019-080). The need for informed consent was waived due to the retrospective nature of the study.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or median (95% confidence interval [CI]) and compared using the Student t-test or Mann–Whitney U test. Differences in categorical variables were compared using the chi-square test. Follow-up information for at least 14 days was compiled for all patients. For patients with more than one positive blood culture within 14 days, only the first result was considered for survival analysis. Patients who were unavailable for follow-up during the 14-day observation period were censored at the last follow-up. For computing the seven-day and 14-day survival curves, patients were censored at day 7 or at day 14, if they survived beyond each time point, respectively. The Kaplan–Meier method was used to estimate survival curves, and the log-rank test was used to evaluate differences in survival among groups in univariate analysis. Hazard ratios (HRs) and 95% CIs were calculated during the multivariate analysis using a Cox proportional hazards model. Multiple collinearity was tested using values of variance inflation factor (VIF).
A minimum P-value approach was used to evaluate the optimal threshold of procalcitonin with dividing the patients to two cohorts. In this approach, the log-rank test was performed for each two cohorts to determine the optimal cutoff value of procalcitonin with the lowest P-value.
Statistical analyses were performed using JMP, version 10.0 for Windows (SAS Institute Inc., Cary, NC, USA), and R (R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/). Results with p <0.05 were considered statistically significant.
RESULTS
Subjects
Patient demographics are presented in Table 1. The mean age of the patients was 72.3 ± 15.1 years; 59.6% of the patients were men. Most blood samples were obtained in the emergency department, followed by gastroenterology, gastrointestinal surgery, respiratory system, and hematology departments. The mean eGFR was 42.0 ± 30.1 mL/min/1.73 m2. The incidence of an eGFR of <30 mL/min/1.73 m2 was 39.5%. The proportions of patients with serum procalcitonin levels 2–100 ng/mL and ≥100 ng/mL were 87.9% and 12.1%, respectively. The incidence of positive blood culture was 42.3%. Seven-day and 14-day survival values were 87.8% and 83.4%, respectively.
Table 1.
Patient demographics
| Age | 72.3 ± 15.1 | ||
| Sex | Male | 627 | (59.6%) |
| Female | 425 | (40.4%) | |
| Department | Emergency | 622 | (59.1%) |
| Gastroenterology | 71 | (6.7%) | |
| Gastrointestinal surgery | 67 | (6.4%) | |
| Respiratory system | 66 | (6.3%) | |
| Hematology | 62 | (5.9%) | |
| Others | 164 | (15.6%) | |
| Estimated glomerular filtration rate | 42.0 ± 30.1 | ||
| (mL/min/1.73 m2) | <30 | 416 | (39.5%) |
| 30–60 | 221 | (21.0%) | |
| ≥60 | 415 | (39.4%) | |
| Procalcitonin (ng/mL) | 2–10 | 330 | (31.4%) |
| 10–30 | 351 | (33.4%) | |
| 30–100 | 244 | (23.2%) | |
| ≧100 | 127 | (12.1%) | |
| Blood culture | Positive | 445 | (42.3%) |
| Seven-day survival | 87.8% | ||
| Fourteen-day survival | 83.4% | ||
Relationship between procalcitonin levels and the incidence of positive blood culture and 14-day survival
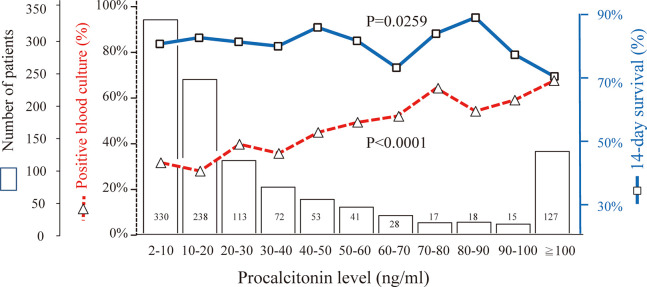
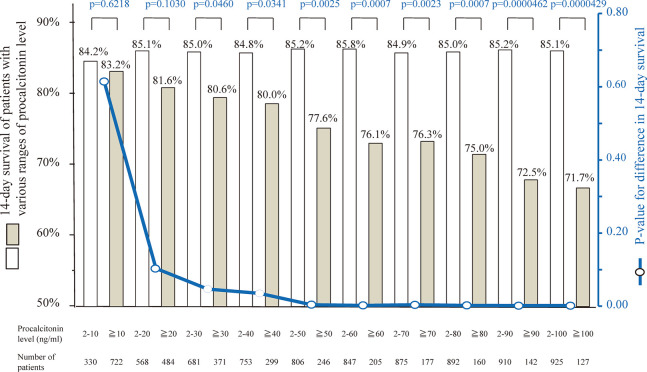
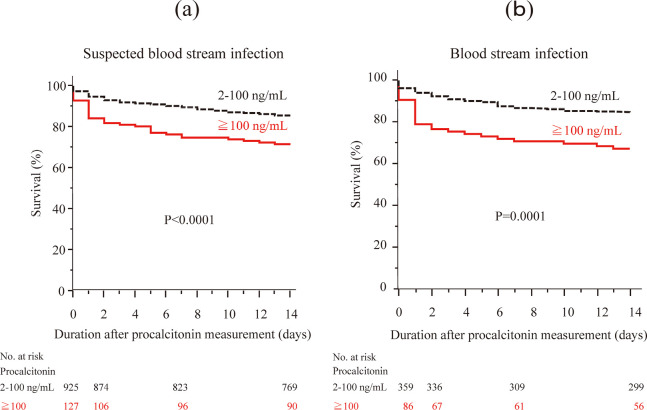
Figure 1 shows the number of patients, incidence of positive blood culture, and 14-day survival of each procalcitonin level. The incidence of positive blood culture and 14-day survival were significantly related to procalcitonin levels (p<0.0001 and p=0.0259, respectively). The more the procalcitonin level was elevated, the higher was the incidence of positive blood culture and the lower was the 14-day survival. When we examined various cutoff values of procalcitonin between 10 and 100 ng/mL, the 14-day survival was significantly different in the cutoff values between 30 and 100 ng/mL (p-values between 0.0000429 and 0.0460). The p-value of 14-day survival differences was lowest between patients with procalcitonin 2–100 ng/mL and those with ≥100 ng/mL (85.1% and 71.7%, p=0.0000429, Figure 2 and 3(a)). Based on the results, we determined ≥100 ng/mL as the significant elevated level of serum procalcitonin for survival of patients with suspected BSI.
Fig. 1.
Number of patients, incidence of positive blood culture, and 14-day survival in eleven ranges of procalcitonin levels
The incidence of positive blood culture and 14-day survival were significantly related to procalcitonin level (p<0.0001 and p=0.0259, respectively); the more the procalcitonin level was elevated, the higher was the incidence of positive blood culture and the lower was the 14-day survival.
Fig. 2.
Fourteen day survival and the p-value of differences classified by 10 cutoff values
Boxes indicate the 14-day survival of patients with each procalcitonin level classified by cutoff values of 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 ng/mL. Fourteen-day survival was significantly different in terms of cutoff values between 30 and 100 ng/mL. The p-value of differences in 14-day survival was lowest between patients with procalcitonin 2–100 ng/mL and those with ≥100 ng/mL (85.1% and 71.7%, p=0.0000429), and the 14-day survival was lowest in patients with procalcitonin ≥100 ng/mL.
Fig. 3.
Short-term survival according to the serum procalcitonin levels
(a) 1052 patients with suspected blood stream infection, (b) 445 patients with positive blood culture.
Comparison of demographic characteristics between patients with procalcitonin of 2–100 ng/mL and ≥100 ng/mL
Based on the above results, the study population was divided into 2 groups: patients with procalcitonin 2–100 ng/mL (n=925) and ≥100 ng/mL (n=127). Comparison of patient demographic characteristics is presented in Table 2. There was no significant difference in age, sex, and the departments in which patients were treated. The mean eGFR in patients with procalcitonin ≥100 ng/mL was significantly lower than that in those with 2–100 ng/mL (24.4 ± 18.2 vs 44.4 ± 30.6 mL/min/1.73 m2, p<0.0001). The incidence of positive blood cultures was significantly higher in patients with procalcitonin ≥100 ng/mL than in those with 2–100 ng/mL (67.7% vs 38.8%, p<0.0001).
Table 2.
Comparison of patient demographics between procalcitonin levels 2.0–99 ng/mL and ≧100 ng/mL
| Procalcitonin | p | ||||||
| 2–100ng/mL | ≥100ng/mL | ||||||
| (n=925) | (n=127) | ||||||
| Age | 72.3 ± 15.3 | 72.5 ± 13.6 | 0.8677 | ||||
| Sex | Male | 555 | (60.0%) | 72 | (56.7%) | 0.4763 | |
| Female | 370 | (40.0%) | 55 | (43.3%) | |||
| Department | |||||||
| Emergency | 538 | (58.2%) | 84 | (66.1%) | 0.1724 | ||
| Gastroenterology | 66 | (7.1%) | 5 | (3.9%) | |||
| Gastrointestinal surgery | 58 | (6.3%) | 9 | (7.1%) | |||
| Respiratory system | 59 | (6.4%) | 7 | (5.5%) | |||
| Hematology | 60 | (6.5%) | 2 | (1.6%) | |||
| Others | 144 | (15.6%) | 20 | (15.7%) | |||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | |||||||
| 44.4 ± 30.6 | 24.4 ± 18.2 | <0.0001 | |||||
| <30 | 322 | (34.8%) | 94 | (74.0%) | <0.0001 | ||
| 30–60 | 212 | (22.9%) | 9 | (7.1%) | |||
| ≥60 | 391 | (42.3%) | 24 | (18.9%) | |||
| Blood culture | |||||||
| Positive | 359 | (38.8%) | 86 | (67.7%) | <0.0001 | ||
| Negative | 566 | (61.2%) | 41 | (32.3%) | |||
| Seven-day survival | 89.6% | 74.8% | <0.0001 | ||||
| Fourteen-day survival | 85.2% | 71.7% | <0.0001 | ||||
Bold values indicate significant differences (p<0.05).
Prognostic factor in patients with suspected BSI
Univariate and multivariate analyses in 1052 patients with suspected BSI showed that an eGFR of <30 mL/min/1.73 m2 and a procalcitonin level of ≥100 ng/mL were significant independent unfavorable prognostic factors (Table 3). Patients with eGFR of <30 mL/min/1.73 m2 had 1.95 times higher risk of early death than those with ≥60 mL/min/1.73 m2. Patients with procalcitonin ≥100 ng/mL had 1.68 times higher risk of early death than those with 2–100 ng/mL.
Table 3.
Univariate and multivariate analyses of 14-day survival of 1052 patients with suspected bloodstream infection
| Univariate analysis | Multivariate analysis | |||||||
| n | 14-day survival (%) |
p | Hazard ratio |
95% confidence interval |
p | |||
| Age | <75 years | 502 | 84.9% | 0.2572 | 1 | |||
| ≧75 years | 550 | 82.3% | 1.20 | 0.88–1.65 | 0.2425 | |||
| Sex | Male | 627 | 82.8% | 0.4564 | 1 | |||
| Female | 425 | 84.7% | 1.21 | 0.89–1.67 | 0.2197 | |||
| Department | ||||||||
| Emergency | 622 | 82.3% | 0.2662 | 1 | ||||
| Gastroenterology | 71 | 90.0% | 0.60 | 0.25–1.20 | 0.1611 | |||
| Gastrointestinal surgery | 67 | 88.1% | 0.80 | 0.35–1.55 | 0.5253 | |||
| Respiratory system | 68 | 86.6% | 0.79 | 0.37–1.48 | 0.4867 | |||
| Hematology | 62 | 77.3% | 1.70 | 0.91–2.95 | 0.0875 | |||
| Others | 162 | 84.0% | 0.90 | 0.57–1.37 | 0.6392 | |||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | ||||||||
| <30 | 416 | 77.6% | <0.0001 | 1.95 | 1.25–3.16 | 0.0030 | ||
| 30–60 | 221 | 86.7% | 1.17 | 0.73–1.93 | 0.5268 | |||
| ≥60 | 415 | 88.7% | 1 | |||||
| Procalcitonin (ng/mL) | ||||||||
| 2–100 | 925 | 85.2% | <0.0001 | 1 | ||||
| ≥100 | 127 | 71.7% | 1.68 | 1.11–2.47 | 0.0138 | |||
| Blood culture | ||||||||
| Positive | 445 | 81.1% | 0.0515 | 1.21 | 0.88–1.66 | 0.2304 | ||
| Negative | 607 | 85.3% | 1 | |||||
Bold values indicate significant differences (p<0.05).
Comparison of microorganisms isolated from blood cultures and primary infectious foci in patients with BSI
Microorganisms isolated from blood cultures and primary infectious foci causing bacteremia were compared between patients with procalcitonin 2–100 ng/mL (n=359) and those with ≥100 ng/mL (n=86) (Table 4). There were no significant differences in distribution of microorganisms between the two groups. If a blood culture yielded organisms commonly considered as blood culture contaminants (eg, coagulase-negative Staphylococci, Corynebacterium species, Bacillus species, or Cutibacterium acnes),20-24 the numbers of contaminated culture were 12 (3.3%) and 3 (3.4%), respectively in patients with procalcitonin 2–100 ng/mL and those with ≥100 ng/mL. There was a marginal significance in the incidence of primary infectious foci; the incidence of urinary tract or abdominal cavity infection was higher in patients with procalcitonin ≥100 ng/mL than in those with 2–100 ng/mL.
Table 4.
Comparison of microbacterium isolated from blood cultures in 445 patients
| Procalcitonin | p | ||||
| 2–100ng/mL | ≥100ng/mL | ||||
| (n=359) | (n=86) | ||||
| Aerobic/anaerobic | |||||
| Aerobic | 324 | (90.3%) | 78 | (90.7%) | 0.1564 |
| Anaerobic | 23 | (6.4%) | 8 | (9.3%) | |
| Aerobic and anaerobic | 12 | (3.3%) | 0 | ||
| Gram stain | |||||
| Gram-negative rods (GNRs) | 207 | (57.7%) | 52 | (66.5%) | 0.4163 |
| Gram-positive cocci (GPC) | 95 | (26.5%) | 15 | (17.4%) | |
| Gram-positive rods (GPRs) | 13 | (3.6%) | 6 | (7.0%) | |
| Fungi | 7 | (1.9%) | 2 | (2.3%) | |
| Multiple bacteria | 36 | (10.0%) | 11 | (12.8%) | |
| Others | 1 | (0.3%) | 0 | ||
| Gram-negative rods (GNRs) (n=262) | |||||
| Escherichia coli | 112 | (54.1%) | 25 | (48.1%) | 0.767 |
| Klebsiella pneumoniae | 34 | (16.4%) | 10 | (19.2%) | |
| Pseudomonas aeruginosa | 6 | (2.9%) | 3 | (5.8%) | |
| Klebsiella oxytoca | 4 | (1.9%) | 0 | ||
| Proteus mirabilis | 4 | (1.9%) | 1 | (1.9%) | |
| Others | 47 | (22.7%) | 13 | (25.0%) | |
| Gram-positive cocci (GPC) (n=110) | |||||
| Staphylococcus aureus | 22 | (23.2%) | 5 | (33.3%) | 0.6661 |
| Streptococcus pneumoniae | 13 | (13.7%) | 3 | (20.0%) | |
| Streptococcus dysgalactiae subsp. equisimilis | 13 | (13.7%) | 1 | (6.7%) | |
| Coagulase-negative Staphylococci | 6 | (6.3%) | 0 | ||
| Others | 41 | (43.2%) | 6 | (40.0%) | |
| Gram-positive rods (GPRs) (n=19) | |||||
| Bacillus cereus | 3 | (23.1%) | 0 | 0.1124 | |
| Bacillus species excluding Bacillus cereus | 1 | (7.7%) | 3 | (50.0%) | |
| Clostridium perfringens | 2 | (15.4%) | 0 | ||
| Clostridium species excluding Clostridium perfringens | 2 | (15.4%) | 0 | ||
| Eubacterium limosum | 0 | 1 | (16.7%) | ||
| Cutibacterium acnes | 1 | (7.7%) | 0 | ||
| Corynebacterium striatum | 1 | (7.7%) | 0 | ||
| Others | 3 | (23.1%) | 2 | (33.3%) | |
| Fungi (n=9) | |||||
| Candida parapsilosis | 2 | (28.6%) | 0 | 0.0611 | |
| Candida glabrata | 4 | (57.1%) | 0 | ||
| Candida albicans | 0 | 1 | (50.0%) | ||
| Candida tropicalis | 0 | 1 | (50.0%) | ||
| Scedosporium prolificans | 1 | (14.3%) | 0 | ||
| Primary infectious foci causing bacteremia | |||||
| Urinary tract | 124 | (34.5%) | 36 | (41.9%) | 0.0977 |
| Respiratory system | 48 | (13.4%) | 13 | (15.1%) | |
| Abdominal cavity | 26 | (7.2%) | 13 | (15.1%) | |
| Biliary system | 54 | (15.0%) | 7 | (8.1%) | |
| Gastrointestinal tract | 28 | (7.8%) | 5 | (5.8%) | |
| Others | 79 | (22.0%) | 12 | (14.0%) | |
Short-term prognostic factors of patients with positive blood cultures
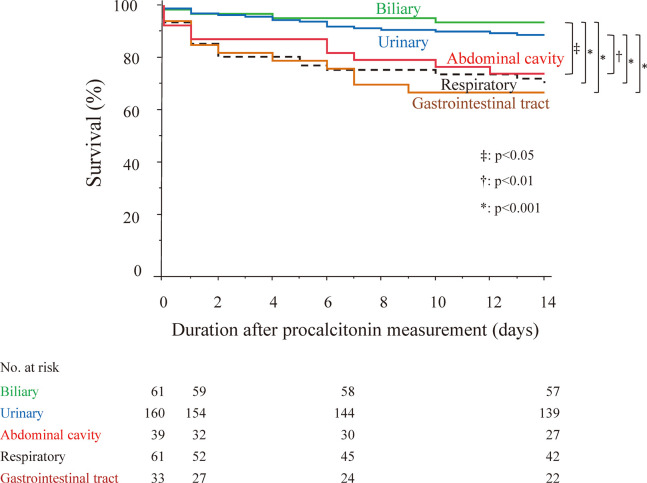
Univariate analysis in 445 patients with a positive blood culture showed that short-term survival was significantly different for eGFR, procalcitonin level (Figure 3(b)), primary infectious foci, and Gram staining of microorganisms (Table 5). Subsequent multivariate analysis showed that eGFR <30 mL/min/1.73 m2, procalcitonin ≥100 ng/mL, and primary infectious foci were significant independent prognostic factors for short-term survival (Table 5 and Figure 4). The values of VIF were below 2. Patients with primary infectious foci in the respiratory system or gastrointestinal tract had significantly higher risk of early death (hazard ratio: 4.12 and 3.83, respectively) than those with primary infectious foci in the urinary tract.
Table 5.
Univariate and multivariate analyses of short-term survival in 445 patients with positive blood cultures
| Univariate analysis | Multivariate analysis | ||||||
| n | 14-day
survival (%) |
p | Hazard
ratio |
95%
confidence interval |
p | ||
| Age | |||||||
| <75 years | 200 | 81.4% | 0.82502 | 1 | |||
| ≧75 years | 245 | 80.7% | 1.19 | 0.75–1.89 | 0.4637 | ||
| Sex | |||||||
| Male | 248 | 80.9% | 0.9387 | 1 | |||
| Female | 197 | 81.1% | 1.06 | 0.66–1.67 | 0.8139 | ||
| Department | |||||||
| Emergency | 316 | 80.9% | 0.5528 | 1 | |||
| Gastrointestinal surgery | 16 | 68.8% | 1.16 | 0.39–2.77 | 0.7617 | ||
| Gastroenterology | 23 | 91.3% | 0.57 | 0.09–1.90 | 0.4061 | ||
| Hematology | 22 | 72.7% | 1.46 | 0.53–3.44 | 0.4345 | ||
| Respiratory system | 19 | 84.2% | 0.45 | 0.11–1.31 | 0.126 | ||
| Others | 49 | 82.2% | 0.70 | 0.30–1.41 | 0.331 | ||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | |||||||
| <30 | 192 | 82.4% | 0.0003 | 2.32 | 1.17–5.00 | 0.0156 | |
| 30–60 | 178 | 87.0% | 1.26 | 0.60–2.84 | 0.5503 | ||
| ≥60 | 85 | 87.0% | 1 | ||||
| Procalcitonin (ng/mL) | |||||||
| 2–100 | 359 | 84.3% | 0.0001 | 1 | |||
| ≥100 | 86 | 67.4% | 2.07 | 1.25–3.38 | 0.0055 | ||
| Primary infectious foci causing bacteremia | |||||||
| Urinary tract | 160 | 88.7% | <0.0001 | 1 | |||
| Respiratory system | 61 | 70.3% | 4.12 | 1.93–8.74 | 0.0003 | ||
| Abdominal cavity | 39 | 73.8% | 2.18 | 0.79–5.63 | 0.1298 | ||
| Biliary system | 61 | 93.4% | 0.69 | 0.19–1.94 | 0.5074 | ||
| Gastrointestinal tract | 33 | 66.7% | 3.83 | 1.68–8.31 | 0.0019 | ||
| Others | 91 | 74.7% | 3.56 | 1.71–7.47 | 0.0008 | ||
| Gram stain | |||||||
| Gram-negative rods (GNRs) | 259 | 81.9% | 0.0231 | 1 | |||
| Gram-positive cocci (GPC) | 110 | 78.0% | 0.79 | 0.43–1.45 | 0.454 | ||
| Gram-positive rods (GPRs) | 19 | 78.9% | 0.55 | 0.13–1.66 | 0.3118 | ||
| Fungi | 9 | 44.5% | 1.78 | 0.56–4.70 | 0.3024 | ||
| Multiple bacteria | 47 | 74.5% | 1.40 | 0.61–2.89 | 0.4068 | ||
| Others* | 1 | 100.0% | <0.0001 | 0–9.54 | 0.4981 | ||
| Aerobic/anaerobic | |||||||
| Aerobic | 402 | 81.5% | 0.5397 | 1 | |||
| Anaerobic | 31 | 73.8% | 1.31 | 0.47–3.39 | 0.5941 | ||
| Aerobic and anaerobic | 12 | 87.8% | 0.81 | 0.12–3.42 | 0.7976 | ||
*: including oral cavity, pharynx, skin, muscle, fascia, bone, spinal fluid, catheter.
Bold values indicate significant differences (p<0.05).
Fig. 4.
Short-term survival according to the primary infectious foci causing bacteremia in 445 patients with positive blood culture
Green: biliary tract
Blue: urinary tract
Red: abdominal cavity
Black: respiratory system
Brown: gastrointestinal tract
DISCUSSION
This study analyzed 1052 patients with suspected BSI and demonstrated the following findings:
1. The incidence of positive blood culture and 14-day survival were significantly related to procalcitonin levels: the more the procalcitonin level was elevated, the higher was the incidence of positive blood culture and the lower was the 14-day survival.
2. The significant elevated level of serum procalcitonin levels was ≥100 ng/mL based on survival analyses.
3. Multivariate analysis in 1052 patients with suspected BSI showed that an eGFR of <30 mL/min/1.73 m2 and a procalcitonin level of ≥100 ng/mL were significant independent unfavorable prognostic factors.
4. There were no significant differences in microorganisms between patients with procalcitonin 2–100 ng/mL and those with ≥100 ng/mL in patient with positive blood culture.
5. Multivariate analysis conducted in 445 patients with positive blood culture showed that eGFR <30 mL/min/1.73 m2, procalcitonin ≥100 ng/mL, and primary infectious foci in the respiratory system or gastrointestinal tract were significant independent prognostic factors for short-term survival.
Recent studies showed that increased serum procalcitonin levels are associated with poor outcomes in patients with several diseases including sepsis, bacteremia, pneumonia, pancreatitis, intestinal ischemia, peritonitis, ulcerative colitis, trauma, and heart failure.2,4,5,24-31 Conversely, some studies showed that elevated procalcitonin levels were associated with decreased renal function.2,32-34 The results of our study revealed ≥100 ng/mL as the significant elevated level of serum procalcitonin in patients with suspected BSI. The results of the multivariate analysis of survival in 1052 patients with suspected BSI and 445 patients with positive blood culture supports that a procalcitonin level of ≥100 ng/mL and eGFR <30 mL/min/1.73 m2 are clinically important. To the best of our knowledge, this is the first study to investigate an optimal cutoff value of procalcitonin that significantly discriminates poor short-term survival in a large cohort with various clinical settings and suspected BSI.
In this study, the incidence of positive blood cultures was higher in patients with procalcitonin level ≥100 ng/mL than in those with 2–100 ng/mL (67.7% vs 38.8%). In our previous study, the incidence of positive blood cultures was 15.8% of 1331 patients with suspected BSI at the same institute.2 The relatively high incidence of positive blood cultures in the present study can be explained by the differences in the patient cohort; the present study included patients with fever (temperature ≥38°C) and procalcitonin levels of ≥2.0 ng/mL.
The present study showed that microorganisms isolated from blood cultures was similar between patients with procalcitonin level 2–100 ng/mL and those with ≥100 ng/mL. The results were partly inconsistent with those of previous studies, which showed that the procalcitonin levels were higher with gram-negative bacteremia than with gram-positive bacteremia or candidemia,35-40 while some previous studies showed decreased procalcitonin levels with fungal bacteremia.35,37,41,42 The disparity may be accounted for by the differences in the study population, procalcitonin levels, and analysis methods.
Our study showed that the incidence of primary infectious foci of the urinary tract and abdominal cavity was more frequent in patients with procalcitonin ≥100 ng/mL than in those with 2–100 ng/mL. The results were consistent with those of previous studies, which showed that patients with urinary tract infections had the highest procalcitonin levels.38,39
This study had some limitations. First, it was a retrospective, single-institutional study, although it included a large dataset from various clinical settings. The unknown background characteristics that can affect elevated procalcitonin levels may have led to a selection bias. Second, several clinical settings can lead to elevated procalcitonin levels and may reduce the clinical significance of the procalcitonin levels. The settings include major surgery, trauma, medullary thyroid carcinoma, metastatic solid tumor, neuroendocrine neoplasms, intracerebral hemorrhage, coronary atherosclerotic disease, jaundice, heart failure, cardiac arrest, anaphylactic shock, and amphetamine intoxication.43-48 However, these diseases were not investigated in our study. Third, the times to positive blood cultures were not investigated because the blood culture bottles and systems had been changed during the study period. Fourth, the causes of death were not fully investigated. Some patients may have had terminal malignant disease or major surgical complications; therefore, in such patients, the cause of death may not have been bacteremia. Fifth, patients with serum procalcitonin level <2.0 ng/mL or less than 16 years old were not included in the study because of patient selection; the present study used a prospective database that was restored for patients who were ≥16 years old, had fever (temperature ≥38°C) and serum procalcitonin levels ≥2.0 ng/mL according to our infection control team. The patient selection may limit the generalizability of the results. Sixth, several factors affecting patient’s short-term survival were not investigated. More comprehensive analysis including patient’s comorbidity, cardiac function, lung capacity, nutrition, anemia and medication records will provide a robust conclusion on the clinical significance of the procalcitonin levels.
Despite these limitations, the results of our study can be applied to various situations and patient populations because this study was conducted among a relatively large number and variation of patients with suspected BSI. The results can be useful in helping clinicians promptly determine the severity of BSI and identify patients in whom large medical resources should be invested.
In conclusion, procalcitonin ≥100 ng/mL was one of the significant independent unfavorable prognostic factors in patients with suspected BSI or positive blood culture. Primary infectious foci in the respiratory system or gastrointestinal tract were associated with unfavorable short-term survival in patients with positive blood culture.
DECLARATION OF CONFLICTING INTERESTS
There are no potential conflicts of interest to declare, with regard to the research, authorship, and publication of this article.
FUNDING
This research was funded by Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital Research Grant to YO. The funding source had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author (NY) upon reasonable request.
GUARANTOR
NY.
ETHICAL APPROVAL
The study protocol was reviewed and approved by the Institutional Review Boards of Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital (2019-080).
AUTHOR CONTRIBUTIONS
YO and NY researched literature and conceived the study conception and design. Material preparation and data collection were performed by all authors. Analysis was performed by YO and NY. YO was involved in gaining ethical approval. The first draft of the manuscript was written by YO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital Research Grant to YO. We are grateful to Prof Ayumi Shintani (Department of Medical Statistics, Osaka City University Graduate School of Medicine) for helpful statistical analyses.
Abbreviations
- BSI
bloodstream infection
- eGFR
estimated glomerular filtration rate
- CI
confidence interval
- HRs
hazard ratios
- VIF
variance inflation factor
REFERENCES
- 1.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-n. [DOI] [PMC free article] [PubMed]
- 2.Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol. 2014;141(1):43–51. doi: 10.1309/AJCP4GV7ZFDTANGC. [DOI] [PubMed]
- 3.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed]
- 4.Jain S, Sinha S, Sharma SK, et al. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes. 2014;7:458. doi: 10.1186/1756-0500-7-458. [DOI] [PMC free article] [PubMed]
- 5.Schuetz P, Birkhahn R, Sherwin R, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin monitoring sepsis (MOSES) study. Crit Care Med. 2017;45(5):781–789. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed]
- 6.Shi Y, Xu YC, Rui X, Zhang HM, Wang Y, Du W. Procalcitonin kinetics and nosocomial pneumonia in older patients. Respir Care. 2014; 59(8): 1258–1266. doi: 10.4187/respcare.02364. [DOI] [PubMed]
- 7.Pepper DJ, Sun J, Rhee C, et al. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: a systematic review and meta-analysis. Chest. 2019;155(6):1109–1118. doi: 10.1016/j.chest.2018.12.029. [DOI] [PMC free article] [PubMed]
- 8.Jones AE, Fiechtl JF, Brown MD, Ballew JJ, Kline JA. Procalcitonin test in the diagnosis of bacteremia: a meta-analysis. Ann Emerg Med. 2007;50(1):34–41. doi: 10.1016/j.annemergmed.2006.10.020. [DOI] [PubMed]
- 9.Bloos F, Marshall JC, Dellinger RP, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 2011;15(2):R88. doi: 10.1186/cc10087. [DOI] [PMC free article] [PubMed]
- 10.Gendrel D, Raymond J, Assicot M, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997;24(6):1240–1242. doi: 10.1086/513633. [DOI] [PubMed]
- 11.Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007;245(5):745–754. doi: 10.1097/01.sla.0000252443.22360.46. [DOI] [PMC free article] [PubMed]
- 12.Leroy S, Adamsbaum C, Marc E, et al. Procalcitonin as a predictor of vesicoureteral reflux in children with a first febrile urinary tract infection. Pediatrics. 2005;115(6):e706–e709. doi: 10.1542/peds.2004-1631. [DOI] [PubMed]
- 13.Sharma P, Patel K, Baria K, et al. Procalcitonin level for prediction of postoperative infection in cardiac surgery. Asian Cardiovasc Thorac Ann. 2016;24(4):344–349. doi: 10.1177/0218492316640953. [DOI] [PubMed]
- 14.Facy O, Paquette B, Orry D, et al. IMACORS Study. Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery: results from the IMACORS study. Ann Surg. 2016;263(5):961–966. doi: 10.1097/SLA.0000000000001303. [DOI] [PubMed]
- 15.Iida H, Maehira H, Mori H, Tani M. Serum procalcitonin as a predictor of infectious complications after pancreaticoduodenectomy: review of the literature and our experience. Surg Today. 2020;50(2):87–96. doi: 10.1007/s00595-019-01811-y. [DOI] [PubMed]
- 16.Nishiyama N, Yuasa N, Minoshima M, et al. Short-term prognostic factors of patients with fever and elevated serum procalciton in[in Japanese]. Jpn J Environ Infect. 2018;33(1):15–23. doi: 10.4058/jsei.33.15. [DOI]
- 17.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed]
- 18.Oguri T. Clinical microbiology handbook. 4th ed. Tokyo: Miwa Shoten; 2011.
- 19.Jpn Soc Clin Microbiol. A guide to blood cultures. Tokyo: Nankodo; 2013.
- 20.Liaudat S, Dayer E, Praz G, Bille J, Troillet N. Usefulness of procalcitonin serum level for the diagnosis of bacteremia. Eur J Clin Microbiol Infect Dis. 2001;20(8):524–527. doi: 10.1007/s100960100548. [DOI] [PubMed]
- 21.Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis. 2002;35(2):156–161. doi: 10.1086/341023. [DOI] [PubMed]
- 22.Richter SS, Beekmann SE, Croco JL, et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol. 2002;40(7):2437–2444. doi: 10.1128/JCM.40.7.2437-2444.2002. [DOI] [PMC free article] [PubMed]
- 23.Riedel S, Melendez JH, An AT, Rosenbaum JE, Zenilman JM. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol. 2011;135(2):182–189. doi: 10.1309/AJCP1MFYINQLECV2. [DOI] [PubMed]
- 24.Jeong S, Park Y, Cho Y, Kim HS. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin Chim Acta. 2012;413(21–22):1731–1736. doi: 10.1016/j.cca.2012.06.030. [DOI] [PubMed]
- 25.Zhou H, Guo S, Lan T, Ma S, Zhang F, Zhao Z. Risk stratification and prediction value of procalcitonin and clinical severity scores for community-acquired pneumonia in ED. Am J Emerg Med. 2018;36(12):2155–2160. doi: 10.1016/j.ajem.2018.03.050. [DOI] [PubMed]
- 26.Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009;146(1):72–81. doi: 10.1016/j.surg.2009.02.013. [DOI] [PubMed]
- 27.Cosse C, Sabbagh C, Browet F, et al. Serum value of procalcitonin as a marker of intestinal damages: type, extension, and prognosis. Surg Endosc. 2015;29(11):3132–3139. doi: 10.1007/s00464-014-4038-0. [DOI] [PubMed]
- 28.Pupelis G, Drozdova N, Mukans M, Malbrain ML. Serum procalcitonin is a sensitive marker for septic shock and mortality in secondary peritonitis. Anaesthesiol Intensive Ther. 2014;46(4):262–273. doi: 10.5603/AIT.2014.0043. [DOI] [PubMed]
- 29.Koido S, Ohkusa T, Takakura K, et al. Clinical significance of serum procalcitonin in patients with ulcerative colitis. World J Gastroenterol. 2013;19(45):8335–8341. doi: 10.3748/wjg.v19.i45.8335. [DOI] [PMC free article] [PubMed]
- 30.AlRawahi AN, AlHinai FA, Doig CJ, et al. The prognostic value of serum procalcitonin measurements in critically injured patients: a systematic review. Crit Care. 2019;23(1):390. doi: 10.1186/s13054-019-2669-1. [DOI] [PMC free article] [PubMed]
- 31.Aïssou L, Sorbets E, Lallmahomed E, et al. Prognostic and diagnostic value of elevated serum concentration of procalcitonin in patients with suspected heart failure. A review and meta-analysis. Biomarkers. 2018;23(5):407–413. doi: 10.1080/1354750X.2018.1443511. [DOI] [PubMed]
- 32.Wu SC, Liang CX, Zhang YL, Hu WP. Elevated serum procalcitonin level in patients with chronic kidney disease without infection: a case-control study. J Clin Lab Anal. 2020;34(2):e23065. doi: 10.1002/jcla.23065. [DOI] [PMC free article] [PubMed]
- 33.Chun K, Chung W, Kim AJ, et al. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci Rep. 2019;9(1):4777. doi: 10.1038/s41598-019-41291-1. [DOI] [PMC free article] [PubMed]
- 34.Yunus I, Fasih A, Wang Y. The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics. PLoS One. 2018;13(11):e0206527. doi: 10.1371/journal.pone.0206527. [DOI] [PMC free article] [PubMed]
- 35.Oussalah A, Ferrand J, Filhine-Tresarrieu P, et al. Diagnostic accuracy of procalcitonin for predicting blood culture results in patients with suspected bloodstream infection: an observational study of 35,343 consecutive patients (A STROBE-compliant article). Medicine (Baltimore). 2015; 94(44): e1774. doi: 10.1097/MD.0000000000001774. [DOI] [PMC free article] [PubMed]
- 36.Guo SY, Zhou Y, Hu QF, Yao J, Wang H. Procalcitonin is a marker of gram-negative bacteremia in patients with sepsis. Am J Med Sci. 2015;349(6):499–504. doi: 10.1097/MAJ.0000000000000477. [DOI] [PMC free article] [PubMed]
- 37.Li S, Rong H, Guo Q, Chen Y, Zhang G, Yang J. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci. 2016;21:39. doi: 10.4103/1735-1995.183996. [DOI] [PMC free article] [PubMed]
- 38.Yan ST, Sun LC, Jia HB, Gao W, Yang JP, Zhang GQ. Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria. Am J Emerg Med. 2017;35(4):579–583. doi: 10.1016/j.ajem.2016.12.017. [DOI] [PubMed]
- 39.Thomas-Rüddel DO, Poidinger B, Kott M, et al. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care. 2018;22(1):128. doi: 10.1186/s13054-018-2050-9. [DOI] [PMC free article] [PubMed]
- 40.Bassetti M, Russo A, Righi E, et al. Role of procalcitonin in predicting etiology in bacteremic patients: report from a large single-center experience. J Infect Public Health. 2020;13(1):40–45. doi: 10.1016/j.jiph.2019.06.003. [DOI] [PubMed]
- 41.Raineri SM, Cortegiani A, Vitale F, Iozzo P, Giarratano A. Procalcitonin for the diagnosis of invasive candidiasis: what is the evidence? J Intensive Care. 2017;5:58. doi: 10.1186/s40560-017-0252-x. [DOI] [PMC free article] [PubMed]
- 42.Pieralli F, Corbo L, Torrigiani A, et al. Usefulness of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients outside the intensive care unit. Intern Emerg Med. 2017;12(5):629–635. doi: 10.1007/s11739-017-1627-7. [DOI] [PubMed]
- 43.Molter GP, Soltész S, Kottke R, Biedler A, Silomon M. Procalcitonin plasma concentrations and systemic inflammatory response following different types of surgery[in German]. Anaesthesist. 2003;52(3):210–217. doi: 10.1007/s00101-003-0460-8. [DOI] [PubMed]
- 44.Meisner M, Adina H, Schmidt J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit Care. 2006;10(1):R1. doi: 10.1186/cc3910. [DOI] [PMC free article] [PubMed]
- 45.Chen L, Zhang Y, Lin Y, et al. The role of elevated serum procalcitonin in neuroendocrine neoplasms of digestive system. Clin Biochem. 2017;50(18):982–987. doi: 10.1016/j.clinbiochem.2017.06.010. [DOI] [PubMed]
- 46.Kurtul A, Elcik D. Procalcitonin is an independent predictor for coronary atherosclerotic burden in patients with stable coronary artery disease. Int J Cardiol. 2017;236:61–64. doi: 10.1016/j.ijcard.2017.02.061. [DOI] [PubMed]
- 47.Qu J, Feng P, Luo Y, Lü X. Impact of hepatic function on serum procalcitonin for the diagnosis of bacterial infections in patients with chronic liver disease: a retrospective analysis of 324 cases. Medicine (Baltimore). 2016;95(30):e4270. doi: 10.1097/MD.0000000000004270. [DOI] [PMC free article] [PubMed]
- 48.Fries M, Kunz D, Gressner AM, Rossaint R, Kuhlen R. Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation. 2003;59(1):105–109. doi: 10.1016/s0300-9572(03)00164-3. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (NY) upon reasonable request.