Abstract
We conducted a scoping review of the epidemiological literature from the past 50 years to document the contribution of influenza virus infection to extrapulmonary clinical outcomes. We identified 99 publications reporting 243 associations using many study designs, exposure and outcome definitions, and methods. Laboratory confirmation of influenza was used in only 28 (12%) estimates, mostly in case-control and self-controlled case series study designs. We identified 50 individual clinical conditions associated with influenza. The most numerous estimates were of cardiocirculatory diseases, neurological/neuromuscular diseases, and fetal/newborn disorders, with myocardial infarction the most common individual outcome. Due to heterogeneity, we could not generate summary estimates of effect size, but of 130 relative effect estimates, 105 (81%) indicated an elevated risk of extrapulmonary outcome with influenza exposure. The literature is indicative of systemic complications of influenza virus infection, the requirement for more effective influenza control, and a need for robust confirmatory studies.
Keywords: cardiovascular, epidemiology, extrapulmonary, influenza, review
In a scoping review to document associations between influenza and extrapulmonary outcomes, we identified 99 publications reporting 243 associations and 50 distinct clinical conditions. Studies were heterogeneous with limited comparability. These findings emphasize the importance of prevention and need for additional research.
Influenza viruses infect between 5% and 20% of the global population annually [1]. Transmitted by droplets through the respiratory tract, infection results in cough, fever, and other “flu-like” symptoms, which can develop to primary viral or secondary bacterial pneumonia and result in up to 650 000 respiratory deaths annually [2]. In addition to this respiratory burden, associations between seasonal influenza peaks and secondary cardiovascular, renal, and neurological mortality have been observed since almost 100 years ago [3]. Observational studies have described relationships between influenza and conditions including heart disease, exacerbations of lung disease, diabetes, myocarditis, encephalitis, and blood disorders [3–6].
Studies describing these relationships have used different designs (eg, cohort, case-control, time-series), influenza definitions (influenza-like illness [ILI], laboratory-confirmed influenza, diagnosis codes), effect measures (incidence rate ratios, odds ratios, incidence rates), outcomes (deaths, morbidity, hospitalizations), and comparison groups (individuals without influenza, time periods with less/no influenza circulation). Comparability is therefore limited, contributing to uncertainty around the magnitude of association between influenza and these conditions. Previous reviews have focused on qualitative descriptions [6], cardiovascular events [7], and/or specific study designs [8, 9]. We attempted to illustrate the full range of extrapulmonary medical outcomes that have been associated with influenza by conducting a scoping review of the literature from the past 50 years and discussed important remaining evidence gaps to be addressed.
A DESCRIPTIVE SYNTHESIS OF OBSERVATIONAL EVIDENCE
We looked at studies that have reported associations between influenza, as an exposure, and extrapulmonary medical outcomes (excluding typical manifestations of influenza such as pneumonia or broadly defined “respiratory disease”). We applied the PECO (population, exposure, comparison, and outcome) framework [10] to guide in developing our search terms. The population included any community-dwelling or hospitalized individual, irrespective of age. The exposure was influenza, either clinically diagnosed as influenza or ILI, laboratory confirmed at the individual level, or indicated through surveillance data at the population level. The comparison group included non-influenza control populations or control time periods during which influenza was absent. The outcomes were medical events of the cardiovascular, renal, nervous, ophthalmic, musculoskeletal, hepatic, hematological, and selected outcomes of the pulmonary or endocrine systems, identified from a previous review [6], augmented with any study describing mortality. Of pulmonary outcomes, only chronic obstructive pulmonary disease (COPD) and asthma exacerbations, which are not typical manifestations of influenza virus infection, were captured. Study types were limited to observational studies reporting measures of association between influenza and outcomes. No geographical limits were applied but, for reasons of feasibility, only English-language publications were reviewed. Literature published since 1970 was included to capture trends on a broad range of exposure and outcome ascertainments associated with different study designs and increased availability and use of laboratory tests for influenza over time. We excluded studies reporting results of medical interventions (eg, those describing vaccines as exposures, including clinical trials), letters, reviews, editorials, case reports, animal studies, and studies of self-reported medical outcomes. Detailed procedures for the review can be seen in the Supplementary Materials.
CHARACTERIZING THE LITERATURE
Studies, Populations, and Chronology
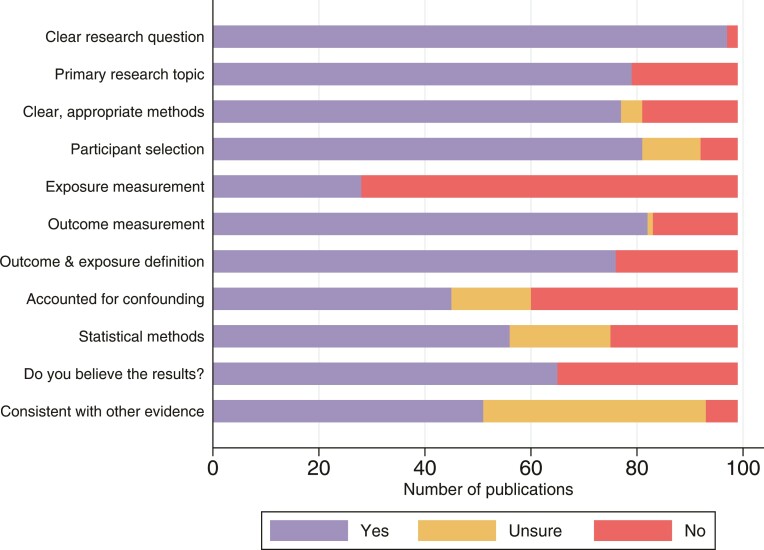
We identified 4136 articles from Embase, 1116 from PubMed, and 147 from reference lists of review articles, resulting in 4172 nonduplicate publications. After title/abstract review, 3975 were excluded, leaving 197 articles for full-text review, from which we identified 99 eligible manuscripts reporting 243 associations between influenza and secondary medical outcomes (see study flowchart in Supplementary Figure 1). The first identified publication was from 1972, with papers increasing in frequency over time, peaking in 2012. Among estimates reported during this period, 207 (85%) were published after 2004 (Table 1). All age groups were included, with 76 estimates (31%) from adults aged ≥18 years; 30 (12%) from children <18 years old, and 44 (18%) describing associations between maternal influenza exposure and fetal disorders. Of the 243 estimates, 96 (40%) were generated from North America (76 from the United States and 20 from Canada), 96 (40%) from Europe (32 of which were from the United Kingdom), and 51 (21%) from Asian countries. We identified no studies from Africa or the Middle East. The general quality of the studies was assessed based on criteria modified from the Critical Appraisal Skills Programme checklists for cohort and case control studies [11] detailed in Supplementary Table 1. Despite most studies having a clear research question, other aspects such as statistical methods, exposure measurement, or considerations regarding confounders and biases were not as well characterized (Figure 1).
Table 1.
Characteristics of Included Effect Estimates
| Characteristic | Cancer (n = 16) |
Cardiocirculatory (n = 90) | Fetal/Newborn (n = 29) |
Infections (n = 14) |
Neurological/Neuromuscular (n = 39) | Other Outcomes (n = 55) | Total (N = 243) |
|---|---|---|---|---|---|---|---|
| Publication year (tertiles) | |||||||
| 1970–1987 | 3 (19) | 10 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 13 (5) |
| 1988–2003 | 2 (13) | 4 (4) | 4 (14) | 1 (7) | 10 (26) | 2 (4) | 23 (9) |
| 2004–2019 | 11 (69) | 76 (84) | 25 (86) | 13 (93) | 29 (74) | 53 (96) | 207 (85) |
| Influenza definition | |||||||
| Laboratory confirmed | 1 (6) | 12 (13) | 3 (10) | 3 (21) | 7 (18) | 2 (4) | 28 (12) |
| PCR | 0 (0) | 0 (0) | 3 (10) | 1 (7) | 2 (5) | 1 (2) | 7 (3) |
| Othera | 1 (6) | 12 (13) | 0 (0) | 2 (14) | 5 (13) | 1 (2) | 21 (9) |
| ILI/SARI/ICD code | 9 (56) | 28 (31) | 18 (62) | 1 (7) | 22 (56) | 17 (31) | 95 (39) |
| Positivity rate | 3 (19) | 46 (51) | 6 (21) | 10 (71) | 6 (15) | 34 (62) | 105 (43) |
| Combination/other | 3 (19) | 4 (4) | 2 (7) | 0 (0) | 4 (10) | 2 (4) | 15 (6) |
| Age group | |||||||
| Maternal exposure and fetal disorder | 0 (0) | 0 (0) | 29 (100) | 0 (0) | 11 (28) | 4 (7) | 44 (18) |
| Children (<18 y) | 5 (31) | 0 (0) | 0 (0) | 9 (64) | 8 (21) | 8 (15) | 30 (12) |
| Adults (≥18 y) | 5 (31) | 44 (49) | 0 (0) | 4 (29) | 3 (8) | 20 (36) | 76 (31) |
| Mixed/unspecified | 6 (38) | 46 (51) | 0 (0) | 1 (7) | 17 (44) | 23 (42) | 93 (38) |
| Study design | |||||||
| Case-control | 7 (44) | 4 (4) | 9 (31) | 2 (14) | 12 (31) | 1 (2) | 35 (14) |
| Cohort | 3 (19) | 10 (11) | 11 (38) | 2 (14) | 4 (10) | 6 (11) | 36 (15) |
| Excess model/time series | 6 (38) | 68 (76) | 9 (31) | 10 (71) | 17 (44) | 48 (87) | 158 (65) |
| Self-controlled case series | 0 (0) | 8 (9) | 0 (0) | 0 (0) | 6 (15) | 0 (0) | 14 (6) |
| Effect measure | |||||||
| Odds ratio | 7 (44) | 12 (13) | 20 (69) | 1 (7) | 16 (41) | 0 (0) | 56 (23) |
| Rate ratio | 2 (13) | 24 (27) | 1 (3) | 0 (0) | 7 (18) | 3 (5) | 37 (15) |
| Risk ratio | 4 (25) | 8 (9) | 8 (28) | 3 (21) | 10 (26) | 3 (5) | 36 (15) |
| Hazard ratio | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (0) |
| Incidence rate | 1 (6) | 26 (29) | 0 (0) | 9 (64) | 3 (8) | 20 (36) | 59 (24) |
| Mortality rate | 2 (13) | 6 (7) | 0 (0) | 0 (0) | 2 (5) | 6 (11) | 16 (7) |
| Percentage | 0 (0) | 10 (11) | 0 (0) | 1 (7) | 0 (0) | 14 (25) | 25 (10) |
| Number | 0 (0) | 4 (4) | 0 (0) | 0 (0) | 1 (3) | 8 (15) | 13 (5) |
Table shows No. (%) of effect estimates, stratified by clinical outcome group.
Abbreviations: ICD, International Classification of Diseases; ILI, influenza-like illness; PCR, polymerase chain reaction; SARI, severe acute respiratory illness.
Laboratory-confirmed “Other” includes serology, rapid tests, viral culture, direct fluorescent antibody staining, and unspecified laboratory-confirmed methods.
Figure 1.
Assessment of study quality. The study quality assessment tool is shown in Supplementary Table 1.
Influenza Definitions and Study Types
There was considerable heterogeneity in the definition of influenza applied in the 243 estimates included in this review, generally reflecting poor ascertainment of influenza as an exposure (Table 1, Figure 2). Influenza positivity rate from surveillance data was the most common, applied in 105 of 243 (43%) studies, which almost exclusively (102/105) used time-series/excess modeling methods. Clinical diagnosis of influenza captured through International Classification of Diseases (ICD) or other codes accounted for 95 (39%) effect estimates. Laboratory confirmation (polymerase chain reaction [PCR], serology, rapid tests, culture or other) was applied for only 28 (12%) estimates, never before 2004, and most commonly in case-control (15/35 case-control estimates [43%]) and self-controlled case series (8/14 [57%]) studies. While laboratory confirmation is the gold standard for defining influenza exposure, clinical diagnoses may in some contexts represent a good proxy: In Australia and Canada, for example, ICD-10 codes for influenza have shown a positive predictive value for laboratory confirmation of 84%–94% [12, 13], although coding practices are likely to vary by country, hospitals, and over time, and validation may be warranted. As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza viruses begin to cocirculate, laboratory confirmation of respiratory pathogens could become routine care, enabling studies using specific case definitions.
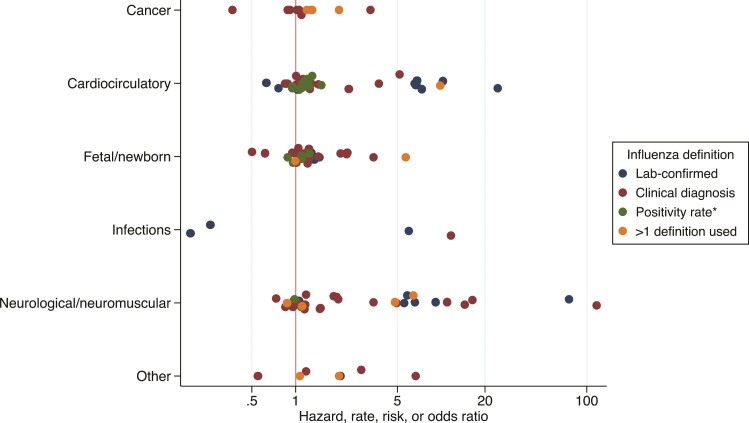
Figure 2.
Reported sizes of relative effect measures from identified studies. *Positivity rate as determined from public health surveillance data.
The types of effect measure reported varied by study design. Self-controlled case series studies report incidence rate ratios and case-control studies normally report odds ratios. But there was considerable heterogeneity in effects reported from excess models/time series studies which included incidence/mortality rates, rate/risk ratios, percentage differences, and number of events (differences). These values masked additional heterogeneity in terms of exposure ascertainment. For example, case-control studies defined influenza exposure using laboratory confirmation or diagnosis codes, and considered an exposure time window that could vary from days [14] to several years [15]. A matched case-control study in the United Kingdom looked at clinician-diagnosed influenza among patients with and without epilepsy, allowing for a look-back period of over 2 years [15]. Time series studies also used various definitions for influenza exposure including the number of hospitalizations per unit increase in influenza incidence rate per 100 000 population to elucidate any association to asthma exacerbation [16]; fluctuation in influenza virus circulation to assess short-term variation in population-level rates of preterm birth, stillbirth, and perinatal death in Ontario [17]; and the association of monthly COPD admissions with influenza admission incidence [18]. Studies, even using the same design and with similar outcomes, were therefore seldom comparable.
EXTRAPULMONARY MEDICAL OUTCOMES ASSOCIATED WITH INFLUENZA
We segregated estimates of influenza on extrapulmonary outcomes into 6 clinical outcome groups following medical review (S. S. C. and T. B.-S.): (1) Cardiocirculatory events including atrial fibrillation, heart failure, myocarditis, and other health events involved 90 of 243 estimates of effect (37% of all estimates); (2) neurological/neuromuscular diseases including Guillain-Barré syndrome, dementia, and epilepsy represented 39 of 243 estimates (16%); (3) fetal/newborn disorders included cleft lip or palate, congenital abnormalities, and mental retardation with 29 of 243 estimates (12%); (4) cancer included a variety of blood and other cancers with 16 of 243 estimates (7%); (5) a category for infections (other than influenza) where associations were reported with bacterial meningitis, invasive pulmonary aspergillosis, otitis media, and others represented 14 of 243 estimates (6%); and finally, (6) a miscellaneous group comprised outcomes such as asthma and COPD exacerbations, chronic fatigue syndrome, and other occurrences such as natality decline and hip fracture (Table 1).
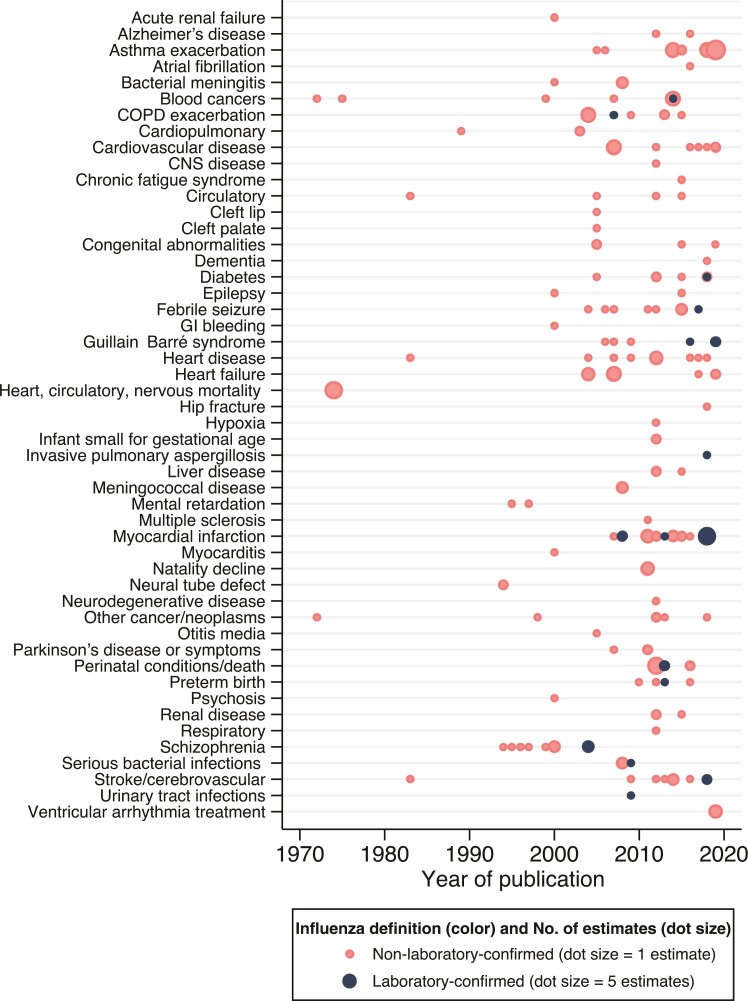
Overall, the outcome groups encompassed 50 categories of diseases, several of which were overlapping due to definitions applied in underlying studies (eg, “heart failure” and “cardiovascular disease”) (Figure 3; Supplementary Table 2). Myocardial infarction was reported most frequently (25 of 243 effect measures [10%]), followed by asthma exacerbations (23 estimates [9%]), heart failure, and perinatal conditions/death (12 estimates [5%] each). Potential mechanisms for some of these more common outcomes have been previously discussed including stimulation of inflammatory pathways, atherosclerotic-plaque disruption, exertion of excessive metabolic demands, antigenic cross-reactivity between influenza and proinflammatory plaque antigens, and the development of cardiac lesions and associated myocardial disruption [19, 20]. But we also observed conditions such as psychosis, neurological complications, mental retardation, or liver disease whose potential biological associations are less clear but for which possible pathways have been discussed [6]. Nonetheless, it is important to stress that some conditions previously described as associated with influenza may have been missed either because we excluded case series or case reports, or because our search terms were not exhaustive. For example, there are studies exploring the association of influenza and interstitial lung disease exacerbations [21] that were not identified in our search but highlight the complex downstream consequences of influenza virus infection, particularly in high-risk population groups. Also, until recently, the associations between influenza and thrombotic events have been mostly limited to case series, especially published in association with the 2009 influenza pandemic, and more recent studies fell out of our investigation period [22].
Figure 3.
Extrapulmonary medical outcomes associated with influenza, by publication year. The frequency weight of number of estimates included for each condition is illustrated by the size of the dot, as indicated in the legend. Three captured outcomes were indirectly related to the search criteria. Hip fracture was included from a study assessing a hypothesis that influenza may impact the risk of falls and fractures during acute illness due to unsteady gait or dizziness [42]. Hypoxia in newborn babies was related to maternal influenza exposure in utero and was therefore not considered a typical manifestation of influenza infection [43]. Natality decline was documented following pandemics and consistent with influenza causing first-trimester miscarriage [30]. Abbreviations: CNS, central nervous system; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
The breadth of observed outcomes was defined by some underlying patterns. The high number of neurological/neuromuscular conditions resulted from 10 estimates on schizophrenia from maternal influenza exposure derived from 1994 to 2004 and 8 pediatric febrile seizure studies from 2004 to 2017. We chose not to exclude influenza pandemic periods, and a total of 41 (17%) estimates were derived from the 1918 (H1N1), 1957–1958 (H2N2), 1968 (H3N2), or 2009 (H1N1pdm09) pandemic periods in which study designs tended to be more ecological—for example, comparing outcomes in pandemic vs nonpandemic birth cohorts. Twenty-eight estimates were derived from the 2009 pandemic, the majority describing fetal medical events (such as preterm birth, perinatal conditions, and small size for gestational age). These designs often used nonspecific influenza exposure definitions and may be susceptible to ecological fallacy and other biases; observed population-level effects in this context may not equate to individual-level risk following influenza infection [23].
QUANTITATIVE ASSESSMENT OF RELATIONSHIPS
A total of 130 effect estimates were reported using relative measures (hazard, rate, risk, or odds ratios) that we considered broadly comparable and therefore suitable for quantitative description. Of note, 27 of 130 (21%) of these estimates were based on laboratory-confirmed influenza mostly involving cardiocirculatory or neurological/muscular disorders. Only 3 clinical groups (congenital abnormalities, seizures, and blood cancers) had ≥3 relative estimates and these were extracted from different age groups and disparate study types. This heterogeneity precluded any meaningful meta-analysis as illustrated by the hazard, rate, risk or odds ratio point estimates presented in Figure 2, and we did not generate summary effect estimates for any clinical group.
Observed relative effect sizes of influenza on the various clinical outcomes varied from 0.19 to 116.7 with a median of 1.16 (interquartile range, 0.56–18.6). Many more studies reported statistically significant (P < .05) positive results (76 estimates with effect size >1) than significantly negative (4 estimates <1), with a marked clustering around 1: 52% of point estimates (67/130) fell between 0.8 and 1.2 indicating small effect sizes, almost half of which (30 [45%]) were significant at the 95% level. Of the 130 relative effect estimates, 105 (81%) were >1, indicating an elevated risk of extrapulmonary outcomes with influenza exposure (Figure 2). The lowest effect estimate was a risk ratio generated from a case-control study of influenza and serious bacterial infection in children [24], and the highest from a self-controlled case series study associating influenza diagnosis with febrile seizures [25].
There was no obvious influence of study design on effect size, and the high effect sizes observed in some studies were difficult to explain. Of the 3 studies reporting odds/rate ratios >20, 2 described relationships between influenza and febrile seizures [25, 26] and 1 on myocardial infarction [27], conducted using different designs and in different countries. A self-controlled case series estimated an incidence rate ratio for febrile seizures of 116.7 (95% confidence interval, 62.8–216.9) on the same day as diagnosis with pandemic H1N1(pdm09) influenza in Norway in children aged <45 months, a transient, brief elevation in risk which declined sharply within 1 week [25]. A case-control study identified a 79.4-fold (95% CI: 10.6–10 164) elevated odds of PCR-confirmed influenza virus infection—generated from <30 influenza cases—in febrile seizure patients aged <6 years compared with healthy controls [26]. Febrile seizures are a known complication of influenza A/H1N1(pdm09) virus, seasonal influenza, and other viral infections, and elevated risks concurrent with infection are reflective of very low background rates of febrile seizures in healthy children [28, 29]. Another case-control study identified an odds ratio of 25.5 (95% CI: 8.3–78.3) of influenza B infection in patients with newly diagnosed myocardial infarction of compared with randomly sampled outpatients in Harbin, China [27]. Influenza was defined using a cross-sectional serological threshold, cases were more likely to be male and aged >60 than controls, and while these confounders were included in multivariable analysis, remaining unmeasured confounding may have contributed to the large observed effect. Some of these estimates were imprecise and may have arisen by chance, emphasizing the importance of appropriately powered studies, and replication, to confirm estimates which may be derived from specific exposure events that are not reflective of wider population-level risk.
WHAT CAN WE ACTUALLY SAY ABOUT THE IMPACT OF INFLUENZA ON EXTRAPULMONARY MEDICAL OUTCOMES? INSIGHTS AND GAPS TO BE ADDRESSED
Our scoping review intended to understand the evidence supporting different extrapulmonary complications that have been associated with influenza virus infection to inform public health decision making and generate hypotheses for future studies. We planned a sensitive search and, accordingly, identified a broad range of studies using a wide variety of exposure and case definitions, age groups, control populations, statistical methods, and time periods. We could not conduct a meta-analysis of observed effect sizes due to this heterogeneity and lack of comparability between even similar study designs. Influenza definitions were especially variable. Nonspecific exposure definitions, such as “time when influenza was circulating,” risk being confounded by other contributors to medical outcomes like co-circulating pathogens and environmental (eg, cold weather) and behavioral factors, which were not always fully accounted for statistical analyses and could give to rise to type I errors, whereby associations that are not real are detected.
Although underlying study quality was generally assessed as good, the ecological characteristics of some studies and lack of pathophysiological mechanisms supporting observed effects reduced confidence in some associations. For instance, 1 study published in 2011 looked at monthly birth rates from 1911 through 1930 in Scandinavian countries and the United States and associated observed birth rate depressions with miscarriages during the first trimester of pregnancy and exposure to the 1918 pandemic influenza [30]. Another study from 2005 found a positive association between severe anophthalmos/microphthalmos prevalence and seasonal influenza (rate ratio, 1.41 [95% CI: 1.08–1.84]), suggestive of a teratogenic role of influenza infection during pregnancy [31]. In a nested case-control study, a large US birth cohort (1959–1966) was followed up for psychiatric disorders 30–38 years later to associate influenza antibody in maternal serum during pregnancy with subsequent development of schizophrenia in offspring. The risk of schizophrenia was increased 7-fold for influenza exposure during the first trimester, representing the first serologic evidence that prenatal influenza could play a role in schizophrenia [32]. Such claims could have significance in prioritization of public health interventions to prevent influenza at the community level and to reinforce the benefits of maternal influenza vaccination. Yet, the lack of robust studies and consistent findings undermine the use of these data for policy making.
Other extrapulmonary associations are described more consistently in the literature. For instance, the association of influenza and cardiovascular diseases such as acute myocardial infarction and stroke have been documented through ecological and population-level observational study designs for many decades [3, 33]. More recent self-controlled case series using laboratory-confirmed influenza as a specific exposure have provided increasingly confirmatory evidence that influenza virus infection can trigger these events, identifying an approximately 6-fold increased rate of myocardial infarction and approximately 3- to 10-fold higher rates of stroke in the week following influenza infection [5, 34, 35]. If influenza virus infection causes cardiovascular events, influenza vaccines should prevent them. A meta-analysis of randomized influenza vaccine studies identified a significant and substantial reduction in cardiovascular events of 46% in influenza vaccine recipients [36]. This was followed by a randomized controlled trial in high-risk populations that found, after early termination due to coronavirus disease 2019 (COVID-19), influenza vaccine recipients experienced 28% lower risk of a composite endpoint of all-cause death, myocardial infarction, or stent thrombosis than placebo recipients [37]. These trials provide a high level of evidence that influenza is an important trigger of cardiovascular events. However, the size of protective effect could imply pathogen-independent biological pathways, which require further investigation through mechanistic and additional clinical studies [20].
A similar range of extrapulmonary manifestations has been documented during the COVID-19 pandemic as associated with SARS-CoV-2. The fast-emerging literature on COVID-19 cases suggest dissemination and replication of SARS-CoV-2 outside the lungs or an effect of the immune response as a sequalae of the disease involving hematologic, cardiovascular, renal, gastrointestinal and hepatobiliary, endocrinologic, neurologic, ophthalmologic, and dermatologic systems [38]. In contrast to influenza, where despite annual epidemics and previous pandemics from which to learn, we have conflicting study results and limited capacity to understand the full array of complications, the COVID-19 data are robust and of unprecedented volume, alluding to the modest understanding we have of respiratory viruses in general and the broader extent of their related disease burden implications.
Influenza has not been widely circulating in many countries during the COVID-19 pandemic, and vaccine coverage rates in some settings have been declining, perhaps as a consequence of perceived reduced severity of influenza in comparison to COVID-19 [39]. The lack of natural or vaccine-derived immunity will result in a more susceptible population and the possibility of severe influenza epidemics when COVID-19 social distancing measures are relaxed and influenza circulation resumes. The uncertain breadth and magnitude of influenza’s extrapulmonary complications is problematic because it contributes to underestimation of the full benefit of influenza vaccines, which could prevent these events in addition to the respiratory events for which they are primarily indicated. Confirmatory studies would be helpful to understand the strength of association between influenza and extrapulmonary complications, including the use of sensitivity analyses and/or designs that reduce or eliminate ecological confounders observed in earlier studies [40]. The self-controlled case series design—documented only during the latter period of our review—measures the change in outcome rate associated with a transient exposure, accounts for confounding factors that remain constant over the observation period, and when using laboratory-confirmed influenza as a specific exposure, provides a convincing level of evidence of influenza’s contribution to subsequent medical events [34, 41]. Moreover, as most observational studies rely on administratively assigned diagnostic codes, attention needs to be given to improve ascertainment and validation of definitions for study outcomes. Additional mechanistic studies to explain unexpected associations are needed; and comparison of the rates of outcomes from randomized vaccine studies can provide further confidence in hypothesized causal pathways [37].
CONCLUSIONS
This scoping review identified an extremely broad range of extrapulmonary medical outcomes associated with influenza, indicative of systemic complications, and the requirement for more effective influenza control. To support policy recommendations and adherence to influenza vaccination, more clinical and epidemiological studies should be done to document the spectrum of complications associated with influenza and the full extension of associated burden.
Supplementary Material
Contributor Information
Joshua Nealon, Sanofi, Lyon, France; School of Public Health, University of Hong Kong, Hong Kong Special Administrative Region, China.
Nieves Derqui, Sanofi, Lyon, France; Medical Research Council Centre for Global Infectious Disease Analysis, School of Public Health, Imperial College London, London, United Kingdom.
Caroline de Courville, Sanofi, Lyon, France.
Tor Biering-Sørensen, Department of Cardiology, Copenhagen University Hospital–Herlev and Gentofte, Copenhagen, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Benjamin J Cowling, School of Public Health, University of Hong Kong, Hong Kong Special Administrative Region, China.
Harish Nair, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom.
Sandra S Chaves, Sanofi, Lyon, France.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Patient consent. This study is a literature review and does not include factors necessitating patient consent.
Financial support. This study received no funding except for salaries.
Potential conflicts of interest. J. N. used to work for, and holds shares in, Sanofi. C. d. C. and S. S. C. are employees of Sanofi. T. B.-S. sits on the Steering Committee of the Amgen-financed GALACTIC-HF trial and Boston Scientific–financed LUX-Dx TRENDS trial and has contributed to advisory boards or received research grants or honoraria from Sanofi, GE Healthcare, Amgen, GSK, Novartis, and Bayer. B. J. C. has received honoraria from AstraZeneca, GlaxoSmithKline, Moderna, Roche, and Sanofi. H. N. reports grants from Innovative Medicines Initiative, the National Institute for Health Research, the World Health Organization, and Pfizer; consulting fees from the Bill & Melinda Gates Foundation; honoraria from AstraZeneca (all to the institution); and participation in data and safety monitoring boards/advisory boards of Sanofi, ReViral, Novavax, and GSK, outside the submitted work. N. D. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Somes MP, Turner RM, Dwyer LJ, Newall AT. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine 2018; 36:3199–207. [DOI] [PubMed] [Google Scholar]
- 2. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Reports (1896–1970) 1932; 47:2159–79. [Google Scholar]
- 4. Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol 2004; 160:492–502. [DOI] [PubMed] [Google Scholar]
- 5. Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51:1701794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses 2017; 11:372–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009; 9:601–10. [DOI] [PubMed] [Google Scholar]
- 8. Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart 2015; 101:1738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caldeira D, Rodrigues B, David C, Costa J, Pinto FJ, Ferreira JJ. The association of influenza infection and vaccine with myocardial infarction: systematic review and meta-analysis of self-controlled case series. Expert Rev Vaccines 2019; 18:1211–7. [DOI] [PubMed] [Google Scholar]
- 10. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 2014; 14:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Critical Appraisal Skills Programme . CASP case control study and cohort study checklist. Oxford, UK: Critical Appraisal Skills Programme; 2021. [Google Scholar]
- 12. Hamilton MA, Calzavara A, Emerson SD, et al. Validating International Classification of Diseases, 10th Revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS One 2021; 16:e0244746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore HC, Lehmann D, de Klerk N, et al. How accurate are International Classification of Diseases-10 diagnosis codes in detecting influenza and pertussis hospitalizations in children? J Pediatric Infect Dis Soc 2014; 3:255–60. [DOI] [PubMed] [Google Scholar]
- 14. Hara K, Tanabe T, Aomatsu T, et al. Febrile seizures associated with influenza A. Brain Dev 2007; 29:30–8. [DOI] [PubMed] [Google Scholar]
- 15. Wilson JC, Toovey S, Jick SS, Meier CR. Previously diagnosed influenza infections and the risk of developing epilepsy. Epidemiol Infect 2015; 143:2408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altzibar JM, Tamayo-Uria I, De Castro V, et al. Epidemiology of asthma exacerbations and their relation with environmental factors in the Basque country. Clin Exp Allergy 2015; 45:1099–108. [DOI] [PubMed] [Google Scholar]
- 17. Fell DB, Buckeridge DL, Platt RW, Kaufman JS, Basso O, Wilson K. Circulating influenza virus and adverse pregnancy outcomes: a time-series study. Am J Epidemiol 2016; 184:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerke AK, Tang F, Yang M, Foster ED, Cavanaugh JE, Polgreen PM. Predicting chronic obstructive pulmonary disease hospitalizations based on concurrent influenza activity. COPD 2013; 10:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med 2019; 380:171–6. [DOI] [PubMed] [Google Scholar]
- 20. Aidoud A, Marlet J, Angoulvant D, Debacq C, Gavazzi G, Fougere B. Influenza vaccination as a novel means of preventing coronary heart disease: effectiveness in older adults. Vaccine 2020; 38:4944–55. [DOI] [PubMed] [Google Scholar]
- 21. Li L, Wang C, Sun L, Zhang X, Yang G. Clinical characteristics and prognostic risk factors of mortality in patients with interstitial lung diseases and viral infection: a retrospective cohort study. J Med Microbiol 2021; 70:001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stals MAM, Grootenboers MJJH, van Guldener C, et al. Risk of thrombotic complications in influenza versus COVID-19 hospitalized patients. Res Pract Thromb Haemost 2021; 5:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenland S, Robins J. Invited commentary: ecologic studies—biases, misconceptions, and counterexamples. Am J Epidemiol 1994; 139:747–60. [DOI] [PubMed] [Google Scholar]
- 24. Krief WI, Levine DA, Platt SL, et al. Influenza virus infection and the risk of serious bacterial infections in young febrile infants. Pediatrics 2009; 124:30–9. [DOI] [PubMed] [Google Scholar]
- 25. Bakken IJ, Aaberg KM, Ghaderi S, et al. Febrile seizures after 2009 influenza A (H1N1) vaccination and infection: a nationwide registry-based study. BMC Infect Dis 2015; 15:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pokorn M, Jevsnik M, Petrovec M, et al. Respiratory and enteric virus detection in children. J Child Neurol 2017; 32:84–93. [DOI] [PubMed] [Google Scholar]
- 27. Guan XR, Li X, Xin XM, et al. Influenza virus infection and risk of acute myocardial infarction. Inflammation 2008; 31:266–72. [DOI] [PubMed] [Google Scholar]
- 28. Surana P, Tang S, McDougall M, Tong CY, Menson E, Lim M. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr 2011; 170:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child 2007; 92:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bloom-Feshbach K, Simonsen L, Viboud C, et al. Natality decline and miscarriages associated with the 1918 influenza pandemic: the Scandinavian and United States experiences. J Infect Dis 2011; 204:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busby A, Dolk H, Armstrong B. Eye anomalies: seasonal variation and maternal viral infections. Epidemiology 2005; 16:317–22. [DOI] [PubMed] [Google Scholar]
- 32. Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 2004; 61:774–80. [DOI] [PubMed] [Google Scholar]
- 33. Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 35. Ohland J, Warren-Gash C, Blackburn R, et al. Acute myocardial infarctions and stroke triggered by laboratory-confirmed respiratory infections in Denmark, 2010 to 2016. Euro Surveill 2020; 25:1900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–20. [DOI] [PubMed] [Google Scholar]
- 37. Frobert O, Gotberg M, Erlinge D, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021; 144:1476–84. [DOI] [PubMed] [Google Scholar]
- 38. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26:1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaccines Europe . Open letter calling for urgent action to address alarmingly low coverage rates for influenza vaccination. 2022. https://www.vaccineseurope.eu/news/articles/open-letter-calling-for-urgent-action-to-address-alarmingly-low-coverage-rates-for-influenza-vaccination. Accessed 7 March 2022.
- 40. Zhang X, Stamey JD, Mathur MB. Assessing the impact of unmeasured confounders for credible and reliable real-world evidence. Pharmacoepidemiol Drug Saf 2020; 29:1219–27. [DOI] [PubMed] [Google Scholar]
- 41. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016; 354:i4515. [DOI] [PubMed] [Google Scholar]
- 42. McConeghy KW, Lee Y, Zullo AR, et al. Influenza illness and hip fracture hospitalizations in nursing home residents: are they related? J Gerontol A Biol Sci Med Sci 2018; 73:1638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hansen C, Desai S, Bredfeldt C, et al. A large, population-based study of 2009 pandemic influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. J Infect Dis 2012; 206:1260–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.