Key Points
Question
What is the durability associated with 2 doses of the BNT162b2 COVID-19 vaccine against Delta- and Omicron-related emergency department and urgent care encounters among adolescents aged 12 to 17 years, and is a third dose associated with improved protection?
Findings
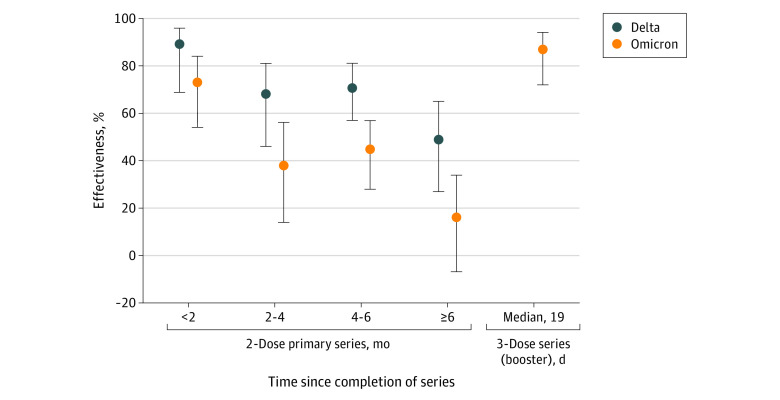
In this case-control study including 3168 adolescents, estimated effectiveness of 2 doses of BNT162b2 was highest against both Delta (89%) and Omicron (73%) less than 2 months after vaccination but waned to 49% against Delta and 16% against Omicron at 6 months and beyond. Estimated effectiveness of 3 doses of BNT162b2 against Omicron was 87%.
Meaning
These findings suggest that third doses are needed to achieve peak protection for adolescents and may help mitigate against a future surge in cases.
This case-control study estimates vaccine effectiveness of 2 doses of BNT162b2 COVID-19 vaccine against emergency department (ED) and urgent care encounters for the Delta and Omicron variants of SARS-CoV-2 among adolescents aged 12 to 17 years in the US.
Abstract
Importance
Data about the duration of protection of 2 and 3 doses of BNT162b2 in children and adolescents are needed to help inform recommendations for boosters in this age group.
Objective
To evaluate vaccine effectiveness (VE) and durability associated with 2 doses of BNT162b2 against Delta- and Omicron-related emergency department (ED) and urgent care (UC) encounters among adolescents aged 12 to 17 years and to estimate VE associated with 3 doses against these same outcomes.
Design, Setting, and Participants
This test-negative case-control study was conducted at Kaiser Permanente Southern California, an integrated health care system using electronic health records in the US. Participants included Kaiser Permanente Southern California members ages 12 to 17 years with an ED or UC encounter from November 1, 2021, through March 18, 2022, for acute respiratory infection who were tested for SARS-CoV-2 via a reverse transction–polymerase chain reaction test. Analyses were conducted from March 21 to June 22, 2022.
Exposures
BNT162b2 vaccination status ascertained from electronic health records and state registry data.
Main Outcomes and Measures
The main outcome was VE associated with BNT162b2 against ED and UC encounters related to Delta or Omicron variant SARS-CoV-2 infection.
Results
Analyses were conducted among 3168 adolescents, including 1004 with ED visits and 2164 with UC visits. Median (IQR) age was 15 (13-16) years, and 1461 (46.1%) were boys. In adjusted analyses, VE associated with 2 doses of BNT162b2 against ED or UC encounters was highest within the first 2 months for both Delta (89% [95% CI, 69% to 96%]) and Omicron (73% [95% CI, 54% to 84%]) variants but waned to 49% (95% CI, 27% to 65%) for the Delta variant and 16% (95% CI, −7% to 34%) for the Omicron variant at 6 months and beyond. A third dose of BNT162b2 was associated with improved protection against the Omicron variant (87% [95% CI, 72% to 94%]) after a median (IQR) of 19 (9-32) days after dose 3.
Conclusions and Relevance
These findings suggest that 2 doses of the BNT162b2 COVID-19 vaccine were associated with high levels of protection against ED and UC encounters related to the Delta and Omicron variants of SARS-CoV-2 in the first few months after vaccination. However, effectiveness waned over time, especially against Omicron. A third dose of BNT162b2 was associated with improved protection against Omicron beyond that seen initially after 2 doses, underscoring the importance of boosters for adolescents aged 12 to 17 years.
Introduction
SARS-CoV-2 infections are generally mild in adolescents, but severe outcomes, including multisystem inflammatory syndrome, hospitalization, or death, can occur. Additionally, many children report long-term sequelae after COVID-19,1 and the pandemic has exacted a heightened psychosocial burden on children.1,2
The BNT162b2 vaccine (Pfizer/BioNTech) is recommended as a 2-dose primary series with a third dose administered at least 5 months after the second dose for adolescents aged 12 to 17 years of age in the US.3 Limited data describing vaccine effectiveness (VE) of BNT162b2 in this age group are available,4,5,6,7,8,9,10,11 and to our knowledge, only 2 preprint reports11,12 and 2 published reports13,14 have estimated VE against Omicron. Both preprint reports11,12 evaluated VE primarily against infection or symptomatic COVID-19 only. In the 2 published reports, VE estimates against Omicron-related end points were limited by small sample size, and only 1 study described the pattern of waning prior to 5 months after dose 214 or evaluated a third dose.13 We evaluated VE and durability associated with 2 doses of BNT162b2 and preliminary effectiveness associated with 3 doses against emergency department (ED) or urgent care (UC) encounters among adolescents aged 12 to 17 years during periods when the Delta and Omicron variants of SARS-CoV-2 were predominant.
Methods
The protocol for this case-control study was reviewed and approved by the Kaiser Permanente Southern California (KPSC) institutional review board, which waived requirement for informed consent per 45CFR 46.116. This study was registered on ClinicalTrials.gov (NCT04848584). This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Study Design and Participants
Using methods described previously,15 we estimated variant-specific VE using a test-negative case-control study in the KPSC health system. Briefly, we included adolescents aged 12 to 17 years with an ED or UC encounter (without subsequent hospitalization) from November 1, 2021, through March 18, 2022, with a diagnosis of acute respiratory infection (ARI) and a reverse transcription–polymerase chain reaction (RT-PCR) nucleic acid amplification test for SARS-CoV-2 from a sample collected within 14 days prior through 3 days after the visit. ARI codes are provided in eTable 1 in the Supplement. As described previously,15 isolates were characterized as Delta or Omicron based on a combination of S-gene target failure status and date of diagnosis.15 Because of the timing of booster dose availability for adolescents, the sample size was insufficient to evaluate VE associated with a third dose against Delta. For evaluating VE associated with a third dose against Omicron, analyses were restricted to December 27, 2021, to March 18, 2022, when more than 90% of samples were Omicron.
Exposures
Adolescents were considered vaccinated with 2 doses at least 14 days after receipt of a second dose of BNT162b2, and they were considered boosted at least 14 days after receipt of a third dose, administered at least 5 months after the second dose. Participants who never received a COVID-19 vaccine were considered unvaccinated. Participants were excluded if they received only 1 dose of BNT162b2, if they had not yet reached 14 days after receipt of the second dose of BNT162b2, if they had not yet reached 14 days after receipt of the third dose of BNT162b2, if they received a third dose of BNT162b2 less than 5 months after their second dose, or if they received any COVID-19 vaccine other than BNT162b2. Vaccination status was captured through KPSC medical records, which incorporate California Immunization Registry data.16
Outcomes
Cases were patients with RT-PCR test results positive for SARS-CoV-2. Controls were patients whose RT-PCR results were negative for SARS-CoV-2 and had not had any positive SARS-CoV-2 test results within 30 days prior to the ED or UC encounter. Multiple events per individual were included if events took place more than 30 days apart.
Statistical Analysis
Crude and adjusted 2- and 3-dose VE against ED and UC encounters were estimated and compared using odds ratios (ORs) and 95% CIs from logistic regression models. For 2-dose estimates, VE was stratified by time since receipt of the second dose. VE was calculated as (1 − OR) × 100%, with 95% CIs calculated using the Wald method. Adjusted models included age, sex, race and ethnicity, body mass index, prior documented positive RT-PCR test result, a pediatric comorbidity index,17 and encounter date as a linear trend. In subanalyses, VE was also estimated for those without a prior documented positive RT-PCR test result. Race and ethnicity were self-reported as Asian or Pacific Islander, Black, Hispanic, White and other or unknown (including American Indian or Alaska Native, multiple races or ethnicities, unknown, and those who self-identified as other). We included race and ethnicity in analysis because they are associated with vaccination and COVID-19 outcomes.
P values were 2-sided, and statistical significance was set at P = .05. Analyses were performed using SAS statistical software version 9.4 (SAS Institute). Analyses were conducted from March 21 to June 22, 2022.
Results
There were 3168 adolescents aged 12 to 17 years with an ED or UC encounter for ARI tested via RT-PCR for SARS-CoV-2 who met study selection criteria. Of 3168 patients included, 51 patients (1.6%) had 2 qualifying encounters included, but no patients had repeat positive test results more than 30 days apart. A total of 19 patients (0.6%) were immunocompromised at the time of test collection. Overall, 1004 patients (31.7%) had ED encounters and 2164 patients (68.3%) had UC encounters. Median (IQR) age was 15 (13-16) years and 1461 (46.1%) were boys (Table). Overall, 978 patients (30.9%) had test results positive for SARS-CoV-2, and among these, 262 patients (26.8%) were infected with the Delta variant and 716 patients (73.2%) were infected with the Omicron variant. In the full cohort, 1907 patients (60.2%) had received 2 doses of BNT162b2, 109 patients (3.4%) had received 3 doses of BNT162b2, and 1152 patients (36.4%) were unvaccinated. Median (range) time since vaccination was 159 (0-303) days for 2-dose analyses and 19 (0-69) days for 3-dose analyses. For 3-dose analyses, 109 patients with an ED or UC encounter for ARI with RT-PCR collection date of on or after December 27, 2021, were included.
Table. Characteristics of Adolescents Aged 12 to 17 Years by Vaccination and Outcome Status, November 1, 2021, Through March 18, 2022.
| Characteristic | Vaccination status | SARS-CoV-2 test results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | |||||||
| Unvaccinated | 2 doses | 3 doses | Total | Negative | Delta | Omicron | Total | |||
| With ARI diagnosis | 1152 (36.4) | 1907 (60.2) | 109 (3.4) | 3168 | NA | 2190 (69.1) | 262 (8.3) | 716 (22.6) | 3168 | NA |
| Age at index | ||||||||||
| Mean (SD) | 14.5 (1.73) | 14.9 (1.67) | 15.4 (1.52) | 14.8 (1.71) | NA | 14.8 (1.73) | 14.9 (1.68) | 14.8 (1.64) | 14.8 (1.71) | NA |
| Median | 15 | 15 | 16 | 15 | NA | 15 | 15 | 15 | 15 | NA |
| 12 | 217 (18.8) | 228 (12.0) | 5 (4.6) | 450 (14.2) | <.001 | 328 (15.0) | 31 (11.8) | 91 (12.7) | 450 (14.2) | .16 |
| 13 | 173 (15.0) | 230 (12.1) | 7 (6.4) | 410 (12.9) | 289 (13.2) | 35 (13.4) | 86 (12.0) | 410 (12.9) | ||
| 14 | 175 (15.2) | 262 (13.7) | 22 (20.2) | 459 (14.5) | 299 (13.7) | 37 (14.1) | 123 (17.2) | 459 (14.5) | ||
| 15 | 192 (16.7) | 350 (18.4) | 16 (14.7) | 558 (17.6) | 372 (17.0) | 43 (16.4) | 143 (20.0) | 558 (17.6) | ||
| 16 | 212 (18.4) | 409 (21.4) | 21 (19.3) | 642 (20.3) | 441 (20.1) | 61 (23.3) | 140 (19.6) | 642 (20.3) | ||
| 17 | 183 (15.9) | 428 (22.4) | 38 (34.9) | 649 (20.5) | 461 (21.1) | 55 (21.0) | 133 (18.6) | 649 (20.5) | ||
| Sex | ||||||||||
| Girls | 608 (52.8) | 1037 (54.4) | 62 (56.9) | 1707 (53.9) | .56 | 1169 (53.4) | 138 (52.7) | 400 (55.9) | 1707 (53.9) | .47 |
| Boys | 544 (47.2) | 870 (45.6) | 47 (43.1) | 1461 (46.1) | 1021 (46.6) | 124 (47.3) | 316 (44.1) | 1461 (46.1) | ||
| Race and ethnicity | ||||||||||
| Asian or Pacific Islander | 31 (2.7) | 182 (9.5) | 13 (11.9) | 226 (7.1) | <.001 | 143 (6.5) | 18 (6.9) | 65 (9.1) | 226 (7.1) | <.001 |
| Black | 96 (8.3) | 112 (5.9) | 5 (4.6) | 213 (6.7) | 133 (6.1) | 15 (5.7) | 65 (9.1) | 213 (6.7) | ||
| Hispanic | 633 (54.9) | 1083 (56.8) | 62 (56.9) | 1778 (56.1) | 1210 (55.3) | 160 (61.1) | 408 (57.0) | 1778 (56.1) | ||
| White | 309 (26.8) | 398 (20.9) | 22 (20.2) | 729 (23) | 557 (25.4) | 47 (17.9) | 125 (17.5) | 729 (23) | ||
| Other or unknowna | 83 (7.2) | 132 (6.9) | 7 (6.4) | 222 (7.0) | 147 (6.7) | 22 (8.4) | 53 (7.4) | 222 (7.0) | ||
| BMI categories for children, percentile | ||||||||||
| Underweight, <5th | 36 (3.1) | 61 (3.2) | 4 (3.7) | 101 (3.2) | .003 | 65 (3.0) | 9 (3.4) | 27 (3.8) | 101 (3.2) | .03 |
| Normal weight, 5th to <85th | 628 (54.5) | 903 (47.4) | 50 (45.9) | 1581 (49.9) | 1137 (51.9) | 118 (45.0) | 326 (45.5) | 1581 (49.9) | ||
| Overweight, 85th to <95th | 172 (14.9) | 340 (17.8) | 15 (13.8) | 527 (16.6) | 359 (16.4) | 51 (19.5) | 117 (16.3) | 527 (16.6) | ||
| Obese, ≥95th | 291 (25.3) | 577 (30.3) | 39 (35.8) | 907 (28.6) | 598 (27.3) | 81 (30.9) | 228 (31.8) | 907 (28.6) | ||
| Unknown | 25 (2.2) | 26 (1.4) | 1 (0.9) | 52 (1.6) | 31 (1.4) | 3 (1.1) | 18 (2.5) | 52 (1.6) | ||
| Pediatric Comorbidity Index (weighted) | ||||||||||
| 0 | 535 (46.4) | 811 (42.2) | 44 (40.4) | 1390 (43.9) | <.001 | 954 (43.6) | 128 (48.9) | 308 (43.0) | 1390 (43.9) | .58 |
| 1 | 271 (23.5) | 406 (21.3) | 17 (15.6) | 694 (21.8) | 474 (21.6) | 56 (21.4) | 164 (22.9) | 694 (21.9) | ||
| 2 | 104 (9.0) | 194 (10.2) | 7 (6.4) | 305 (9.6) | 213 (9.7) | 22 (8.4) | 70 (9.8) | 305 (9.6) | ||
| 3 | 47 (4.1) | 91 (4.8) | 11 (10.1) | 149 (4.7) | 98 (4.5) | 15 (5.7) | 36 (5.0) | 149 (4.7) | ||
| ≥4 | 195 (16.9) | 405 (21.2) | 30 (27.5) | 630 (19.9) | 451 (20.6) | 41 (15.6) | 138 (19.3) | 630 (19.9) | ||
| Documented prior SARS-CoV-2 infection | ||||||||||
| No | 997 (86.5) | 1734 (90.9) | 90 (82.6) | 2821 (89.0) | <.001 | 1893 (86.4) | 256 (97.7) | 672 (93.9) | 2821 (89.0) | <.001 |
| Yes | 155 (13.5) | 173 (9.1) | 19 (17.4) | 347 (11.0) | 297 (13.6) | 6 (2.3) | 44 (6.1) | 347 (11.0) | ||
| Encounter | ||||||||||
| Emergency department | 412 (35.8) | 553 (29.0) | 39 (35.8) | 1004 (31.7) | NA | 572 (26.1) | 73 (27.9) | 359 (50.1) | 1004 (31.7) | NA |
| Urgent care | 740 (64.2) | 1354 (71.0) | 70 (64.2) | 2164 (68.3) | NA | 1618 (73.9) | 189 (72.1) | 357 (49.9) | 2164 (68.3) | NA |
Abbreviations: ARI, acute respiratory infection; BMI, body mass index; NA, not applicable.
Including American Indian or Alaska Native, multiple races or ethnicities, unknown, and those who self-identified as other race or ethnicity.
In adjusted analyses, VE associated with 2 doses of BNT162b2 against Omicron-related ED or UC encounters for ARI was 73% (95% CI, 54% to 84%) less than 2 months after the second dose, 38% (95% CI, 14% to 56%) at 2 to 3 months after the second dose, 45% (95% CI, 28% to 57%) at 4 to 5 months after the second dose, and 16% (95% CI, −7% to 34%) at 6 months after dose 2 and beyond (Figure; eTable 2 in the Supplement). VE associated with 3 doses of BNT162b2 against Omicron was 87% (95% CI, 72%-94%) after a median (IQR) time of 19 (9-32) days after dose 3. Against Delta, the VE associated with 2 doses of BNT162b2 was 89% (95% CI, 69% to 96%) at less than 2 months after the second dose and 49% (95% CI, 27% to 65%) at 6 months or longer after the second dose.
Figure. Adjusted Vaccine Effectiveness Associated With BNT162b2 Against Emergency Department and Urgent Care Encounters (Without Subsequent Hospitalization) for Delta- or Omicron-Related Acute Respiratory Illness.
Vaccine effectiveness (dots) and 95% CIs (whiskers) are based on results from multivariable logistic regression analysis comparing adolescents aged 12 to 17 years who received the 2-dose primary or a third dose of BNT162b2 compared with individuals who were unvaccinated, adjusted for age, sex, race and ethnicity, body mass index, prior positive results on a SARS-CoV-2 test, pediatric comorbidity index, and admission date. Sample size was insufficient to estimate effectiveness of 3 doses of BNT162b2 against the Delta variant.
No meaningful differences in VE estimates were observed when analyses were restricted to patients without a prior documented positive RT-PCR test result (2821 patients [89%]) (eTable 3 in the Supplement).
Discussion
The findings of this case-control study suggest that 2 doses of BNT162b2 were associated with preventing ED and UC encounters for ARI caused by either the Delta or Omicron variants of SARS-CoV-2 in the first few months after vaccination. However, this effectiveness waned over time, although waning was more pronounced for the Omicron variant. A third dose of BNT162b2 was associated with improved protection beyond that seen initially after 2 doses. These findings underscore the important role of booster doses to enhance protection against COVID-19 for adolescents aged 12 to 17 years.
A recent Centers for Disease Control and Prevention (CDC) report13 evaluated effectiveness of 2 doses of BNT162b2 against Omicron-related ED or UC encounters stratified by less than 150 days (ie, approximately 5 months) vs 150 days or longer after dose 2, with VE estimated at 45% (95% CI, 30% to 57%) among adolescents aged 12 to 15 years and 34% (95% CI, 8% to 53%) among adolescents aged 16 to 17 years at less than 150 days, and no VE against Omicron in either age group thereafter. Studies among adults have also shown similar findings, namely that 2 doses of an mRNA vaccine likely provide only limited and short-lived protection against Omicron-related illness and that a third dose significantly improves protection against this variant of concern.18,19 Our study estimated that VE against Omicron-related ED or UC encounters less than 2 months after receipt of a second dose was 73% (95% CI, 54% to 84%) but with significant waning thereafter. Given improved VE observed early after a third dose in our study (87% [95% CI, 72% to 94%] against Omicron) and according to CDC data (86% [95% CI, 73% to 93%] against Delta or Omicron among adolescents aged 12 to 17 years),13 a shorter interval for administration of a booster dose may be warranted to ensure protection against a seasonal surge or uptick in cases driven by BA.2 or future variants. However, future studies describing duration of protection associated with a third dose in adolescents are needed.
Limitations
This study has some limitations. Our study is subject to known limitations of observational studies, which we have detailed in previous reports.15,16,20 Briefly, we were not able to capture data describing social or health behaviors. Additionally, sample size limitations precluded inclusion of all potentially confounding variables in adjusted analyses. While individuals who are vaccinated may be more likely to seek care or testing for SARS-CoV-2, the test-negative design helps mitigate against bias caused by differences in health care–seeking behavior, including propensity to test.21,22,23,24 We attempted to control for prior SARS-CoV-2 infection but likely missed some infections, given that many tests may be conducted at home and thus not reported or included in medical records. This is likely to be especially true during the Omicron period when at-home tests became widely available. If undocumented prior infection occurred more often among individuals who were not vaccinated, this could lead to an underestimation of VE or an overestimate of the degree of waning over time.25 Stratified analyses among patients without prior documented infection demonstrated nearly identical VE estimates. Additionally, severe COVID-19 outcomes, such as hospitalization, were rare in our study population, and we were unable to evaluate VE against severe illness. A recent CDC study13 suggested that 2 doses of BNT162b2 was associated with high levels of protection against hospitalization in this age group but did not evaluate Omicron specifically. Hence, more data estimating VE against severe end points in children and adolescents, especially for Omicron, are needed.
Conclusions
This case-control study found that although 2 doses of BNT162b2 were associated with preventing Delta- and Omicron-related ED and UC encounters in adolescents aged 12 to 17 years relatively soon after vaccination, waning of vaccine-induced immunity, especially against Omicron, supports the need for a booster in this age group. Initial VE associated with a third dose was 87% against Omicron-related ED and UC encounters, but more data about the durability of a third dose are needed. Our findings underscore the need for booster doses in this population. In the absence of new variant-adapted mRNA vaccines that may extend duration of protection against omicron or future variants, boosters of the current formulation may be needed sooner than is currently recommended (at ≥5 months after dose 2) in the event of a surge in cases.
eTable 1. Acute Respiratory Infection Codes, November 1, 2021, to March 18, 2022
eTable 2. Adjusted Effectiveness Associated With BNT162b2 Against Emergency Department or Urgent Care Encounters (Without Subsequent Hospitalization) for Delta- or Omicron-Related Acute Respiratory Illness
eTable 3. Unadjusted and Adjusted Effectiveness Associated With BNT162b2 Against Emergency Department or Urgent Care Encounters (Without Subsequent Hospitalization) for Delta- or Omicron-Related Acute Respiratory Illness Among Patients Without Prior Documented SARS-CoV-2 Infection
References
- 1.Chow EJ, Englund JA. Severe Acute Respiratory Syndrome Coronavirus 2 Infections in Children. Infect Dis Clin North Am. 2022;36(2):435-479. doi: 10.1016/j.idc.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rankin DA, Talj R, Howard LM, Halasa NB. Epidemiologic trends and characteristics of SARS-CoV-2 infections among children in the United States. Curr Opin Pediatr. 2021;33(1):114-121. doi: 10.1097/MOP.0000000000000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . COVID-19 vaccine: interim COVID-19 immunization schedule for ages 5 years and older. Accessed March 17, 2022. https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
- 4.Thakkar PV, Zimmerman KO, Brookhart MA, Erickson TR, Benjamin DK Jr, Kalu IC; ABC Science Collaborative . COVID-19 incidence among sixth through twelfth grade students by vaccination status. Pediatrics. 2022;149(5):e2022056230. doi: 10.1542/peds.2022-056230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks KJ, Whitaker M, Anglin O, et al. ; COVID-NET Surveillance Team . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):271-278. doi: 10.15585/mmwr.mm7107e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutrick K, Rivers P, Yoo YM, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12-17 years—Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1761-1765. doi: 10.15585/mmwr.mm705152a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson SM, Newhams MM, Halasa NB, et al. ; Overcoming COVID-19 Investigators . Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12-18 years—United States, June-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(42):1483-1488. doi: 10.15585/mmwr.mm7042e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prunas O, Weinberger DM, Pitzer VE, Gazit S, Patalon T. Waning effectiveness of the BNT162b2 vaccine against infection in adolescents. medRxiv. Preprint posted online January 5, 2022. doi: 10.1101/2022.01.04.22268776 [DOI] [PMC free article] [PubMed]
- 9.Glikman D, Stein M, Shinwell ES. Vaccinating children and adolescents against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—the Israeli experience. Acta Paediatr. 2021;110(9):2496-2498. doi: 10.1111/apa.15982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis BY, Barda N, Leshchinsky M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med. 2021;385(22):2101-2103. doi: 10.1056/NEJMc2114290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews N, Stowe J, Kirsebom F, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of BNT162b2 (Comirnaty, Pfizer-BioNTech) COVID-19 booster vaccine against covid-19 related symptoms in England: test negative case-control study. medRxiv. Preprint posted online November 15, 2021. doi: 10.1101/2021.11.15.21266341 [DOI]
- 12.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5-11 and 12-17 years in New York after the emergence of the Omicron variant. medRxiv. Preprint posted online February 28, 2022. doi: 10.1101/2022.02.25.22271454 [DOI]
- 13.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing covid-19–associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years—VISION Network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):352-358. doi: 10.15585/mmwr.mm7109e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell AAKF, Kirsebom F, Stowe J, et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22(5):581-583. doi: 10.1016/S1473-3099(22)00177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 (Pfizer–BioNTech) mRNA COVID-19 vaccine against omicron-related emergency department and hospital admission in a large US health system: a test-negative design. Lancet Respir Med. 2022;10(7):689-699. doi: 10.1016/S2213-2600(22)00101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407-1416. doi: 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JW, Bourgeois FT, Haneuse S, et al. Development and validation of a pediatric comorbidity index. Am J Epidemiol. 2021;190(5):918-927. doi: 10.1093/aje/kwaa244 [DOI] [PubMed] [Google Scholar]
- 18.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532-1546. doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartof SY, Slezak JM, Puzniak L, et al. Immunocompromise and durability of BNT162b2 vaccine against severe outcomes due to omicron and delta variants. Lancet Respir Med. 2022;10(7):e61-e62. doi: 10.1016/S2213-2600(22)00170-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9:100198. doi: 10.1016/j.lana.2022.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18(37):20585. doi: 10.2807/1560-7917.ES2013.18.37.20585 [DOI] [PubMed] [Google Scholar]
- 22.Haber M, An Q, Foppa IM, Shay DK, Ferdinands JM, Orenstein WA. A probability model for evaluating the bias and precision of influenza vaccine effectiveness estimates from case-control studies. Epidemiol Infect. 2015;143(7):1417-1426. doi: 10.1017/S0950268814002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165-2168. doi: 10.1016/j.vaccine.2013.02.053 [DOI] [PubMed] [Google Scholar]
- 24.Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol. 2016;45(6):2060-2074. doi: 10.1093/ije/dyw124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsitch M. Challenges of vaccine effectiveness and waning studies. Clin Infect Dis. 2019;68(10):1631-1633. doi: 10.1093/cid/ciy773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Acute Respiratory Infection Codes, November 1, 2021, to March 18, 2022
eTable 2. Adjusted Effectiveness Associated With BNT162b2 Against Emergency Department or Urgent Care Encounters (Without Subsequent Hospitalization) for Delta- or Omicron-Related Acute Respiratory Illness
eTable 3. Unadjusted and Adjusted Effectiveness Associated With BNT162b2 Against Emergency Department or Urgent Care Encounters (Without Subsequent Hospitalization) for Delta- or Omicron-Related Acute Respiratory Illness Among Patients Without Prior Documented SARS-CoV-2 Infection