Abstract
Background:
Prevalence of antibody-mediated autoimmune encephalitis (AE) is reported to be comparable to infectious encephalitis in Western populations. We evaluated the frequency and significance of AE and neuronal autoantibodies in comparison to infectious etiologies among patients presenting with encephalitis in a South Asian population.
Methods:
Ninety-nine consecutive patients with a clinical diagnosis of encephalitis/meningoencephalitis admitted to two of the largest tertiary-care hospitals in Sri Lanka were studied. PCR and ELISA were used to screen viruses while Gram stain and culture were used to screen bacteria. Sera were tested for antibodies binding to primary embryonic rat hippocampal neuronal cultures and cell-based assays for antibodies to NMDAR, LGI1, CASPR2, Contactin2, AMPAR, GABAAR, GABABR, aquaporin-4 and MOG.
Results:
Patient ages ranged from 1 month to 73 years (mean = 24.91; SD = 21.33) with a male: female ratio of 1.75:1. A viral etiology was identified in 27.3% and bacterial meningoencephalitis was diagnosed in 17.1%. Sera of nine patients had antibodies binding to live primary neurons, but only five had specific antibodies to CASPR2 (n = 1), NMDAR (n = 2) or GABABR-antibodies (n = 2). Moreover, the patients with CASPR2 antibodies and NMDAR-antibodies were also positive for dengue antibodies. Only the two patients with NMDAR-antibodies had features and responses to immunotherapy consistent with AE.
Conclusions:
Identified infectious forms of meningoencephalitis (44.4%) greatly exceeded the occurrence of neuronal autoantibodies (9.1%) and AE (2%) in Sri Lanka, and this may be common in those regions where infections are prevalent.
Keywords: Autoimmune encephalitis, meningoencephalitis, NMDAR, Sri Lanka
INTRODUCTION
Encephalitis and meningoencephalitis are clinical syndromes that denote underlying cerebral inflammation. Although infections, particularly viruses, are considered the most important etiological agents, the cause of encephalitis remains unidentified in over 50% of the cases.[1] Among the 'non-infectious' group of encephalitis, treatment responsive autoimmune encephalitis (AE) mediated by potentially pathogenic autoantibodies directed against neuronal cell surface and synaptic proteins are increasingly recognized.[2,3] In temperate regions, the incidence and prevalence of AE has been reported to be comparable to viral encephalitis.[4] However, research on AE from South Asia remains sparse,[5] but infectious etiologies are assumed to predominate.[6] Although neuronal autoantibodies can occur as para- or post-infectious epiphenomena, only about one-third would result in AE.[7] Thus, the detection of neuronal autoantibodies in patients with postulated infectious encephalitis is likely to pose both diagnostic and therapeutic challenges. It is hypothesized that infectious encephalitides are likely to exceed AE and that neuronal autoantibodies induced as epiphenomena of infections are likely to be mostly clinically insignificant in regions with high prevalence of infections. The prevalence of pathogenic and non-pathogenic neuronal autoantibodies in infectious encephalitis in a region with a high prevalence of infections such as South Asia remains unknown. Thus, we studied the sera of our recent study on the viral etiologies of encephalitis/meningoencephalitis,[6] to determine the prevalence of neuronal autoantibodies and AE in comparison to identified infectious etiologies.
METHODS
Patients and samples
Consecutive patients with a clinical syndrome of encephalitis/meningoencephalitis diagnosed according to criteria of the Consensus Statement of the International Encephalitis Consortium[8] were prospectively recruited from patients admitted to the largest tertiary-care hospitals in Sri Lanka for adults (National Hospital of Sri Lanka, bed-strength 3900) and children (Lady Ridgeway Hospital for Children, bed-strength 900) during a period of 21 months. Patients with an alternative diagnosis that could mimic encephalitis such as psychiatric illness, metabolic disorders, epilepsy, post-anoxia, vasculitis, stroke and septicemia were excluded. The computed minimum sample size to achieve a precision of 0.05 and Zα value of 1.96 was 87 for the initial study of viral etiologies in encephalitis, of which, this study is an extension.[6] Diagnosis of encephalitis was made by Board-certified specialists in Neurology, Pediatric Neurology, Internal Medicine or Pediatrics. Written informed consent was obtained from either the patient, next-of-kin or guardian. Serum and/or cerebrospinal fluid (CSF) were obtained from all patients when these specimens were collected as part of their diagnostic work up. Demographic, clinical and laboratory data including CSF analysis, blood investigations, brain imaging and electroencephalogram (EEG) results were recorded from hospital records.
Ethical considerations
The study was approved by the Ethics Review Committees of the Faculty of Medicine, University of Colombo, Sri Lanka and that of the two hospitals.
Laboratory analyses
Bacteriological screening
CSF was tested using Gram stain and culture on enriched culture media.
Virus screening
Serum and CSF were tested. Polymerase reaction (PCR) assays were performed to detect Herpes simplex (HSV), Varicella zoster (VZV), Epstein Barr (EBV) and cytomegalo (CMV) viruses while reverse transcriptase-PCR was used to detect Dengue, Japanese encephalitis (JEV) and West Nile (WNV) viruses. Enzyme-linked immunosorbent assays were used to detect specific IgM and IgG antibodies against HSV, Dengue, JEV and WNV. Plaque reduction neutralization test (PRNT) was performed at the National WNV reference laboratory of the National University of Singapore only for samples that were positive for WNV-specific IgM.
Autoantibody assays
Of the autoantibodies associated with encephalitis, only ones with established pathogenic roles or with potential for pathogenicity by virtue of targeting neuroglial cell surface or synaptic proteins were selected for testing. Autoantibody assays were performed in the Neuroimmunology laboratory of the Nuffield Department of Clinical Neurosciences in Oxford, United Kingdom. CSF were no longer available. Sera were tested on live cell-based assays (CBA) for NMDAR, LGI1, CASPR2 and Contactin2 antibodies performed according to previously described standard protocols.[9] To expand the range of possible autoantibodies, sera were also tested on primary embryonic rat hippocampal neuronal cultures for surface binding antibodies, as previously described.[10] Only those that showed neuronal and glial surface-binding antibodies were tested on CBA for AMPAR, GABAAR, GABABR, aquaporin-4 and MOG antibodies. Binding was graded as 0 (negative) to 4 (strongly positive) by two independent observers and considered positive only if results agreed. In primary neuronal cultures and CBA, positive controls and negative controls (healthy sera) were tested concurrently.
Diagnosis of AE
Since this study aimed to determine whether the neuronal cell surface/synaptic autoantibodies were pathogenic, the mere detection of antibodies were not taken as indicative of AE. The diagnosis of AE was based on the presence of established diagnostic criteria for 'probable' AE[11] and supported by a positive response to immunotherapy.
RESULTS
A total of 108 patients were recruited over a period of 21 months, but 9 were subsequently excluded due to inadequate clinical data for analysis. Patient ages ranged from 1 month to 73 years (mean = 24.91; SD = 21.33) with 41.4% being less than 12 years of age. The male to female ratio was 1.75:1 in both adults and children. All 99 patients were tested for both geographically restricted and globally prevalent viruses known to cause encephalitis. A viral etiology was identified in only 27.3% patients with encephalitis, details of which have been previously published.[7,12] In addition, a diagnosis of bacterial meningoencephalitis was made in 17.1% based on typical CSF profiles (high protein, neutrophil pleocytosis and low CSF:serum glucose ratio) supported by peripheral blood neutrophilic leukocytosis and elevated inflammatory markers.[13] However, bacteriological staining of CSF, and culture of blood and CSF failed to isolate any specific organisms.
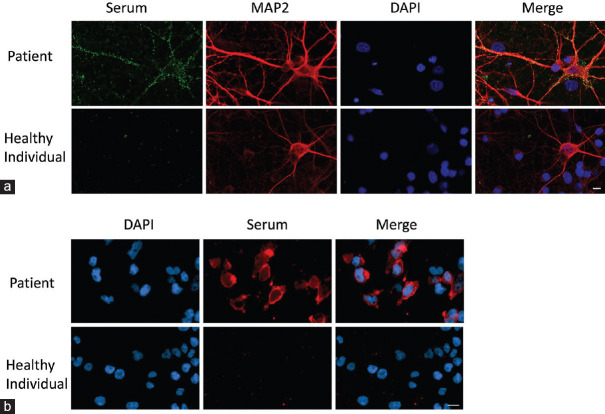
Sera of 4 patients showed high-positive (grade 3 – 4) neuronal staining while 5 showed low-positive (grade 2) neuronal staining on live primary embryonic rat hippocampal neuronal cultures [Figure 1a]. Sera of five patient were positive for specific encephalitogenic autoantibodies on CBA: one with CASPR2-antibodies (1%), two with NMDAR antibodies (2%) and two with GABABR antibodies (2%) [Figure 1b and Table 1]. Only two were positive on both primary neuronal cultures and CBAs: Patient 2 (NMDAR antibodies) and Patient 5 (GABABR antibodies). Patient 4 serum bound to glial cells in the hippocampal cultures but was positive for GABABR antibodies. Patient 1 (CASPR2 antibodies), and Patient 3 (NMDAR antibodies) did not have serum antibodies to the primary cultures. All other sera, including the four with positive neuronal antibodies, were negative for AMPAR, GABAAR, aquaporin-4 or MOG antibodies. Unfortunately, CSF was no longer available for antibody testing.
Figure 1.
(a) Primary embryonic rat hippocampal neurons in culture labelled with MAP2 (neuronal stain) and DAPI (nuclear stain). Patient serum antibodies are detected binding to the surface of neuronal cell bodies and processes (top row). In contrast, healthy control serum does not show any binding in the neuronal cultures (bottom row). Scale bar = 10 mm. (b) Representative immunofluorescence images of a cell-based assay. HEK cells transiently transfected with NR1 and labelled with DAPI (nuclear stain) shows cell surface binding with patient serum (top row). Healthy control serum does not show any binding (bottom row). Scale bar = 10 mm
Table 1.
Clinical profiles, investigation results and treatment outcomes of patients with serum encephalitogenic autoantibodies directed against cell surface and synaptic proteins
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Neuronal antibodies detected using CBA | CASPR2 | NMDAR | NMDAR | GABABR | GABABR |
| Staining on primary neuronal cultures | None | Neuronal | None | Glial | Neuronal |
| Gender, Age (years) | M, 58 | F, 3.5 | M, 39 | M, 19 | M, 59 |
| Prodrome or concurrent symptoms | Chills, myalgia | Irritable, aggressive, insomnia | Mutism | None | arthralgia and myalgia |
| Fever | Y (3 days) | N | N | Y (5 days) | Y (1 day) |
| Headache | Y | N | N | N | Y |
| Altered level of consciousness | Y (confusion) | Y (abnormal behaviour 15 days) | Y (abnormal behaviour) | Y (confusion) | Y (confusion) |
| Seizures | N | Y | Y (right focal seizures) | Y (GTCS) | Y (GTCS) |
| GCS | 14/15 | 9/15 | 9/15 | 8/15 | 13/15 |
| CT brain | Normal | Normal | Not done | Normal | Normal |
| MRI brain | Not done | Not done | Normal | Not done | Not done |
| EEG | Generalized slow wave discharges | Right-sided slow waves with abnormal sleep EEG. Rhythmic sharps were in bilateral mid-frontal regions | Generalized slow wave discharges | Generalized slow wave discharges | Low voltage cerebral activity |
| Cerebrospinal fluid | |||||
| Colour | Colourless | Colourless | Colourless | Colourless | Colourless |
| Protein (mg/dl) | 25 | 45 | 59 | 49 | 52 |
| Glucose: CSF/plasma | 4.8/6.0 | 4.0/7.6 | 5.7/9.4 | 4.2/7.2 | 4.0/7.8 |
| Lymphocytes | 08 | 25 | 15 | 10 | 08 |
| Polymorphs | 00 | 00 | 00 | 20 | 25 |
| Gram stain | Negative | Negative | Negative | Negative | Negative |
| Culture and AFB | Sterile | Sterile | Sterile | Sterile | Sterile |
| Screen for viruses | Dengue IgM antibodies in serum 11.88 and in CSF 17.26 panbio units | Dengue IgM antibodies in serum 2.96 and in CSF 14.03 panbio units | Negative | Negative | Negative |
| Treatment | Intravenous ceftriaxone and aciclovir for 10 days | Intravenous aciclovir for 14 days. | Intravenous ceftriaxone and aciclovir for 14 days. Intravenous methyl prednisolone 1 g/d for 3 days. | Intravenous dexamethasone for 4 days, ceftriaxone and aciclovir for 10 days. | Intravenous ceftriaxone and aciclovir for 14 days. |
| Intravenous methyl prednisolone 30 mg/kg for 3 days. | |||||
| Duration of hospital stay | 14 days | 34 days | 17 days | 12 days | 15 days |
| Outcome at discharge from hospital | GCS 15/15 and return to premorbid level of cognitive functions. | GCS 15/15 and return to premorbid level of cognitive functions. | GCS 15/15 and return to premorbid level of cognitive functions. | GCS 15/15 and return to premorbid level of cognitive functions. | GCS 15/15 and return to premorbid level of cognitive functions. |
| Final diagnosis | Dengue encephalitis | NMDARAb encephalitis | NMDARAb encephalitis | Bacterial meningoencephalitis | Bacterial meningoencephalitis |
Cut off values for Dengue IgM antibodies: positive >11; equivocal 9–11; negative <9. AFB – Acid fast bacilli. CBA – cell-based assay. GTCS – generalised tonic-clonic seizures. Y – Yes; N – No.
The clinical profiles, relevant investigation results and treatment outcomes of the patients with serum CBA positive antibodies are shown in Table 1. The antibody results were not available at the time of diagnosis. Patient ages ranged from 3.5 to 59 years with four of five patients being male. Patient 2 and Patient 3 fulfilled criteria for a diagnosis of 'probable NMDAR-antibody encephalitis' at presentation, which included cognitive dysfunction, seizures, dyskinesias, mutism, altered level of consciousness, abnormal EEG, CSF pleocytosis and exclusion of other etiologies.[11] The cranial CT scan was normal in Patient 2 while the cranial MRI was normal in Patient 3 as seen with most NMDAR-antibody encephalitides. These two patients were treated with high-dose intravenous steroids. All patients received intravenous antibiotics and aciclovir and/or a short course of dexamethasone, which is the default practice when a definitive etiological diagnosis is not evident.
In addition, dengue IgM antibodies were detected in significant titers in Patient 1 (serum and CSF) and Patient 2 (CSF). Although Patient 1 had leukocytosis at presentation, he developed leucopenia and thrombocytopenia during the ensuing days consistent with dengue fever complicated by encephalitis, but without evidence of plasma leakage. All his counts subsequently normalized. The clinical profile, peripheral leukocytosis, and CSF pleocytosis noted in Patient 4 and Patient 5 were consistent with partially treated bacterial meningoencephalitis. These two patients had received antibiotics for five to six days before lumbar punctures were performed. All patients with encephalitogenic antibodies demonstrated abnormal EEG activity consistent with cerebral inflammation, but only Patient 2 had epileptic discharges. All patients recovered normal consciousness and premorbid cognitive status at time of discharge. Only Patient 2 had a prolonged hospital stay while the others were discharged from hospital in 12 to 17 days.
DISCUSSION
Among the 99 patients presenting to hospital with a clinical syndrome of encephalitis or meningoencephalitis, an infectious etiology was identified in 44.4%, NMDAR-antibody encephalitis was diagnosed in 2% and the etiology remained undetermined in 53.6%. The etiology of encephalitis in the undetermined group was assumed to be infectious given the high prevalence of infections in the region. Although neuronal surface and synaptic protein binding autoantibodies were detected in 9.1% of patients, established pathogenic antibodies of recognized AE were identified in only 5% among them. However, a definitive diagnosis of AE was established only among the two patients with NMDAR antibodies who fulfilled the established diagnostic criteria[11] and responded to immunotherapy. Thus, our data suggest that neuronal cell surface/synaptic protein binding autoantibodies to recognized and yet unrecognized epitopes occur in about one tenth of patients presenting with encephalitis, and that often these autoantibodies occur as para- or post-infectious epiphenomena without an identified pathogenic role.
Although in this study AE was diagnosed in only 2% of unselected encephalitis patients, a recent study which selected patients with suspected AE based on established diagnostic criteria[11] found up to 50% of patients to have AE and 44.6% of them to have detectable pathogenic antibodies to recognized AE in CSF.[5] The higher yield in the cited study is likely due to exclusion of patients with likely infectious etiologies, which probably accounts for most of the encephalitis in regions with a high prevalence of infections.
There are many clinical and investigational overlaps between infectious and AE while sensitivities of diagnostic assays are not optimal. Moreover, the therapeutic use of corticosteroids also in CNS infections, the empirical use of antibiotics and the limited availability of reliable diagnostic tests are likely to further blur the distinction between infectious and AE. In this study, a definitive diagnosis of AE was stringently established based on established diagnostic criteria,[11] detection of pathogenic autoantibodies and response to high dose intravenous corticosteroids. Thus, it is possible that a few seronegative AE may have been misclassified as 'infectious' or 'etiology undetermined' encephalitis.
Curiously, the two patients diagnosed with NMDAR-antibody encephalitis in our study recovered within days with high dose intravenous steroids alone without requiring further immunosuppression, but similar responses have been previously reported.[14] Since the antibody status was not known at the time of diagnosis, no attempts were made to screen for underlying tumors. However, tumors are rare among pediatric patients who develop NMDAR-antibody encephalitis.[15]
Interestingly, Patient 2 was also positive for dengue antibodies [Table 1]. Dengue is recognized as a leading cause of encephalitis in endemic regions and encephalitis may present as a primary manifestation of the infection.[16,17,18] Dengue has emerged as the most important vector-borne disease accounting for recurrent epidemics in Sri Lanka,[19] with encephalitis as a frequent presentation.[6] Thus, it is plausible that patients with AE may have co-infection with dengue virus in an endemic region, but it is tempting to postulate that the AE may have been triggered as a post-infectious phenomenon of dengue as has been described with HSV and Japanese encephalitis.[7,20] Such postulations would require further study.
None of the patients with GABABR or CASPR2 antibodies, had clinical or investigational characteristics consistent with a diagnosis of limbic encephalitis,[11] but had alternative diagnoses - Patient 1: dengue encephalitis, Patient 4 and Patient 5: partially treated bacterial meningoencephalitis. These patients recovered with antiviral and antibiotic medication without requirement of immunotherapies. Thus, it is likely that these autoantibodies occurred as a para- or post-infectious epiphenomenon with no pathogenic consequences at the time of the study. Virus-induced antibody generation is likely to be a widespread mechanism and patients may require long-term follow up to define the significance and pathogenic potential of these autoantibodies detected during or after infections.
There are some limitations to our study. First, our data suffers from the inherent bias of hospital-based studies and is likely to be an underestimate. Second, although patients were recruited prospectively, specimen analyses were done later and therefore the results were not known at the time of diagnosis and treatment, leading to lack of long-term follow-up data or screening for tumors. Furthermore, since there was no long-term follow up of patients, the significance of the detected autoantibodies could not be defined. Third, as CSF was not available at the time of analysis, we had to rely on serum testing which could have underestimated NMDAR antibodies (although this is not common with live CBAs) or, conversely, detected neuronal autoantibodies that did not have the potential to cross the blood brain barrier.[21] Fourth, had cranial magnetic resonance imaging been performed on all patients, we may have identified more patients of seronegative AE. Finally, an infectious etiology may have been more commonly identified had more specialized microbiological investigations been utilized.
CONCLUSIONS AND RECOMMENDATIONS
This study informs clinicians that an infectious etiology remains the commonest identified cause of encephalitis in regions with a high prevalence of infections and that both pathogenic and non-pathogenic neuronal autoantibodies can occur as post- or para-infectious epiphenomena in encephalitis, and recommends that the occurrence of neuronal autoantibodies be interpreted in the clinical context of established diagnostic criteria for AE.[11,22]
Abbreviations
AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, CASPR2: Contactin-associated protein-like 2, CBA: Cell-based assay, CMV: Cytomegalovirus, CRP: C-reactive protein, CSF: Cerebrospinal fluid, CT: Computerized tomography, EEG: Electroencephalogram, EBV: Epstein Barr virus, ELISA: Enzyme-linked immunosorbent assays, ESR: Erythrocyte sedimentation rate, GABAB: γ-aminobutyric acid B, HEK: Human embryonic kidney, HSV: Herpes simplex virus, JEV: Japanese encephalitis virus, LE: Limbic encephalitis, LGI1: Leucine-rich glioma-inactivated protein 1, MRI: Magnetic resonance imaging, MOG: Myelin oligodendrocyte glycoprotein, NMDAR: N-methyl D-aspartate receptor, PCR: Polymerase chain reaction, RT-PCR: Reverse transcriptase polymerase chain reaction, VGKC: Voltage-gated potassium channel, VZV: Varicella zoster virus, WNV: West Nile virus
DECLARATIONS
Ethics approval and consent to participate
Ethics approval was obtained from Ethics Review Committees of Faculty of Medicine, University of Colombo, National Hospital of Sri Lanka and Lady Ridgeway Hospital for Children. Written informed consent was obtained from either the patient or guardian.
Consent to publish
Not applicable.
Availability of data and material
All relevant data generated or analyzed during this study are included in this published article.
Financial support and sponsorship
This work was partly supported by the National Research Council, Sri Lanka (NRC Grant No. 11-75).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom is gratefully acknowledged for providing reagents and facilities to carry out the neuronal and cell-based assays.
REFERENCES
- 1.Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 2.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378:840–51. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 4.Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83:166–77. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickramasinghe N, Dasanayake D, Malavige N, de Silva R, Chang T. Autoimmune encephalitis in a South Asian population. BMC Neurol. 2021;21:203. doi: 10.1186/s12883-021-02232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohitharajah J, Malavige N, Arambepola C, Wanigasinghe J, Gamage R, Gunaratne P, et al. Viral aetiologies of acute encephalitis in a hospital-based South Asian population. BMC Infect Dis. 2017;17:303. doi: 10.1186/s12879-017-2403-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–72. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–28. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters P, Woodhall M, O'Connor KC, Reindl M, Lang B, Sato DK, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2:e89. doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaudoin GM, 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7:1741–54. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 11.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohitharajah J, Malavige GN, Chua AJ, Ng ML, Arambepola C, Chang T. Emergence of human West Nile Virus infection in Sri Lanka. BMC Infect Dis. 2015;15:305. doi: 10.1186/s12879-015-1040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benninger F, Steiner I. CSF in acute and chronic infectious diseases. Handb Clin Neurol. 2017;146:187–206. doi: 10.1016/B978-0-12-804279-3.00012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanigasinghe J, Chang T, Vincent A. Treatment-responsive, reversible, autoimmune encephalitis in a child. Ceylon Med J. 2012;57:90–1. doi: 10.4038/cmj.v57i2.4466. [DOI] [PubMed] [Google Scholar]
- 15.Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013;12:157–65. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solbrig MV, Perng GC. Current neurological observations and complications of dengue virus infection. Curr Neurol Neurosci Rep. 2015;15:29. doi: 10.1007/s11910-015-0550-4. [DOI] [PubMed] [Google Scholar]
- 17.Withana M, Rodrigo C, Chang T, Karunanayake P, Rajapakse S. Dengue fever presenting with acute cerebellitis: A case report. BMC Res Notes. 2014;7:125. doi: 10.1186/1756-0500-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Silva NL, Weeratunga P, Umapathi T, Malavige N, Chang T. Miller Fisher syndrome developing as a parainfectious manifestation of dengue fever: A case report and review of the literature. J Med Case Rep. 2019;13:120. doi: 10.1186/s13256-019-2066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeewandara C, Gomes L, Paranavitane SA, Tantirimudalige M, Panapitiya SS, Jayewardene A, et al. Change in dengue and Japanese encephalitis seroprevalence rates in Sri Lanka. PLoS One. 2015;10:e0144799. doi: 10.1371/journal.pone.0144799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti-N-methyl-D-aspartate receptor encephalitis. J Neurol. 2017;264:1127–31. doi: 10.1007/s00415-017-8501-4. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019;18:1045–57. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 22.Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27:361–8. doi: 10.1097/WCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data generated or analyzed during this study are included in this published article.