Abstract
Dexamethasone acts as an immunosuppressive drug and has been used recently in the management of specific coronavirus disease 2019 (COVID-19) cases; however, various adverse effects could limit its use. In this work, we studied the mitigation effects of black pepper oil (BP oil) on glycemic parameters, dyslipidemia, oxidative and nitrosative stress and pancreatic fibrosis in dexamethasone-treated rats. Animals were divided into five groups that were treated with vehicle, dexamethasone (10 mg/kg, SC) or black pepper oil (BP oil, 0.5 mL, or 1 mL/kg) or metformin (50 mg/kg) plus dexamethasone for 4 consecutive days. Serum insulin, blood glucose, total cholesterol, triglycerides, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) were higher in the dexamethasone group vs the control group and decreased in BP oil and metformin groups relative to the dexamethasone group. Pancreatic nitric oxide, inducible nitric oxide synthase and malondialdehyde levels were increased in the dexamethasone group vs the control group and decreased in BP oil and metformin groups relative to the dexamethasone group. Pancreatic endothelial nitric oxide synthase and reduced glutathione were declined in the dexamethasone group vs the control group. They were increased in BP oil and metformin groups relative to the dexamethasone group. Moreover, the pancreatic islets diameter and collagen deposition were assessed and found to be higher in the dexamethasone group vs the control group. BP oil and metformin groups showed to regress this effect. In conclusion, BP oil may alleviate hyperglycemia, hyperinsulinemia, insulin resistance, dyslipidemia and pancreatic structural derangements and fibrosis by suppressing oxidative stress, increasing endogenous antioxidant levels, modulating nitric oxide signaling, preventing pancreatic stellate cells transition and collagen deposition.
Keywords: Dexamethasone, Nitric oxide, Oxidative stress, iNOS, Pancreas, Collagen
1. Introduction
Glucocorticoids are commonly utilized to treat a variety of disorders and recognized among the highly effective anti-inflammatory and immunosuppressive medications [1]. Dexamethasone belongs to this drug family and has recently been widely approved to treat the hospitalized, critically ill patients with coronavirus disease 2019 (COVID 19) [2]. However, glucocorticoids are linked to variety of adverse effects, such as new onset hyperglycemia in patients without diabetes mellitus (DM) history or hard-to-control hyperglycemia in people with DM. Glucocorticoid-induced diabetes mellitus (GIDM) is a potentially dangerous condition that prevails within clinical practice and practically affects all medical professions. The lack of well-defined management procedures and the broad changes in postprandial hyperglycemia raise new challenges to properly manage GIDM [4], [3]. Dexamethasone induced diabetes may be attributed to either peripheral insulin resistance (IR) or pancreatic damage. Previous studies also showed that dexamethasone caused pancreatic cell apoptosis by increasing reactive oxygen species (ROS) [5]. Acute exposure of pancreatic beta-cells to glucocorticoids can increase insulin production and beta-cell hyperplasia to offset glucocorticoid-induced insulin resistance and maintain physiological plasma glucose levels. However, persistent glucocorticoid exposure can impair insulin production and secretion and cause apoptotic cell death in beta cells [3].
Glucocorticoids, among them dexamethasone, increase the oxidative stress at the cellular level in response to the stress conditions by increasing the mitochondrial membrane potential and mitochondrial oxidation as well [6]. This in turn stimulates the cellular metabolic rate, ATP production, and the sequential generation of several reactive radical and non-radical molecules. Oxidative stress is associated also with nitrosative stress, which is marked by elevated levels of reactive nitrogen species (RNS) including nitric oxide (NO), nitrogen dioxide (NO2), peroxynitrite (ONOO) and dinitrogen trioxide (N2O3) [7]. Several disease processes and states have been linked to oxidative and nitrosative stresses, including ageing, ischemia/reperfusion injury, hypertension, atherosclerosis, diabetic neuropathies, renal disorders, neurological diseases such as Alzheimer's disease and other types of dementia, and malignancies [9], [8], [10]. Several essential oils with antioxidant properties showed promising mitigation effects against the deleterious effects of glucocorticoids induced oxidative stress [12], [11].
The king of spices, black pepper (Piper nigrum L.), belongs to the family Piperaceae and is one of the commonly used spices around the globe. The plant is native to southern India; however, the production of black pepper condiment takes place in many other countries such as Thailand, Sri Lanka, China, where it is included as a crucial ingredient in many culinary delights of Asian countries [13]. P. nigrum is a perennial climbing vine, known to grow well in shady areas on supporting poles or trees and has three putative parents, according to the biosystematics and morphological studies, namely, P. trichostchyon, P. galeatum, and P. wightii. The plant has important applications in the perfumes and preservatives industries as well as many potential uses in folklore medicine. It is used traditionally as stomachic to improve appetite and assist digestion, antidote for worms, soothing agent to relieve hemorrhoids, anti-cough, antipyretic, and for treating epilepsy and dysentery as well [14].
Phytochemical analysis of P. nigrum fruits showed that the alkaloid piperine and the essential oil are the major bioactive components. Piperine along with the essential oil are responsible for the pungency and the aroma of the black pepper. Several therapeutic benefits of black pepper including anticancer, antimicrobial, antioxidant, anti-inflammatory, and analgesic properties are attributed mainly to piperine and/or the essential oil [15], [16], [17]. High amounts of carbohydrates, fats, and proteins, significant amounts of minerals such as calcium, magnesium, phosphorus, and potassium, in addition to a relatively high content of vitamins B1, B2, B3, and C were detected in the fruits. Moreover, myricetin, catechin, and quercetin, as well as the carotenoids (β-carotene and lutein) were identified in significant concentrations [18].
In the current work, we profiled the volatile constituents of black pepper (P. nigrum) fruits essential oil and explored its protective effects against dexamethasone induced pancreatic damage and glucocorticoid induced diabetes. We used metformin as a reference drug, where previous studies in human and animal models showed that metformin inhibits important gluconeogenic enzymes and fatty acid production, resulting in improved glucose tolerance and insulin sensitivity under glucocorticoid treatment and protects pancreas against dexamethasone induced oxidative damage [19], [12].
2. Materials and methods
2.1. Plant material, oil hydro-distillation and GC-MS analysis
Fruits of black pepper were bought from a commercial market at Zagazig city, Sharquia, Egypt. Fruits (4 Kg) were ground into a coarse powder, then Clevenger type apparatus was used to run hydro-distillation for 2 h. The obtained oil was stored, until further analysis, in a dark vial at the freezer. A Shimadzu GC-MS/QP2010 (Kyoto, Japan) coupled with Rtx®-5MS fused bonded column (30 m length, 0.25 mm internal diameter and 0.25 µm film thickness, Restek, USA) was used to assign the volatile components of the oil at the same conditions [12].
2.2. Experimental animals and design
Thirty adult male, Albino Wistar rats, weighing 180–200 g were utilized for the experiments in the current study. The rats were obtained from the Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharquia, Egypt. Rats were housed at 21 ± 2 °C and were subjected to normal light/dark cycle (12/12 h). Food and water were offered as per the standard procedure (ad libitum). Dexamethasone (10 mg/kg body weight, EPICO Co., 10th of Ramadan city, Egypt) was dissolved in normal saline, and subcutaneously injected daily at the same time for 4 consecutive days to induce pancreatic damage [12]. Animals were divided randomly into 5 groups (n = 6). Normal control (NC) group: rats only received the vehicle. Pancreatic damage group (PD): rats received subcutaneously a daily dose of dexamethasone (10 mg/kg) for 4 days. PD + metformin group: rats with induced PD were treated orally with metformin (50 mg/kg, GlaxoSmithKline pharmaceutical CO, Cairo, Egypt) in 10% gum acacia. PD + black pepper oil low dose (BP oil 0.5) group: rats with induced PD were treated orally with black pepper oil (0.5 mL/kg/day) diluted with olive oil. PD + black pepper oil high dose (BP oil 1) group: rats with induced PD were treated orally with black pepper oil (1 mL/kg/day) diluted with olive oil. Oral gavage was used to deliver the vehicle, metformin, and the black pepper oil to the rats once daily (at 8 AM), 1 h before dexamethasone injection for 4 consecutive days. Vehicles volumes were saline 1 mL/kg SC, olive oil and 10% gum acacia 10 mL/kg by oral gavage. The weight of the rats was determined at the beginning and again at the end of the experimental procedure.
2.3. Sample collection
On the fifth day of the study, rats had been fasted for 8 h to assess the fasting blood glucose level that was measured by an automatic blood glucose meter (Bionime, Taiwan) with blood samples taken from the tail tip. Animals were anaesthetized later by intraperitoneal (IP) injection of thiopental (40 mg/kg) to collect the blood (3 mL/rat) from the orbital sinus. The collected blood was left to clot at 2 °C for 20 min then centrifuged at 4000 g for 20 min to separate the serum that was stored at − 80 °C until performing the biochemical analysis. Thereafter, animals were euthanized via cervical dislocation. The pancreas was cautiously dissected and washed in cold saline. One part of the pancreas was fixed in neutral formalin (10%) for 24 h at room temperature for histopathological and immunohistochemical analyses. The second part was frozen in liquid nitrogen and kept at − 80 °C for the oxidative and nitrosative stress markers evaluation.
2.4. Biochemical analysis
A rat insulin kit (My BioSource, CO, San Diego, CA, USA, Catalog number, MBS281388) was used to measure the serum insulin level by ELISA. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was used to calculate the insulin resistance index from the formula: HOMA-IR = blood glucose (mg/dL) × fasting insulin (μU/mL) ÷ 405 [20]. A kit supplied by Spinreact (Spinreact, SA, Girona, Spain) was used to colorimetrically determine the total cholesterol (TC), high density lipoprotein (HDL), and serum triglycerides (TG) levels. A rat reduced glutathione ELISA Kit (Catalog Number: E02G0367, ShangHai BlueGene Biotech CO., LTD, Shanghai, China), a rat malondialdehyde ELISA Kit (Catalog No. LS-F28018, LifeSpan BioScienes, Seattle, USA) and Assay Designs™ Nitric Oxide Assay Kit (Catalog No. 25–0550, Assay Designs, MI, USA) were used to measure the reduced glutathione (GSH), malondialdehyde (MDA) and nitric oxide (NO) levels, respectively in the pancreas tissues according to the manufacturer's instructions.
2.5. Histological study
For routine histological study, after proper fixation of pancreas in 10% formaldehyde, pancreas was dehydrated in an alcohol series of 100%, 90%, 70%, 50%, cleared in xylene, infiltrated and embedded in paraffin and then sectioned (5 µm thick) using a rotary microtome (LEICA RM 2125 UK). They were further deparaffinized, stained with hematoxylin and eosin (H&E) (Bancroft and Layton [21]). For detection of collagen, the tissues were stained with Mallory's trichrome stain. The Slides were examined under a light microscope (Primo star, ZEISS, China). The photos were taken using (Axiocam ERc 5 s, ZEISS, China) camera, at Histology Department, faculty of Medicine for Girls, Al Azhar University.
2.6. Immunohistochemical study
2.6.1. Endothelial and inducible nitric oxide synthases (eNOS & iNOS)
Pancreatic sections were processed and immunostained using peroxidase labeled streptavidin-biotin for eNOS and iNOS using eNOS polyclonal antibodies (dilution 1:100, Abcam, Cat: ab5589, Cambridge, UK) and iNOS polyclonal antibody (diluted 1:100; Abcam, Cat: ab15323, Cambridge, UK) [22]. Positive slide was provided by the manufacture. Negative control sections were prepared with omission of the primary antibody [23].
2.7. Morphometric study
The following parameters were measured using Image J software (National Institute of Health, Bethesda, MD, USA). A picture of known distance in micrometer was used for setting scale and conversion of values from pixels to micrometers. The size of the islet of Langerhans was measured in six H&E-stained sections from six individual rats in each group at a magnification of x20. The findings were presented as the average islet of Langerhans diameter per µm². we also determined the percentage of stained collagen fibers in the islets of Langerhans in six sections from six separate rats per group under x20 magnification. The findings were presented as the average collagen percentage per µm².
Brown cytoplasmic response was the optical density of the positive eNOS immunostaining reaction. The optical density of eNOS immune expression was measured in six sections from six separate rats per group using the color image analysis equipment (a Leica Qwin 500 image analyzer coupled to a Leica microscope) at a magnification of x400 [24].
Brown cytoplasmic reaction indicated a percentage of positive immunostaining for the iNOS immunoreaction. The optical density of iNOS immune expression in six sections from six separate rats from each group were measured using the color image analysis equipment (a Leica Qwin 500 image analyzer coupled to a Leica microscope) at a magnification of x400 [25].
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (San Diego, CA, USA). Difference among the groups was assigned by One Way ANOVA and Tukey post hoc tests. One Way ANOVA test followed by Dunnett's multiple comparisons test were adopted to analyze the histopathological changes. All data were expressed as means ± standard error of mean. P-value ˂ 0.05 was accepted as statistically significant.
3. Results and discussion
3.1. Volatile constituents
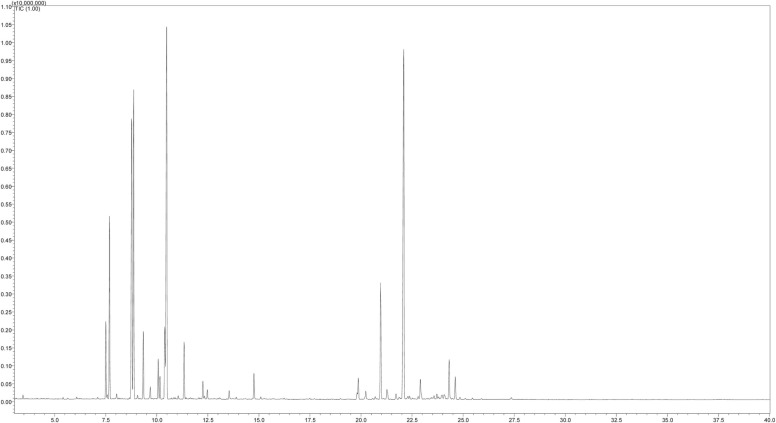
In the current study, the GC-MS analysis of the black pepper oil furnished 26 volatile compounds, Table 1 and Fig. 1. Out of the identified compounds, 7 compounds dominated the oil. They are E-caryophyllene (20.49%), sabinene (13.11%), β-pinene (12.01%), α-pinene (6.51%), α-copaene (5.21%), α-thujene (2.8%), and myrcene (2.43%). The essential oil yield of black pepper varies depending on the plant collection region, plant part used, and the extraction method. The oil collected from the fruits of India, Bangladesh, and Sri Lanka had β-caryophyllene and limonene as major components along with α-pinene in different proportions. Other components included sabinene, β-bisabolene, α-humulene, α-cadinol, and α-thujene from the oil of the Indian fruits, δ-3-carene, β-phellandrene, and 1-napthalenol from the oil of Bengali fruits, and β-terpenine, β-pinene, α β-phellandrene, and myrcene from the oil of Seri Lanka. However, the essential oil of the fruits from Brazil had δ-3-carene as a major component along with α-pinene, sylvestrene, and germacrene D [26], [27], [28].
Table 1.
Volatile constituents of black pepper oil.
| No | Volatile constituents | Calculated RI | Relative abundance (%) |
|---|---|---|---|
| 1 | α-Thujene | 922 | 2.8 |
| 2 | α-Pinene | 929 | 6.51 |
| 3 | Sabinene | 968 | 13.11 |
| 4 | β-Pinene | 972 | 12.01 |
| 5 | Myrcene | 989 | 2.43 |
| 6 | α-Phellandrene | 1001 | 0.52 |
| 7 | α-Terpinene | 1014 | 1.5 |
| 8 | p-Cymene | 1016 | 0.84 |
| 9 | Limonene | 1027 | 22.00 |
| 10 | E-β-Ocimene | 1045 | 0.13 |
| 11 | γ-Terpinene | 1054 | 2.15 |
| 12 | Terpinolene | 1084 | 0.69 |
| 13 | Linalool derivative | 1091 | 0.36 |
| 14 | allo-Ocimene | 1125 | 0.36 |
| 15 | Terpinen-4-ol derivative | 1164 | 1.08 |
| 16 | δ-Elemene | 1340 | 1.24 |
| 17 | α-Cubebene | 1353 | 0.4 |
| 18 | α-Copaene | 1378 | 5.21 |
| 19 | β-Cubebene | 1389 | 0.64 |
| 20 | Z-Caryophyllene | 1405 | 0.3 |
| 21 | E-Caryophyllene | 1420 | 20.49 |
| 22 | α-Humulene | 1452 | 1.05 |
| 23 | β-Selinene | 1483 | 0.25 |
| 24 | α-Muurolene | 1497 | 0.35 |
| 25 | β-Bisabolene | 1506 | 1.8 |
| 26 | δ-Cadinene | 1518 | 1.06 |
Fig. 1.
GC-MS profile of volatile constituents of black pepper oil.
3.2. In vivo results
3.2.1. Black pepper oil attenuated insulin resistance, hyperinsulinemia, and hyperglycemia
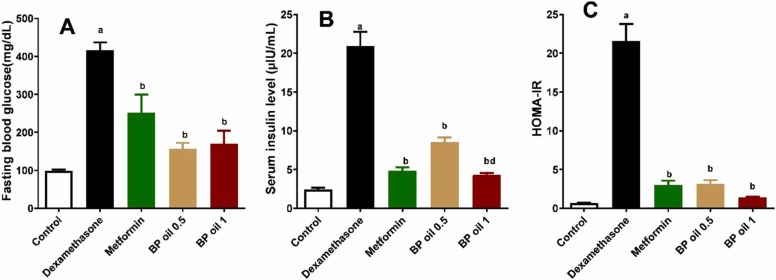
The current study highlighted that animals treated with dexamethasone showed significant (p < 0.05) elevation of their fasting blood glucose, serum insulin levels, and HOMA-IR compared to the control group ( Fig. 2A-C). Co-administration of BP oil in both doses (0.5 and 1 mL/kg) or metformin (50 mg/kg/day) attenuated the effects of dexamethasone, where they significantly (p < 0.05) reduced the levels of the glycemic parameters when compared to the dexamethasone treated animals. The higher dose (1 mL/kg) of BP oil showed better effect on serum insulin level than the lower dose. Interestingly, BP oil, at both dose levels, has equipotent effects to metformin on serum glucose, insulin level and HOMA-IR, respectively (p > 0.05) (Fig. 2). Similar results were observed from Coriander oil [12]. The reduction of insulin resistance caused by BP oil may be responsible for the improvement of hyperglycemia and hyperinsulinemia observed in the current study. A CB2 (cannabinoid type 2 receptor) agonist called β-caryophyllene (BCP) is present in black pepper oil. BCP has been shown to have a beneficial effect on the activity of crucial enzymes that are markedly decreased in diabetes such as hexokinase, pyruvate kinase, and glucose-6-phosphate dehydrogenase (G6PDH), which results in poor glucose uptake in target tissues [29]. We showed previously that BCP mitigated high fructose high fat diet-induced insulin resistance in rats through activation of CB2R [30]. In STZ-induced diabetic mice, BCP administration dramatically lowered blood glucose and enhanced serum insulin levels. It also attenuated hyperglycemia induced oxidative stress in pancreas and other tissues [31]. Another active constituent in black pepper oil is limonene which may be also responsible for improvement of glycemic parameters. Previous studies showed that d-limonene improved insulin resistance in different models. In HFD-LNAME and HFD alone, dietary d-limonene supplementation decreased plasma glucose, insulin, insulin resistance oxidative stress and pancreatic β-cell mass [32], [33]. Linalool also is constituent of BP oil and has diminished hyperglycemia and insulin resistance in animal models of diabetes [34], [35]. Thus the improvement of glycemic parameters observed in the current study is attributed to different beneficial active constituents exist in the essential oil.
Fig. 2.
Effect of the oral administration of black pepper oil (BP 0.5 and 1 mL/kg) on fasting blood glucose (A), serum insulin (B), and HOMA -IR) C) in dexamethasone treated rats. Each column represents the mean ± SEM for six rats. a,b,dp < 0.05 compared with the control, dexamethasone and the low dose of BP oil group, respectively.
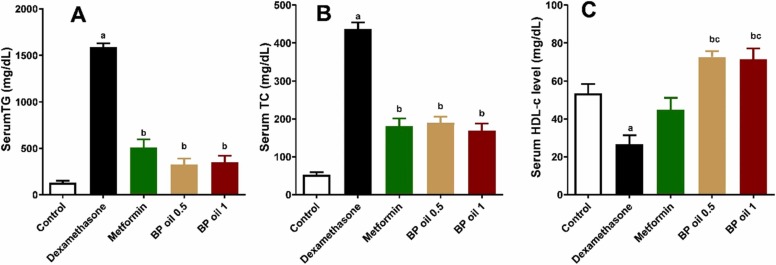
3.2.2. Black pepper oil diminished hypertriglyceridemia, hypercholesterolemia, and increased HDL-c
In the present investigation, dexamethasone administration resulted in significant (p < 0.05) increase in the serum TG and TC levels along with significant decrease (p < 0.05) in the serum HDL-C (Fig. 3A-C). The observed effect of dexamethasone on the lipids profile in this study is attributed to its potential in inducing lipolysis and/or lipogenesis. In adipose tissue, dexamethasone was reported to induce the expression of adipose triglycerides lipase and hormone-sensitive lipase, which are the major lipases responsible for lipolysis in adipocytes [36]. The increase of the free fatty acids enhances the storage of the intracellular lipids in liver and muscles, reduces the glucose uptake, activates the serine kinases, c-Jun N-terminal kinase (JNK) and inhibits the nuclear factor kappa-B kinase (IKK-β), which phosphorylates the serine residues on the insulin receptors, and thus results in a decreased insulin signaling and insulin resistance [37]. However, in liver, administration of glucocorticoids leads to a decrease in lipolysis and an increase in lipogenesis as they inhibit Acyl-CoA dehydrogenase, the key enzyme of β-oxidation, increase the production of VLDL, and induce the key lipogenic enzymes fatty acid synthase and acetyl-CoA-carboxylase [38]. Furthermore, glucocorticoids enhance both the synthesis and secretion of apolipoprotein AI that has a crucial role in lipoprotein receptor recognition and lipoprotein metabolism regulation [39].
Fig. 3.
Effect of the oral administration of black pepper oil (BP 0.5 and 1 mL/kg) on serum triglycerides (TG) (A); serum total cholesterol (TC) (B) and serum high density lipoprotein cholesterol (HDL-C) (C) in dexamethasone treated rats. Each column represents the mean ± SEM for six rats. a,b,cp < 0.05 compared with the control, dexamethasone and metformin groups, respectively.
Co-administration of BP oil at both dose levels (0.5 and 1 mL/kg) or metformin (50 mg/kg/day) restored comparably normal serum TG and TC levels, and even better HDL-C level, relative to the control animals. BP oil’s both doses showed equipotent effect on serum TG and TC (p > 0.05). However, BP oil in both dose levels significantly (p < 0.05) surpassed the effect of metformin on HDL-C ( Fig. 3A-C). Melissa officinalis and coriander essential oils and caryophyllene and linalool (two major compounds identified from the oil) furnished similar activities [40], [12], [31].
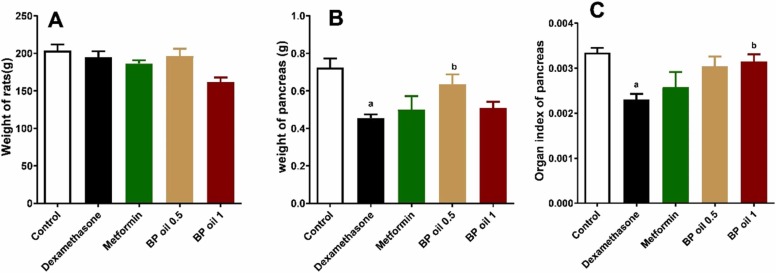
3.2.3. Black pepper oil reversed dexamethasone-induced alterations of pancreas weight
Fig. 4A shows that the body weight of the rats in all treatment groups, including dexamethasone did not significantly change compared to the control group. However, dexamethasone administration revealed significant decrease in the pancreas weight and organ index compared to the control group. Dexamethasone administration decreased both absolute and relative pancreas weight (Fig. 4B and C). This comes in accordance with previous studies, which linked diabetes type 2 to irregular pancreatic border as well as a considerable reduction in pancreatic size [41]. Furthermore, the altered immune responses and oxidative stress state induced by glucocorticoids administration might result in a significant pancreatic atrophy [42]. Although previous studies showed that dexamethasone administration decreased body weight of rats [44], [43], we did not report any changes in body weight of rats in our study. The variation among results may be attributed to the short duration of our study.
Fig. 4.
Effect of the oral administration of Black pepper oil (BP 0.5 and 1 mL/kg) on body weight (A); weight of pancreas (B) and organ index of pancreas (C) in dexamethasone treated rats. Each column represents the mean ± SEM for six rats. a,bp < 0.05 compared with the control and dexamethasone groups, respectively.
Interestingly, co-administration of the BP oil’s lower dose (0.5 mL/kg) significantly (p < 0.05) reversed the effect of dexamethasone on the pancreas weight. On the other hand, only the higher dose of BP oil was able to significantly (p < 0.05) reverse the effect of dexamethasone on the pancreas organ index. Noteworthy, effects revealed by BP oil co-administration in both doses on pancreas weight and organ index were slightly better than those of metformin co-administration (Fig. 4B, C).
3.2.4. Black pepper oil attenuated dexamethasone induced nitrosative stress in pancreas
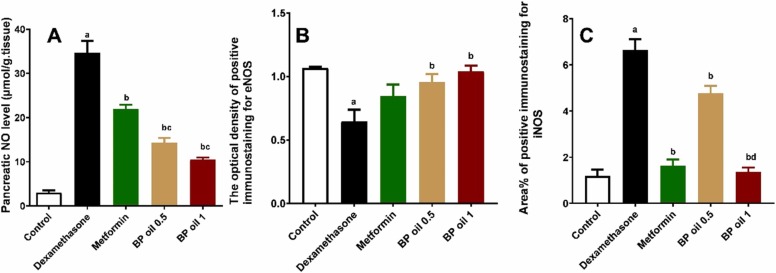
To investigate the mechanism of the protective effects of black pepper oil on dexamethasone induced pancreatic damage, we investigated its effects on NO, eNOS and iNOS. Dexamethasone administration caused a significant (p < 0.05) burst in the pancreatic NO level, presumably by significantly (p < 0.05) reducing eNOS and increasing iNOS levels compared to the control animals ( Fig. 5, Fig. 6, Fig. 7). In a dose dependent manner, co-administration of BP oil resulted in a significant (p < 0.05) reduction in the pancreatic NO and iNOS levels along with significant (p < 0.05) increase in the eNOS level compared to the dexamethasone treated animals. Noteworthy, BP oil in both doses showed slightly better effect than metformin on eNOS level, while only the higher dose (1 mL/kg) showed a comparable effect to metformin on iNOS and a significant (p < 0.05) reduction in iNOS level compared to the lower dose (0.5 mL/kg). However, both dose levels of BP oil showed a significant reduction in pancreatic NO compared to metformin (Fig. 7A–C).
Fig. 5.
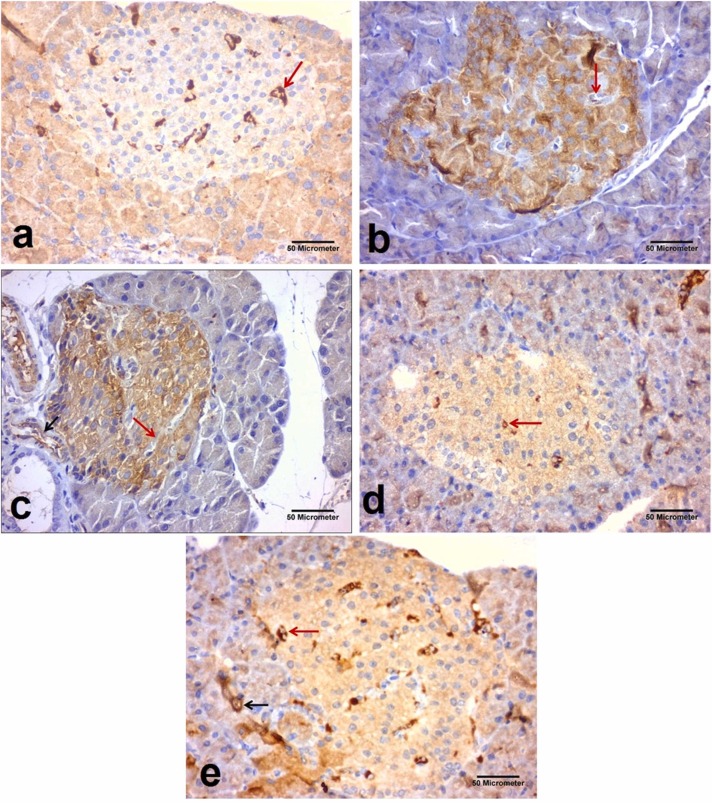
Immunohistochemical staining of eNOS in the pancreatic sections taken from the different experimental groups (x400). eNOS appeared as brown cytoplasmic immunoreactions (Avidine biotin peroxidase stain with Hx counter stain ×400, scale bar = 50 µm); (a): Control group showing strong positive immune-reaction (arrow) for eNOS around the blood vessel of islet of Langerhans (b): Dexamethasone group showing weak positive immune-reaction (arrow) for eNOS around the blood vessel of islet of Langerhans. (c): Metformin group showing strong positive immune reaction for eNOS around the blood vessel inside the islet of Langerhans (red arrow) and also around the blood vessel between pancreatic acini (black arrow). (d): Black pepper oil (0.5 mL/kg) group: showing strong positive immune-reaction (arrows) for eNOS around the blood vessel of islet of Langerhans. (e): Black pepper oil (1 mL/kg) group showing strong positive immune reaction for eNOS around the blood vessel inside the islet of Langerhans (red arrow) and also around the blood vessel between pancreatic acini. (Avidine biotin peroxidase stain with Hx counter stain x400, scale bar=50 µm).
Fig. 6.
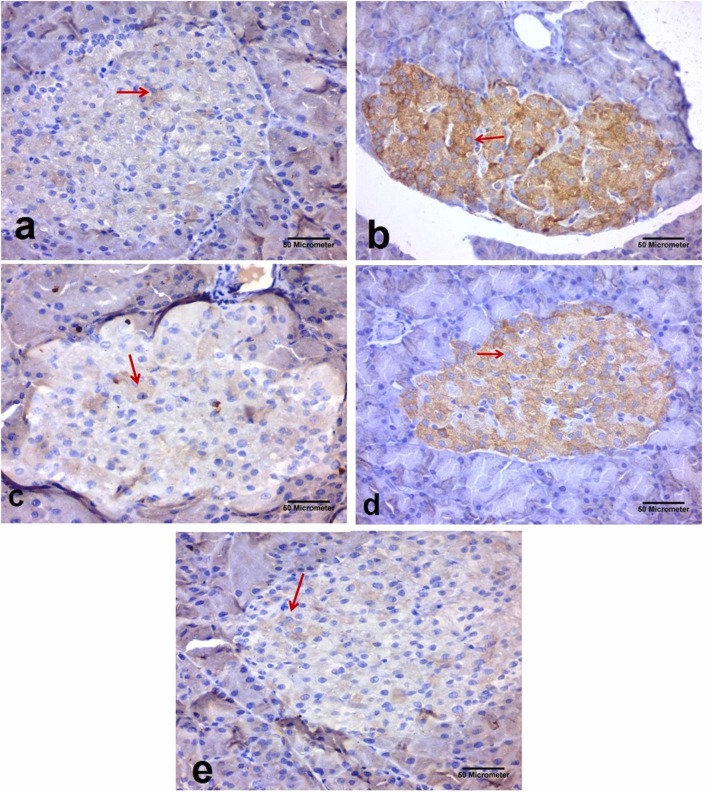
Immunohistochemical staining of iNOS in the pancreatic sections taken from the different experimental groups (x400). iNOS appeared as brown cytoplasmic immunoreactions (Avidine biotin peroxidase stain with Hx counter stain ×400, scale bar = 50 µm); (a): Control group showing scanty immunoexpression of iNOS within the cells of Langerhans islets (arrow) (b): Dexamethasone group showing marked expression of iNOS within most of the cells of Langerhans islets (arrow). (c): Metformin group showing decrease in the expression of iNOS within most of the cells of Langerhans islets (arrow). (d): Black pepper oil (0.5 mL/kg) group: showing decrease in the expression of iNOS within most of the cells of Langerhans islets (arrow). (e): Black pepper oil (1 mL/kg) group showing decrease in the expression of iNOS within most of the cells of Langerhans islets (arrow). (Avidine biotin peroxidase stain with Hx counter stain x400, scale bar=50 µm).
Fig. 7.
Effect of the oral administration of black pepper oil (BP 0.5 and 1 mL/kg) on pancreatic NO level (A); the optical density of positive immunostaining of eNOS (B) and area percentage of positive immunostaining for iNOS (C) in dexamethasone treated rats. Each column represents the mean ± SEM for six rats. a,b,c,dp < 0.05 compared with the control, dexamethasone, metformin and black pepper oil (0.5 mL/kg) groups, respectively.
Endothelial nitric oxide synthase (eNOS) is a constitutive enzyme isoform that normally produces a tonic portion of NO to keep the homeostasis between the endothelium and the surrounding tissues [45]. However, the iNOS is an inducible enzyme that is responsible for the roles of NO in pathological conditions [46]. Typically, iNOS is not expressed in resting cells, but is induced by pro-inflammatory cytokines such as nuclear factor kappa B (NF-κB), tumor necrosis factor alpha, (TNF-α), interleukin 1 alpha (IL-1α), and interleukin 6 (IL-6) [47]. It is reported that the iNOS-induced S-nitrosation of protein kinase B (Akt), insulin receptor,(IR), insulin receptor substrate 1 (IRS-1) in liver and muscle tissues is significantly associated with insulin resistance, especially in obese and aging patients [48]. Furthermore, the excessive generation of NO by iNOS induces nitrosative stress. NO immediately quenches superoxide anions, resulting in the production of highly reactive peroxynitrite (OONO). This causes lipid peroxidation to begin and oxidizes the sulfhydryl group in proteins, and amino acids like cysteine, causing a deleterious effect on several signaling pathways [49].
3.2.5. Black pepper oil mitigated dexamethasone induced oxidative stress in pancreas
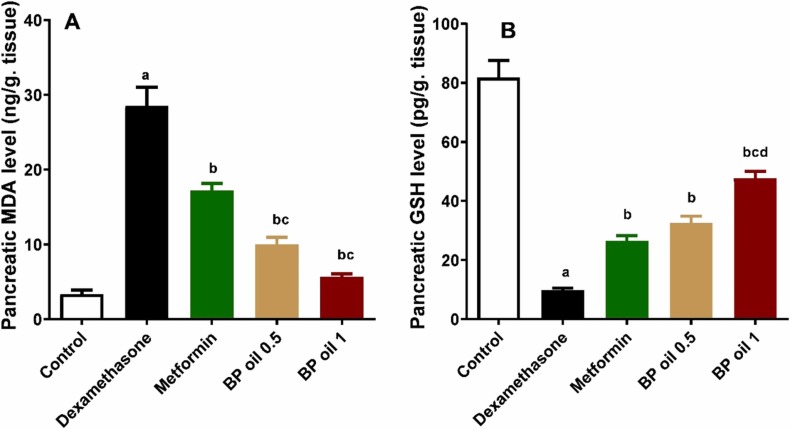
To verify the antioxidant effects of black pepper oil and its contribution to protect against dexamethasone induced pancreatic injury, we investigated the oxidative stress markers levels in pancreas. Dexamethasone administration led to a significant (p < 0.05) increase in MDA and reduction in GSH levels in the pancreatic tissues of the treated animals compared to the control group ( Fig. 8A-B). Co-administration of BP oil showed a significant (p < 0.05) reduction in pancreatic MDA and increase in pancreatic GSH levels in a dose dependent manner compared to the dexamethasone group. Interestingly, both dose levels of BP oil significantly decreased the level of the pancreatic MDA, while only the higher dose of BP oil (1 mL/kg) showed a significant increase in the pancreatic GSH level compared to metformin. The antioxidant effects of black pepper oil may be attributed to β-caryophyllene and limonene and other volatile compounds [51], [50], [31].
Fig. 8.
Effect of the oral administration of Black pepper oil (BP 0.5 and 1 mL/kg) on pancreatic malondialdehyde (MDA) level (A); Pancreatic reduced glutathione (GSH) level (B) in dexamethasone treated rats. Each column represents the mean ± SEM for six rats. a,b,c,dp < 0.05 compared with the control, dexamethasone, metformin and black pepper oil (0.5 mL/kg) groups, respectively.
Oxidative stress, induced mainly by ROS, is among the causes of the dysfunction of beta cells that are characterized by low antioxidant capacity [52]. Beta cells dysfunction is mainly attributed to the enhanced apoptotic events, resulting from the interference of ROS with the mitochondria, and the reduced secretory potential of the beta cells due to the alterations of K (ATP) channels by these reactive species [53]. Moreover, previous studies elaborated the alterations of GLUT4 expression and insulin receptor signaling transduction induced by oxidative stress that ends up in insulin resistance. ROS activate NF-kB, JNK, and p38 MAPK resulting in mitochondrial fission that is mainly associated with insulin resistance in skeletal muscles. Furthermore, ROS have also a direct stimulatory effect on Casein kinase II (CK2) that enhances the lysosomal degradation of GLUT4 [54], [55]. Dexamethasone is reported to induce oxidative stress in skeletal muscles directly through binding to the glucocorticoid receptors and in pancreas, via deactivating PI3K/Akt/Nrf2 signaling pathways, and by inducing glucotoxicity of pancreatic islets [56], [12]. Furthermore, a recent study showed that dexamethasone causes GSH depletion and induces ferroptosis in HT1080 cells in a glucocorticoid receptor dependent manner [2]. This may explain the effects of dexamethasone on the oxidative stress markers observed in the current study.
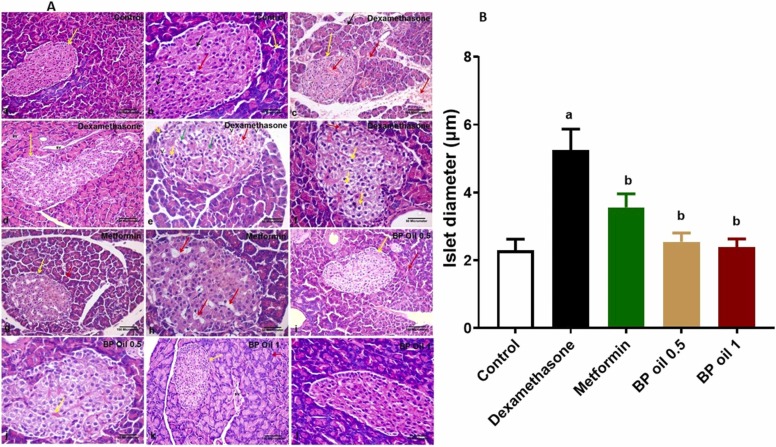
3.2.6. Black pepper oil mitigated dexamethasone induced pancreatic structural alterations
In the current study, we examined the pancreatic sections stained with H&E of different experimental groups and measured the islet’s diameter. Results observed in Fig. 9 revealed that dexamethasone induced multiple vacuolations, hemorrhage and congested blood vessels and increased the diameter of Langerhans islet. The observed effect of dexamethasone was in accordance of our previous findings [12]. The increased islet diameter and β-cell mass may be attributed to the β-cell hyperplasia and, to a lower extent, the β-cell hypertrophy observed in rats receiving high doses of dexamethasone for 5 days mediated by activation of IRS2 pathway [57]. These observed changes may be due to the insulin's diminished peripheral activity that causes compensatory changes in the endocrine pancreas [59], [58]. Black pepper oil at both dose levels and metformin reversed the effects of dexamethasone on the islet’s diameter and reduced the structural derangements induced by dexamethasone in both exocrine and endocrine parts of pancreas. Noteworthy, the high dose of the oil showed similar histological picture to the control group. The beneficial effects of black pepper oil may be associated with its antioxidant potential, modulating nitric oxide signaling and improving various glycemic parameters and decreased insulin resistance [60].
Fig. 9.
(A) Representative photomicrographs of H&E-stained pancreatic sections from the different experimental groups. Control group showing (a) Lightly stained islet of Langerhans (yellow arrow) surrounded by deeply stained pancreatic acini (white arrow). (b) One pale stained islet of Langerhans rich in blood capillaries which are recognized by their flat basophilic endothelial nuclei (red arrow). Endocrine cells have acidophilic cytoplasm and prominent nuclei at the center (black arrow). The pancreatic acini are lined by irregular triangular cells with basely located rounded nuclei (yellow arrow). Notice the apical acidophilic granules and the basal basophilia within the acinar cells. Dexamethasone group showing (c) islet of Langerhans (yellow arrow) with multiple areas of hemorrhage between the endocrine cells and in the connective tissue trabeculae (red arrows). (d) marked increase in the size of islet of Langerhans (yellow arrow) surrounded by pancreatic acini. Some blood vessels distributed throughout the field (Bv). (e) islet of Langerhans with areas of hemorrhage between the endocrine cells (red arrow), some cells appear with vacuolated cytoplasm (yellow arrow), others appear with small dark pyknotic nucleus (green arrows). Multiple vacuolations (V) can be also seen within the islet. (f) islet of Langerhans. Most of islet cells appear with vacuolated cytoplasm (yellow arrow). Congested blood vessel can be also noticed (red arrow). Metformin group showing (g) lightly stained islet of Langerhans (yellow arrow) surrounded by deeply stained pancreatic acini (red arrow), as that of the control group (h) islet of Langerhans restored its normal shape. Although, some vacuolations are still present between the endocrine cells (red arrow). Black pepper oil (BP 0.5, 0.5 mL/kg) group showing (i&j) the histological structure of the pancreas appears as that of the control group; congested blood vessel is still present (yellow arrow). Black pepper oil (BP 1, 1 mL/kg) group showing (k&l): the histological structure of the pancreas appears as that of the control group. [H&E; (a,c, d, g, i, k) x200. scale bar = 100 µm & (b, e, f, h, j, l) x400. scale bar= 50 µm]. (B) bar graph for the islet’s diameter in different treated groups. Each column represents the mean ± SEM for six rats. a,bp < 0.05 compared with the control and dexamethasone groups, respectively.
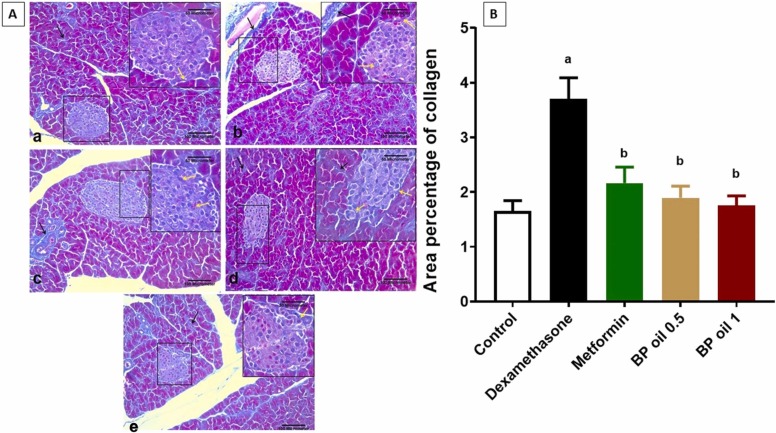
3.2.7. Black pepper oil mitigated dexamethasone induced pancreatic fibrosis
To verify the antifibrotic effects of black pepper oil on pancreas, we investigated its effects on collagen fiber deposition in pancreas. Collagen staining by Mallory’s Trichrome showed increased deposition of collagen around and between the cells of Langerhans islets in the pancreas of dexamethasone treated rats. This effect was mitigated by both metformin and black pepper oil pretreatment ( Fig. 10). Collagen deposition in dexamethasone treated rats may be due to the transition of pancreatic stellate cells (PSCs) to the myofibroblast-like phenotype. These cells produce fibrillary proteins such as collagen [61]. PSCs exposed to hyperglycemia and local hyperinsulinemia become more prone to self-activation [62]. Oxidative stress also plays an important role in the activation of PSCs and the subsequent pancreatic fibrosis. Alleviation of oxidative stress and reduction of hyperglycemia attenuate the activation of PSCs in the islet, thus reducing islet fibrosis [63]. The reduction of the collagen deposition and pancreatic fibrosis caused by the black pepper oil in this work may be attributed to its attenuation on hyperglycemia and oxidative stress.
Fig. 10.
Representative photomicrographs of Mallory’s Trichrome-stained pancreatic sections from the different experimental groups. (a) Control group showing delicate collagen fibers around Langerhans islet (yellow arrow) and between the pancreatic acini (black arrow). (b) Dexamethasone group showing an increased deposition of collagen fibers around and between the cells Langerhans islet (yellow arrow). Marked deposition of collagen around adjacent blood vessel can be also noticed (black arrow). (c) Metformin group showing moderate deposition of collagen fibers around Langerhans islet (yellow arrow) and between the pancreatic acini (black arrow). (d) Black pepper oil (0.5 mL/kg) group: showing delicate collagen fibers around Langerhans islet (yellow arrow). (e) Black pepper oil (1 mL/kg) group showing delicate collagen fibers around Langerhans islet (yellow arrow) and around an adjacent blood vessel (black arrow) as the control group. Mallory's Trichrome x200, scale bar= 100 µm. With inset x400, scale bar= 50 µm. (B) Bar graph for area percentage of collagen fiber deposition in the different treated groups. Each column represents the mean ± SEM for six rats. a,bp < 0.05 compared with the control and dexamethasone groups, respectively.
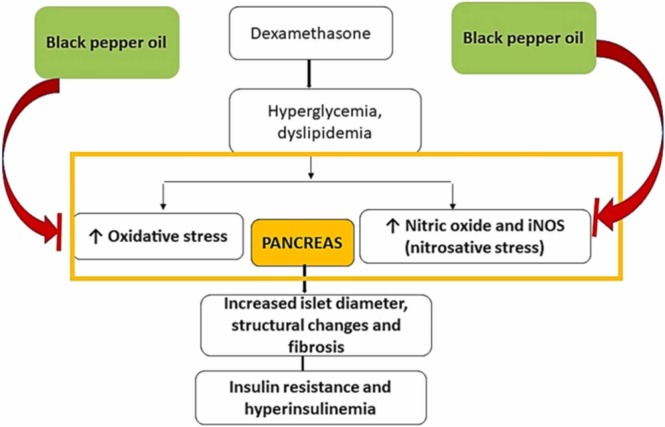
Altogether, black pepper oil exerted its protective effects via suppression of pancreatic oxidative and nitrosative stress and reduction of insulin resistance and its associated metabolic effects. This might be attributed to its diverse volatile compounds, among them E-caryophyllene, sabinene, α- and β-pinenes and, α-copaene, Fig. 11. Noteworthy, similar protective effects were observed from other essential oils such as coriander and Thymus serrulatus [64], [12] . Limitation between this study and the context of BP consumption by humans is that humans consume the whole fruit. In our study, we used the essential oil of black pepper oil, so further experimental and clinical studies should be performed before using the fruits essential oil in clinical settings.
Fig. 11.
Possible mechanisms of the black pepper oil protective effects against dexamethasone induced pancreatic damage. iNOS, inducible nitric oxide synthase.
4. Conclusions
To the best of our knowledge, this is the first study that shows the protective effect of black pepper oil against dexamethasone induced pancreatic damage. We showed that BP oil may alleviate hyperglycemia, hyperinsulinemia, insulin resistance, dyslipidemia and pancreatic structural derangements and fibrosis by suppressing oxidative stress, increasing endogenous antioxidant levels, modulating nitric oxide signaling, preventing pancreatic stellate cells transition and collagen deposition. This indicates a promising potential of black pepper oil in preventing the dexamethasone induced adverse effects and warrants further investigation of other possible mechanisms.
Ethical statement
All protocols of the experiment have been approved by Zagazig University Institutional Animal Care and Use Committee (ZU-IACUC, approval number: ZU-IACUC/3/F/73 /2020).
Author contributions
M.F.M. performed the biological experiments, wrote the manuscript and designed and conceived the study, A.M.E. and N.A. performed biological activities, I.M. and A.M.E. performed extraction and chemical composition. M.A.O.A. wrote the manuscript. M.S. revised the manuscript and designed and conceived the study.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Quatrini L., Ugolini S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell. Mol. Immunol. 2021;18:269–278. doi: 10.1038/s41423-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Mässenhausen A., Zamora Gonzalez N., Maremonti F., Belavgeni A., Tonnus W., Meyer C., Beer K., Hannani M.T., Lau A., Peitzsch M. Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor–induced dipeptidase-1 expression and glutathione depletion. Sci. Adv. 2022;8:eabl8920. doi: 10.1126/sciadv.abl8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J.-X., Cummins C.L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 2022:1–18. doi: 10.1038/s41574-022-00683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh S., Park M.K. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol. Metab. 2017;32:180–189. doi: 10.3803/EnM.2017.32.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo B., Zhang W., Xu S., Lou J., Wang S., Men X. GSK-3β mediates dexamethasone-induced pancreatic β cell apoptosis. Life Sci. 2016;144:1–7. doi: 10.1016/j.lfs.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjelaković G., Beninati S., Pavlović D., Kocić G., Jevtović T., Kamenov Β, Šaranac L., Bjelaković B., Stojanović I., Bašić J. Glucocorticoids and oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2007;18(2):115–128. doi: 10.1515/jbcpp.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- 7.Cikman O., Soylemez O., Ozkan O.F., Kiraz H.A., Sayar I., Ademoglu S., Taysi S., Karaayvaz M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–induced acute pancreatitis: an experimental study on rats. Int. Surg. 2015;100:891–896. doi: 10.9738/INTSURG-D-14-00170.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield D.A., Reed T.T., Perluigi M., De Marco C., Coccia R., Keller J.N., Markesbery W.R., Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraci F.M. Oxidative stress: the curse that underlies cerebral vascular dysfunction? Stroke. 2005;36:186–188. doi: 10.1161/01.STR.0000153067.27288.8b. [DOI] [PubMed] [Google Scholar]
- 10.Forbes J.M., Coughlan M.T., Cooper M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V.S., Inamdar M.N., Viswanatha G.L. Protective effect of lemongrass oil against dexamethasone induced hyperlipidemia in rats: possible role of decreased lecithin cholesterol acetyl transferase activity. Asian Pac. J. Trop. Med. 2011;4:658–660. doi: 10.1016/S1995-7645(11)60167-3. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud M.F., Ali N., Mostafa I., Hasan R.A., Sobeh M. Coriander oil reverses dexamethasone-induced insulin resistance in rats. Antioxidants. 2022;11:441. doi: 10.3390/antiox11030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K.P. Nair, Agronomy and Economy of Black Pepper and Cardamom: The" King” and" Queen” of Spices, 2011.
- 14.Damanhouri Z.A., Ahmad A. A review on therapeutic potential of Piper nigrum L. black pepper): the king of spices. Med. Aromat. Plants. 2014;3:161. [Google Scholar]
- 15.Dorman H.-D., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 16.Jeena K., Liju V.B., Umadevi N.P., Kuttan R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn) J. Essent. Oil Bear. Plants. 2014;17:1–12. [Google Scholar]
- 17.Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 18.Nwofia G.E., Kelechukwu C., Nwofia B.K. Nutritional composition of some Piper nigrum (L.) accessions from Nigeria. Int. J. Med. Aromat. Plants. 2013;3:2249–4340. [Google Scholar]
- 19.Landis D., Sutter A., Fernandez F., Nugent K. The effect of metformin on glucose metabolism in patients receiving glucocorticoids. Am. J. Med. Sci. 2022 doi: 10.1016/j.amjms.2022.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft J.D., Layton C. 8th. Elsevier Health Science; 2018. Bancroft’s theory and practice histological technique; pp. 153–176. [Google Scholar]
- 22.J.D. Bancroft, M. Gamble, Theory and Practice of Histological Techniques. Elsevier Health Sciences, 2008.
- 23.Ali A., Mansour M., Ali S., Noya D. Pancreatic histological changes in adult female albino rats treated with Orlistat and the possible protective role of B- carotene. Egypt. J. Histol. 2020 doi: 10.21608/ejh.2020.40848.1347. [DOI] [Google Scholar]
- 24.Sorour H., Selim M., Almoselhy L., Gouda S. Ameliorative effect of watermelon rind ingestion on the pancreas of diabetic female albino rat (histological, immunohistochemical and morphometric study) Egypt. J. Histol. 2019;42:10–22. [Google Scholar]
- 25.Buchwalow I.B., Böcker W. Springer Science & Business Media; 2010. Immunohistochemistry: Basics and Methods. [Google Scholar]
- 26.Dosoky N.S., Satyal P., Barata L.M., da Silva J.K.R., Setzer W.N. Volatiles of black pepper fruits (Piper nigrum L.) Molecules. 2019;24:4244. doi: 10.3390/molecules24234244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon A.N., Padmakumari K.P. Studies on essential oil composition of cultivars of black pepper (Piper nigrum L.)—V. J. Essent. Oil Res. 2005;17:153–155. [Google Scholar]
- 28.Menon A.N., Padmakumari K.P., Jayalekshmy A. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.) III. J. Essent. Oil Res. 2003;15:155–157. [Google Scholar]
- 29.Basha R.H., Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. Acta Histochem. 2014;116(8):1469–1479. doi: 10.1016/j.acthis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Youssef D.A., El-Fayoumi H.M., Mahmoud M.F. Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: the role of CB2 and PPAR-γ receptors. Biomed. Pharmacother. 2019;110:145–154. doi: 10.1016/j.biopha.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Basha R.H., Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016;245:50–58. doi: 10.1016/j.cbi.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Jing L., Zhang Y., Fan S., Gu M., Guan Y., Lu X., Huang C., Zhou Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013;715(1–3):46–55. doi: 10.1016/j.ejphar.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Santiago J.V.A., Jayachitra J., Shenbagam M., Nalini N. Dietary d-limonene alleviates insulin resistance and oxidative stress-induced liver injury in high-fat diet and L-NAME-treated rats. Eur. J. Nutr. 2012;51(1):57. doi: 10.1007/s00394-011-0182-7. [DOI] [PubMed] [Google Scholar]
- 34.Mahdavifard S., Nakhjavani M. Effect of linalool on the activity of glyoxalase-I and diverse glycation products in rats with type 2 diabetes. J. Mazandaran Univ. Med. Sci. 2020;30(186):24–33. [Google Scholar]
- 35.More T.A., Kulkarni B.R., Nalawade M.L., Arvindekar A.U. Antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: a combinatorial therapy approach. Int. J. Pharm. Pharm. Sci. 2014;6(8):159–163. [Google Scholar]
- 36.Xu C., He J., Jiang H., Zu L., Zhai W., Pu S., Xu G. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 2009;23:1161–1170. doi: 10.1210/me.2008-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H., Moschen A.R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Lemke U., Krones-Herzig A., Diaz M.B., Narvekar P., Ziegler A., Vegiopoulos A., Cato A.C., Bohl S., Klingmüller U., Screaton R.A. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab. 2008;8:212–223. doi: 10.1016/j.cmet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Vegiopoulos A., Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Jun H., Lee J.H., Jia Y., Hoang M.-H., Byun H., Kim K.H., Lee S.-J. Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c–dependent fatty acid synthesis. J. Nutr. 2012;142:432–440. doi: 10.3945/jn.111.152538. [DOI] [PubMed] [Google Scholar]
- 41.Macauley M., Percival K., Thelwall P.E., Hollingsworth K.G., Taylor R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagihashi S. Diabetes and pancreas size, does it matter? J. Diabetes Investig. 2017 doi: 10.1111/jdi.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia H., Yamashita T., Li X., Kato H. Laurel attenuates dexamethasone-induced skeletal muscle atrophy in vitro and in a rat model. Nutrients. 2022;14:2029. doi: 10.3390/nu14102029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamani S.P., Waguia J.K., Miaffo D., Nchouwet M., Kadji C.D., Kamgaing M.W., Djimeli R.D., Ngnitedem J.M., Kamanyi A., Ngnokam S.W. Efficacy of Emilia coccinea aqueous extract on inhibition of α-amylase enzyme activity and insulin resistance in dexamethasone treated-rats. Metab. Open. 2022 doi: 10.1016/j.metop.2022.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okajima Y., Matsuzaka T., Miyazaki S., Motomura K., Ohno H., Sharma R., Shimura T., Istiqamah N., Han S., Mizunoe Y. Morphological and functional adaptation of pancreatic islet blood vessels to insulin resistance is impaired in diabetic db/db mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022;1868 doi: 10.1016/j.bbadis.2022.166339. [DOI] [PubMed] [Google Scholar]
- 46.Lind M., Hayes A., Caprnda M., Petrovic D., Rodrigo L., Kruzliak P., Zulli A. Inducible nitric oxide synthase: good or bad? Biomed. Pharmacother. 2017;93:370–375. doi: 10.1016/j.biopha.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 47.Stuehr D.J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta (BBA) Bioenerg. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho A.C.S., Guedes M.M., de Souza A.L., Trevisan M.T., Lima A.F., Santos F.A., Rao V.S. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Med. 2007;73:1372–1376. doi: 10.1055/s-2007-990231. [DOI] [PubMed] [Google Scholar]
- 49.Singh A., Kukreti R., Saso L., Kukreti S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules. 2022;27:950. doi: 10.3390/molecules27030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldissera M.D., Souza C.F., Grando T.H., Doleski P.H., Boligon A.A., Stefani L.M., Monteiro S.G. Hypolipidemic effect of β-caryophyllene to treat hyperlipidemic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017;390:215–223. doi: 10.1007/s00210-016-1326-3. [DOI] [PubMed] [Google Scholar]
- 51.Calleja M.A., Vieites J.M., Montero-Meterdez T., Torres M.I., Faus M.J., Gil A., Suárez A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013;109:394–401. doi: 10.1017/S0007114512001298. [DOI] [PubMed] [Google Scholar]
- 52.Bhatti J.S., Sehrawat A., Mishra J., Sidhu I.S., Navik U., Khullar N., Kumar S., Bhatti G.K., Reddy P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022 doi: 10.1016/j.freeradbiomed.2022.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Drews G., Krippeit-Drews P., Düfer M. Oxidative stress and beta-cell dysfunction. Pflüg. Arch. Eur. J. Physiol. 2010;460:703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 54.Hurrle S., Hsu W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017;40:257–262. doi: 10.1016/j.bj.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma J., Nakagawa Y., Kojima I., Shibata H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J. Biol. Chem. 2014;289:133–142. doi: 10.1074/jbc.M113.533240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elseady W.S., Abd Ellatif R.A., Estfanous R.S., Emam M.N., Keshk W.A. New insight on the role of liraglutide in alleviating dexamethasone-induced pancreatic cytotoxicity via improving redox status, autophagy flux, and PI3K/Akt/Nrf2 signaling. Can. J. Physiol. Pharmacol. 2021;99:1217–1225. doi: 10.1139/cjpp-2021-0183. [DOI] [PubMed] [Google Scholar]
- 57.Rafacho A., Cestari T.M., Taboga S.R., Boschero A.C., Bosqueiro J.R. High doses of dexamethasone induce increased β-cell proliferation in pancreatic rat islets. Am. J. Physiol. Endocrinol. Metab. 2009;296:E681–E689. doi: 10.1152/ajpendo.90931.2008. [DOI] [PubMed] [Google Scholar]
- 58.Barth R., Ruoso C., Ferreira S.M., de Ramos F.C., Lima F.B., Boschero A.C., Dos Santos G.J. Hepatocyte nuclear factor 4-α (HNF4α) controls the insulin resistance-induced pancreatic β-cell mass expansion. Life Sci. 2022;289 doi: 10.1016/j.lfs.2021.120213. [DOI] [PubMed] [Google Scholar]
- 59.Weir G.C., Laybutt D.R., Kaneto H., Bonner-Weir S., Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50:S154. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- 60.Hashiesh H.M., Meeran M.F., Sharma C., Sadek B., Kaabi J.A., Ojha S.K. Therapeutic potential of β-caryophyllene: a dietary cannabinoid in diabetes and associated complications. Nutrients. 2020;12:2963. doi: 10.3390/nu12102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stassen O.M., Ristori T., Sahlgren C.M. Notch in mechanotransduction–from molecular mechanosensitivity to tissue mechanostasis. J. Cell Sci. 2020;133 doi: 10.1242/jcs.250738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong O.-K., Lee S.-H., Rhee M., Ko S.-H., Cho J.-H., Choi Y.-H., Song K.-H., Son H.-Y., Yoon K.-H. Hyperglycemia and hyperinsulinemia have additive effects on activation and proliferation of pancreatic stellate cells: possible explanation of islet‐specific fibrosis in type 2 diabetes mellitus. J. Cell. Biochem. 2007;101:665–675. doi: 10.1002/jcb.21222. [DOI] [PubMed] [Google Scholar]
- 63.Kim J.-W., Park S.-Y., You Y.-H., Ham D.-S., Lee S.-H., Yang H.K., Jeong I.-K., Ko S.-H., Yoon K.-H. Suppression of ROS production by exendin-4 in PSC attenuates the high glucose-induced islet fibrosis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haile T., Cardoso S.M., de Oliveira Raphaelli C., Pereira O.R., Pereira EdosS V.M., Nora L., Asfaw A.A., Periasamy G., Karim A. Chemical composition, antioxidant potential, and blood glucose lowering effect of aqueous extract and essential oil of thymus Serrulatus Hochst. Ex Benth. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.621536. [DOI] [PMC free article] [PubMed] [Google Scholar]