Abstract
We present the case of acute myocardial infarction secondary to spontaneous coronary artery dissection in a patient 2 weeks post orthotopic heart transplantation. (Level of Difficulty: Advanced.)
Key Words: acute coronary syndrome, cardiac transplant, dissection, myocardial infarction, systolic heart failure
Abbreviations and Acronyms: FMD, fibromuscular dysplasia; ICH, intracranial hemorrhage; LGE, late gadolinium enhancement; LV, left ventricular; SCAD, spontaneous coronary artery dissection; TTE, transthoracic echocardiogram
Central Illustration
History of Presentation
A 50-year-old woman underwent orthotopic heart transplantation for severe nonischemic dilated cardiomyopathy.
Learning Objectives
-
•
To identify differential diagnoses for early graft dysfunction post orthotopic heart transplantation.
-
•
To revise the diagnostic workup for early graft dysfunction post orthotopic heart transplantation.
The operation was uncomplicated with standard bicaval implantation of the donor heart. The ischemic time was 128 minutes. Induction immunosuppression included tacrolimus 3 mg, mycophenolate 1.5 g, methylprednisolone 1 g, and basiliximab 20 mg on days 1 and 4. She was extubated on day 2. Postoperative acute kidney injury necessitated tacrolimus sparing and anti-thymocyte globulin on days 2 to 4. Early brady-arrhythmias and right ventricular dysfunction improved with initial isoprenaline, milrinone, and inhaled nitric oxide, which were weaned and ceased before discharge from the intensive care unit on day 8 posttransplant. Early transthoracic echocardiograms (TTE) demonstrated low-normal left ventricular (LV) systolic function. Routine day 13 predischarge TTE demonstrated new segmental LV systolic dysfunction. There was no history of dyspnea, hypotension, or chest pain.
Past Medical History
The patient was diagnosed with severe dilated cardiomyopathy after the birth of her first child, 11 years before transplantation. An endomyocardial biopsy was unremarkable. Despite optimal medical therapy and cardiac resynchronization therapy, she deteriorated with recurrent hospital admissions for acute decompensated biventricular failure and proceeded to heart transplantation.
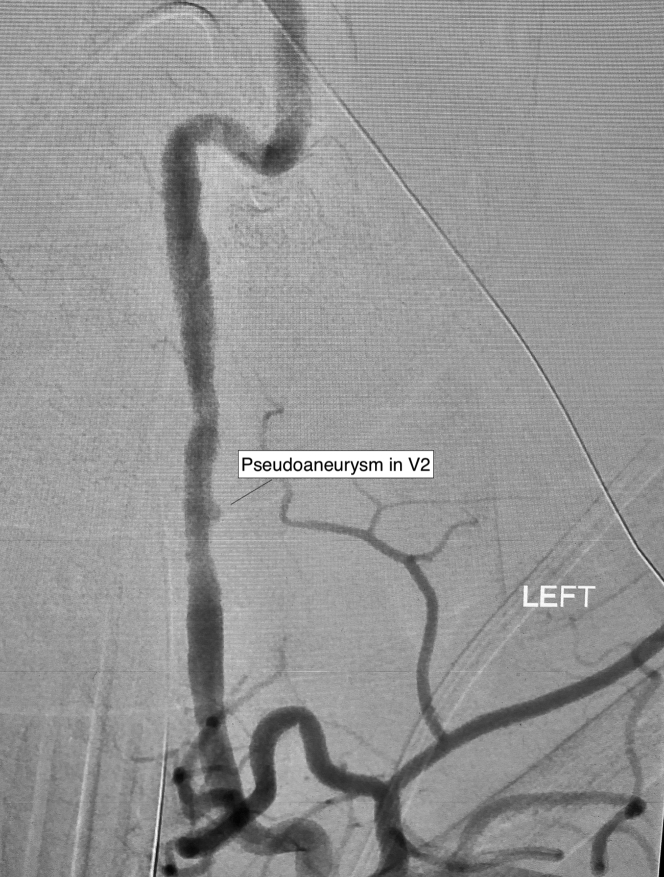
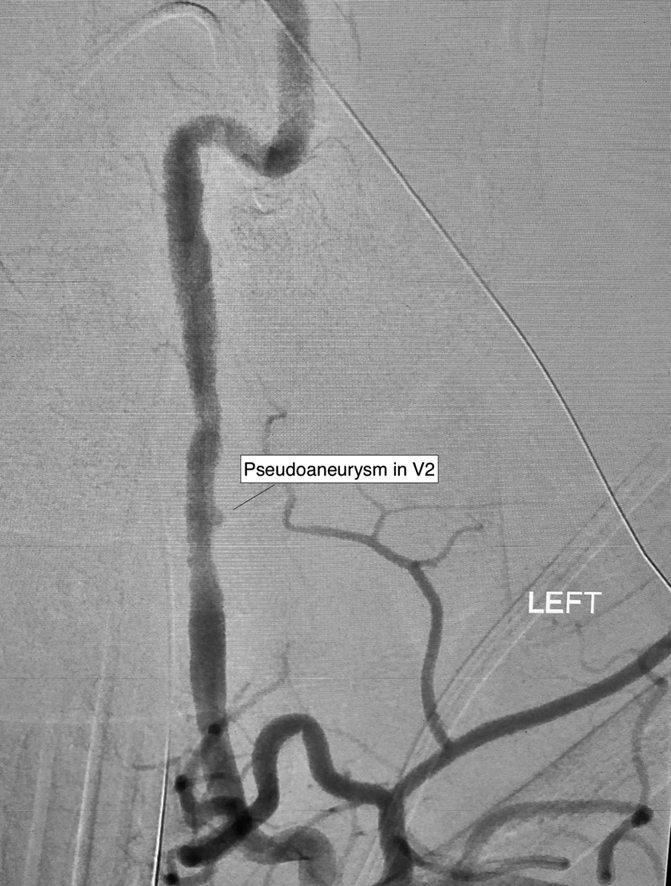
The donor was a woman in her late forties who presented with a grade 4 subarachnoid hemorrhage secondary to a ruptured left middle cerebral artery aneurysm. Initial imaging revealed irregularities in the vertebral arteries and a small pseudoaneurysm in the V2 segment of the left vertebral artery, suspicious for fibromuscular dysplasia (FMD) (Figure 1). Despite repeat interventions, which included clipping, stenting, and coiling, the aneurysm was ultimately unstable, resulting in a catastrophic intracranial hemorrhage and brain death. She was assessed for organ donation. TTE was normal. Because of a history of smoking, coronary angiography was performed, which demonstrated angiographically normal coronary arteries (Video 1).
Figure 1.
Donor Digital Subtraction Angiography of the Left Vertebral Artery
Donor digital subtraction angiography demonstrating irregularities in the vertebral arteries and a small pseudoaneurysm in V2 of the left vertebral artery.
Differential Diagnosis
The leading differential diagnosis for early LV dysfunction post cardiac transplantation is acute cellular or humoral rejection. Other rarer causes include acute myocardial infarction related to embolus, vasospasm, or atherosclerotic plaque rupture from undiagnosed donor coronary artery disease, Takotsubo cardiomyopathy, and acute cytomegalovirus infection.
Investigations
A routine TTE performed on day 13 post-transplant demonstrated a new finding of mild segmental LV systolic dysfunction with antero-apical akinesis (Video 2). The second endomyocardial biopsy was brought forward, which demonstrated no evidence of rejection but instead showed a region of myocyte dropout with an infiltrate of histiocytes, consistent with prior myocardial injury.
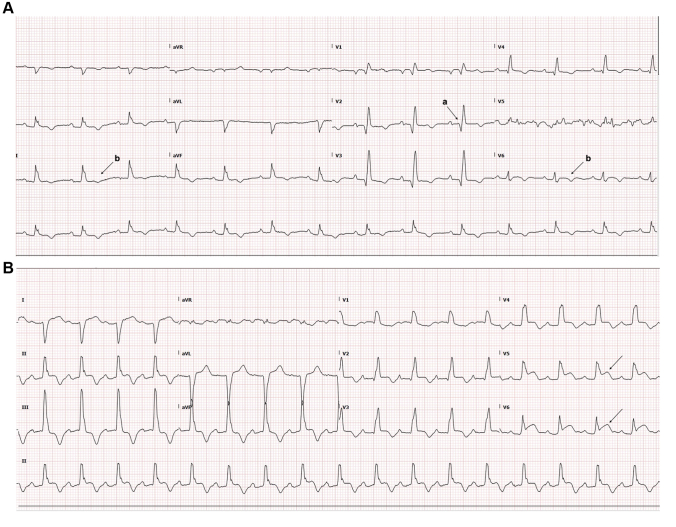
The electrocardiogram demonstrated anterior Q waves and diffuse T-wave inversion (Figure 2A). More significant changes were evident on day 8, with ST-segment elevation in leads V5 and V6, which were noted at the time but not felt to be significant because of an absence of symptoms and overall clinical stability (Figure 2B).
Figure 2.
Electrocardiograms
(A) Electrocardiogram day 13 post-transplant demonstrating anterior Q waves (a) and diffuse T-wave inversion (b). (B) Electrocardiogram day 8 post-transplant showing ST-segment elevation in V5 and V6(arrows).
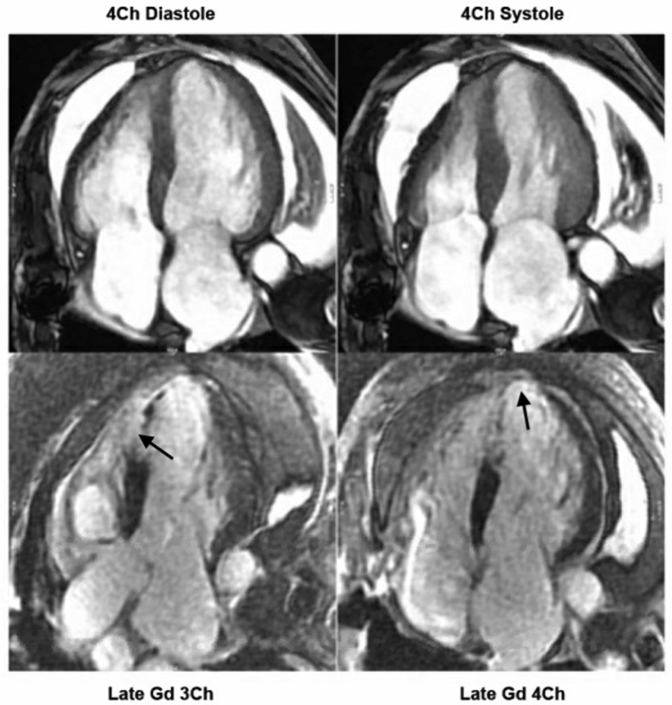
Cardiac magnetic resonance imaging demonstrated wall thinning, hypokinesis, and transmural late gadolinium enhancement (LGE) of the apical and anteroseptal segments of the left ventricle, consistent with a completed anterior myocardial infarction (Figure 3), which prompted further investigation with a coronary angiogram.
Figure 3.
Cardiac Magnetic Resonance Imaging Demonstrating Wall Thinning, Hypokinesis, and Transmural Late Gadolinium Enhancement of the Apical and Anteroseptal Segments of the Left Ventricle
Cardiac magnetic resonance imaging demonstrating wall thinning, hypokinesis, and transmural late gadolinium (Gd) enhancement (arrows) of the apical and anteroseptal segments of the left ventricle. Ch = chamber.
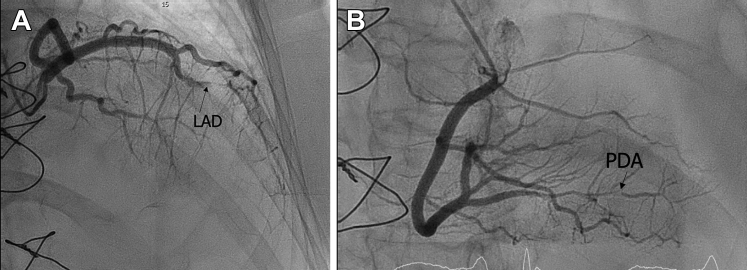
Coronary angiography demonstrated sub-total occlusion of the mid left anterior descending artery with a proximal dissection flap suggestive of spontaneous coronary artery dissection (SCAD) (Figure 4A, Video 3A). There was also a severe stenosis in the distal right-posterior descending artery with evidence of SCAD (Figure 4B, Video 3B). There was otherwise no intracoronary atherosclerotic plaque seen.
Figure 4.
Coronary Angiograms
(A) Coronary angiogram demonstrating a sub-totally occluded mid left anterior descending coronary artery (LAD). (B) Coronary angiogram demonstrating spontaneous coronary artery dissection in the distal posterior descending coronary artery (PDA).
Management
Given the characteristic appearance of SCAD on coronary angiography and transmural LGE on cardiac magnetic resonance imaging confirming a completed infarction, the patient was managed conservatively. Medical therapy included aspirin 100 mg and metoprolol 25 mg twice a day, as well as routine posttransplant rosuvastatin 40 mg for cardiac allograft vasculopathy prevention. Because of early acute kidney injury, angiotensin-converting enzyme inhibitor therapy was delayed for several weeks. Immunosuppression included mycophenolate sodium, everolimus, tacrolimus (from day 5) and prednisolone.
Discussion
SCAD is a rare cause of acute myocardial infarction, resulting from the spontaneous development of an intramural hematoma or intimal tear that causes compression of the true lumen of the coronary artery and consequent myocardial ischemia. This condition is more common in women, particularly in the fourth decade of life, and is strongly associated with FMD.1 Current guidelines support noninterventional management where possible to avoid propagation of the dissection and extension of the intramural haematoma.2
We present the first case of SCAD arising early posttransplant from a donor with probable FMD. To our knowledge, there is only 1 published case of SCAD post heart transplantation, which occurred later, in a 51-year-old woman 10 years posttransplant.3 There have been 3 cases reported of SCAD post other solid organ transplantation that were attributed to calcineurin inhibitor use.4 In this case, the recipient had limited exposure to tacrolimus before the development of SCAD. Potential precipitants for SCAD in this patient included any procedures that increased catecholamine surge, such as the stress from undergoing major cardiac surgery, intraoperative manual handling, or the use of inotropes in intensive care. Given the initial absence of regional wall motion abnormalities and later development of electrocardiogram changes, it is likely the SCAD happened at least 1-week post-transplantation.
The consensus guidelines for diagnosis and management of FMD do not recommend routine screening for SCAD in the absence of symptoms.5 The International Society for Heart and Lung Transplantation guidelines advocate performing coronary angiography only in prospective donors at increased risk of atherosclerosis (ie, age >40 years or significant drug history).6 Locally, the indication for donor coronary angiography includes history of suspected coronary artery disease, LV dysfunction on echocardiogram, or significant cardiovascular risk factors7. In this case, the donor coronary angiogram was normal and therefore did not assist in predicting the development of SCAD.
The implications for screening and selection of prospective donors after fatal intracranial hemorrhage (ICH) remain unclear. ICH represents a common cause of death among heart transplant donors. In 1 large study from the United States examining 23,228 heart transplant recipients, intracranial bleed/stroke was cited as the leading cause of death in approximately 26% of donors.8 Neither the International Society for Heart and Lung Transplantation nor the Transplant Society of Australia and New Zealand guidelines advocate for specific additional screening after death from ICH.6,7
Follow-Up
The patient has since undergone serial TTEs, which have demonstrated some recovery in LV function with an LV ejection fraction of 55% at 3 months posttransplant. The antero-apical akinesis persists.
Conclusions
In this case report, we describe a rare case of SCAD after heart transplantation due to probable undiagnosed FMD in the donor, which to our knowledge, is the first such case reported in the literature. ICH is a frequent cause of death in cardiac donors. Screening this group for FMD before procurement is likely low-yield and logistically impractical. It therefore remains impossible to predict in which recipient SCAD may occur, but it is important to consider this rare cause of acute myocardial infarction and new LV dysfunction in patients post heart transplantation. This case highlights that a high index of suspicion must be maintained in the right clinical context, and consideration of routine predonation coronary angiography or screening of other vascular beds may be appropriate in young donors who have died from a ruptured cerebral aneurysm or dissection in whom FMD is suspected.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Donor coronary angiogram demonstrating normal coronary arteries.
Donor coronary angiogram demonstrating normal coronary arteries.
Transthoracic echocardiogram showing LV antero-apical akinesis.
Transthoracic echocardiogram showing LV antero-apical akinesis.
Coronary angiogram showing SCAD in the (A) mid left anterior descending coronary artery (LAD)
Coronary angiogram showing SCAD in the (B) distal posterior descending coronary artery (PDA).
References
- 1.Saw J., Mancini G.B.J., Humphries K.H. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. doi: 10.1016/j.jacc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Hayes S.N., Kim E.S.H., Saw J., et al. Spontaneous coronary artery dissection: current state of the science: A scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theertham A., Niazi K., Moreyra A. Spontaneous coronary artery dissection in heart transplant recipient. J Am Coll Cardiol. 2020;75(11_Supplement_1):2980. [Google Scholar]
- 4.Tsimikas S., Giordano F.J., Tarazi R.Y., et al. Spontaneous coronary artery dissection in patients with renal transplantation. J Invasive Cardiol. 1999;11:316–321. [PubMed] [Google Scholar]
- 5.Gornik H.L., Persu A., Adlam D., et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. Vasc Med. 2019;24(2):164–189. doi: 10.1177/1358863X18821816. [DOI] [PubMed] [Google Scholar]
- 6.Copeland H., Awori Hayanga J.W., Neyrinck A., et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 2020;39(6):501–517. doi: 10.1016/j.healun.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 7.The Transplantation Society of Australia and New Zealand . TSANZ; 2021. Clinical Guidelines for Organ Transplantation from Deceased Donors. Version 1.5. April. [Google Scholar]
- 8.Srihari K., Lella S.K., Copeland L.A., et al. Donor cause of death in heart transplantation and its effect on post-transplant survival—a UNOS database review. J Card Fail. 2016;22(8):S106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Donor coronary angiogram demonstrating normal coronary arteries.
Donor coronary angiogram demonstrating normal coronary arteries.
Transthoracic echocardiogram showing LV antero-apical akinesis.
Transthoracic echocardiogram showing LV antero-apical akinesis.
Coronary angiogram showing SCAD in the (A) mid left anterior descending coronary artery (LAD)
Coronary angiogram showing SCAD in the (B) distal posterior descending coronary artery (PDA).