Abstract
Background
Dietary changes impact human physiology and immune function and have potential as therapeutic strategies.
Objective
Assess the tolerability of a ketogenic diet (KD) in patients with relapsing multiple sclerosis (MS) and define the impact on laboratory and clinical outcome metrics.
Methods
Sixty-five subjects with relapsing MS enrolled into a 6-month prospective, intention-to-treat KD intervention. Adherence was monitored with daily urine ketone testing. At baseline, fatigue, depression and quality of life (QoL) scores were obtained in addition to fasting adipokines and MS-related clinical outcome metrics. Baseline metrics were repeated at 3 and/or 6 months on-diet.
Results
Eighty-three percent of participants adhered to the KD for the study duration. Subjects exhibited significant reductions in fat mass and showed a nearly 50% decline in self-reported fatigue and depression scores. MS QoL physical health (67±16 vs 79±12, p<0.001) and mental health (71±17 vs 82±11, p<0.001) composite scores increased on-diet. Significant improvements were noted in Expanded Disability Status Scale scores (2.3±0.9 vs 1.9±1.1, p<0.001), 6-minute walk (1631±302 vs 1733±330 ft, p<0.001) and Nine-Hole Peg Test (21.5±3.6 vs 20.3±3.7 s, p<0.001). Serum leptin was lower (25.5±15.7 vs 14.0±11.7 ng/mL, p<0.001) and adiponectin was higher (11.4±7.8 vs 13.5±8.4 μg/mL, p=0.002) on the KD.
Conclusion
KDs are safe and tolerable over a 6-month study period and yield improvements in body composition, fatigue, depression, QoL, neurological disability and adipose-related inflammation in persons living with relapsing MS.
Trial registration information
Registered on ClinicalTrials.gov under registration number NCT03718247, posted on 24 October 2018. First patient enrolment date: 1 November 2018. Link: https://clinicaltrials.gov/ct2/show/NCT03718247?term=NCT03718247&draw=2&rank=1.
INTRODUCTION
Multiple sclerosis (MS) is influenced by genetic and environmental factors. Dietary intake impacts immune profile and function, which includes development of the host immune system, protection against pathogens and maintenance of the immune response.1–3 As a result, researchers have looked toward dietary modification as a way of impacting immune-mediated disorders, such as MS.4–8
Ketogenic diets (KDs) are high-fat, low-carbohydrate, adequate protein diets that mimic a nutritional fasting state. The term ‘ketogenic diet’ is often used loosely to refer to any diet lowering carbohydrate intake; however, for the purposes of this manuscript, we refer to KDs as those which promote biological ketosis that can be measured reliably via the blood or urine. Posited mechanisms by which KDs benefit neuroinflammatory disease are by providing a more efficient source of energy (ie, fatty acids), decreasing oxidative damage associated with metabolic stress, increasing mitochondrial biogenesis pathways and reducing pro-inflammatory cytokine production.9–12
Animal studies highlight the broad neuroprotective and therapeutic properties of KDs and fasting diets.13–15 Mice with experimental autoimmune encephalomyelitis fed a KD experienced reversed motor disability, improved spatial learning and memory, and remyelination of periventricular lesions.15 KDs and ketone metabolites impact the production of pro-inflammatory cytokines and attenuate oxidative stress via several pathways—including actions on the NLRP3 inflammasome,12 nuclear factor-eryhthroid-2-related factor 2 and nuclear factor-ƘB pathway.16 KDs may impact adipocyte-derived cytokines (adipokines), such as leptin and adiponectin. Adipokines serve as an important link between metabolic function, inflammation and the immune response.17–19
The study of KDs in humans with MS is limited. While KDs may offer benefits to patients with MS, there are potential risks associated with the KD’s strict nutritional parameters—including metabolic acidosis, nephrolithiasis, hyperlipidaemia and select nutrient deficiencies. Our group previously performed a pilot feasibility study of a modified Atkins KD (KDMAD) in a cohort of 20 subjects with relapsing MS.20 As a first-step study, it was not powered to determine tolerability or efficacy. Informed by this pilot, we designed and completed a next-step phase II trial to demonstrate the tolerability of a monitored KD over 6 months, while secondarily assessing the impact of the KD on clinical outcomes and pro-inflammatory leptin levels.
METHODS
Study participants
Sixty-five subjects with relapsing-remitting MS per 2017 McDonald criteria, aged 12–55 years, were enrolled.21 Given that MS can affect 3%–5% of patients prior to 18 years of age,22 young adults with MS were included. Most paediatric patients with MS manifest between the ages of 12 and 16 years. As such, they are still undergoing maturational change in body habitus, hormonal alterations associated with puberty and potential changes in immune function.23 Despite these maturational considerations, inclusion of paediatric patients in research is a clear imperative, emphasised further by data showing a strong correlation between childhood obesity and MS risk.24 25 For this study, two paediatric subjects were included (15 and 17 years of age at time of enrolment), representing 3% of the total study cohort.
Subjects had to demonstrate clinical disease stability on their current disease-modifying therapy (if any) for at least 6 months prior to enrolment and have an Expanded Disability Status Scale (EDSS) score of ≤6.0, given the use of ambulatory testing metrics. Subjects were excluded if they had progressive MS, had been on a KD in the past 6 months, were pregnant/planning pregnancy or were underweight by Centers for Disease Control (CDC) guidelines.26 In patients with comorbid conditions that had potential for worsening on KD (eg, hypercholesterolaemia, nephrolithiasis), written permission from their primary physician was required prior to enrolment. Data from the first 20 subjects are expanded in this manuscript with the addition of previously unreported metrics (eg, low contrast visual acuity, accelerometry) in combination with new data from the additional 45 subjects for this phase II study.
Standard protocol approvals, registrations and patient consent
Eligible subjects were identified and contacted through the University of Virginia MS Clinic. All subjects provided informed consent (and assent, when applicable) prior to the start of any study-related procedures. The study was listed on the National Institutes of Health Clinical Trials website.
Sample size
A priori sample size calculations indicate that a sample size of 65 is sufficient to meet the objective of establishing the tolerability of a KD within a population with MS, defined as having at least 42 of 65 (65%) participants, based on epilepsy data27 with >85% adherent days for a 6-month duration. With 65 subjects, the paired t-test has 80% power, with a two-sided significance level of 5%, when the mean change in the total Modified Fatigue Impact Scale is 5.6 points. Similarly, there is 80% power for a mean change of 2.5 ng/mL in serum leptin.
Study procedures
The study entailed a baseline visit (pre-diet) and three study visits (on-diet) at 1, 3 and 6 months. At baseline, demographics and anthropometric data (standardised weight, height and waist circumference) were collected. As a measure of body composition, subjects underwent air displacement plethysmography (BOD POD) to quantify lean mass versus fat mass at baseline and at 6 months on KD.
At baseline, subjects met with the study dietitian (DL-G), who provided education on the initiation of the KDMAD. Subjects were instructed to restrict net carbohydrates to <20 g/day and were encouraged to increase healthy fat intake. Subjects were advised to start a daily multivitamin with minerals and were periodically assessed for dietary calcium intake. If calcium intake from KDMAD was lower than recommended, subjects were advised to take 500–600 mg/day of supplemental calcium. The majority (91%) of MS subjects were already taking vitamin D (at varied doses) at study start and were advised to continue as prescribed by their treating neurologist.
The dietitian contacted subjects at 2 weeks on KDMAD via telephone to troubleshoot diet concerns. Subjects met with the dietitian at all study visits, and at study end, they discussed next diet steps. If a subject wished to continue on KDMAD, they were offered continued dietitian guidance and encouraged to follow closely with their primary doctor. If a subject wished to liberalise their diet, the dietitian provided them with a plan to safely do so.
EDSS evaluation was performed by a single Neurostatus-certified examiner (JNB) at each visit. Subjects had MS Functional Composite (MSFC) testing at baseline and 6 months on-diet. Symbol Digit Modality Test (SDMT), low contrast visual acuity (LCVA) and 6 minute-walk (6MW) were performed pre-diet and at 6 months on-diet. As a marker of real-world physical activity, each participant was sent home with a waist-worn Actigraph accelerometer to be worn continuously over the nondominant hip for 7 days (excluding time spent bathing, swimming and sleeping). Subjects were instructed to wear this prior to diet-start and subjects repeated this assessment at 6 months on-diet.
Patient-reported outcomes were completed at baseline, 3 and 6 months on-diet. These included depression (Beck’s Depression Inventory 1A (BDI)) and fatigue (Modified Fatigue Impact Scale (MFIS)) assessments. The MS Fatigue Severity Scale (MSFSS) and MS Quality of Life-54 (MSQoL-54) were added following enrolment of the first 20 subjects and were thus performed in only the last 45 subjects.
Fasting blood work was completed at baseline, 3 and 6 months on-diet–including a complete metabolic panel, insulin/haemoglobin A1c, lipids, carnitine, 25-hydroxyvitamin D and adipokines (leptin, adiponectin).
Adherence was objectively monitored using daily urine ketone test strips. Subjects were provided with a 6-month supply of Bayer Ketostix and were required to take a photograph of their daily ketone test and send this photographic evidence to the study team each day. The ketone strip was required to be dated in ink to prevent reusing of past test strips. A subject was considered adherent for that study day if the photographed strip demonstrated evidence of ketosis. A negative ketone strip or a day without dated, photographic evidence of ketosis was marked as a non-adherent day. A priori for this study, a subject was considered adherent with the diet intervention if they had >85% adherent days from the 6-month study duration.
Statistical analysis
For primary analysis, an intention-to-treat approach without imputation of missing data was used; thus, the data from all subjects completing the 3-month and 6-month visits were included. Descriptive statistics for baseline and follow-up were calculated and tabulated. Repeated measures models, using a spatial power covariance structure, were used to provide in-group comparisons of changes from baseline to the 3-month and 6-month visits. These models, as well as Spearman correlation coefficients, were planned a priori to assess the association between the change in leptin and body mass index (BMI). A two-sided p-value of <0.05 was defined as statistically significant. All statistical analyses were conducted using SAS V.9.4 PROC MIXED and GAUSS V.21.0 software.
For actigraphy analyses, we screened data for wearing compliance via visual inspection and excluded subjects who wore the sensor for <2 days. We applied a sleep–wake cycle detection algorithm28 to segment resting from active periods. Within active periods, we examined total-wearing time of each day and used only days with ≥10 hours of wear time (ie, valid days) for analysis. We calculated average diurnal activity counts, minutes spent in light physical activity (LPA) and minutes spent in moderate-to-vigorous physical activity (MVPA)+vigorous physical activity (VPA) using defined cut-off points.29 We tested changes from baseline to 6 months in these metrics using linear mixed-effects models. To compare accelerometer-wearing compliance between baseline and 6-month visits, we determined total non-wear time (ie, >90 min of consecutive zeros) within each valid day.30
RESULTS
Enrolment and demographics
Sixty-five subjects enrolled; however, a single subject completed the baseline visit but never started KDMAD due to a change in personal circumstances. Only subjects who began KDMAD and sent at least one dated keto test strip photograph were included in final analyses (n=64) (figure 1). Demographics are provided in table 1.
Figure 1.
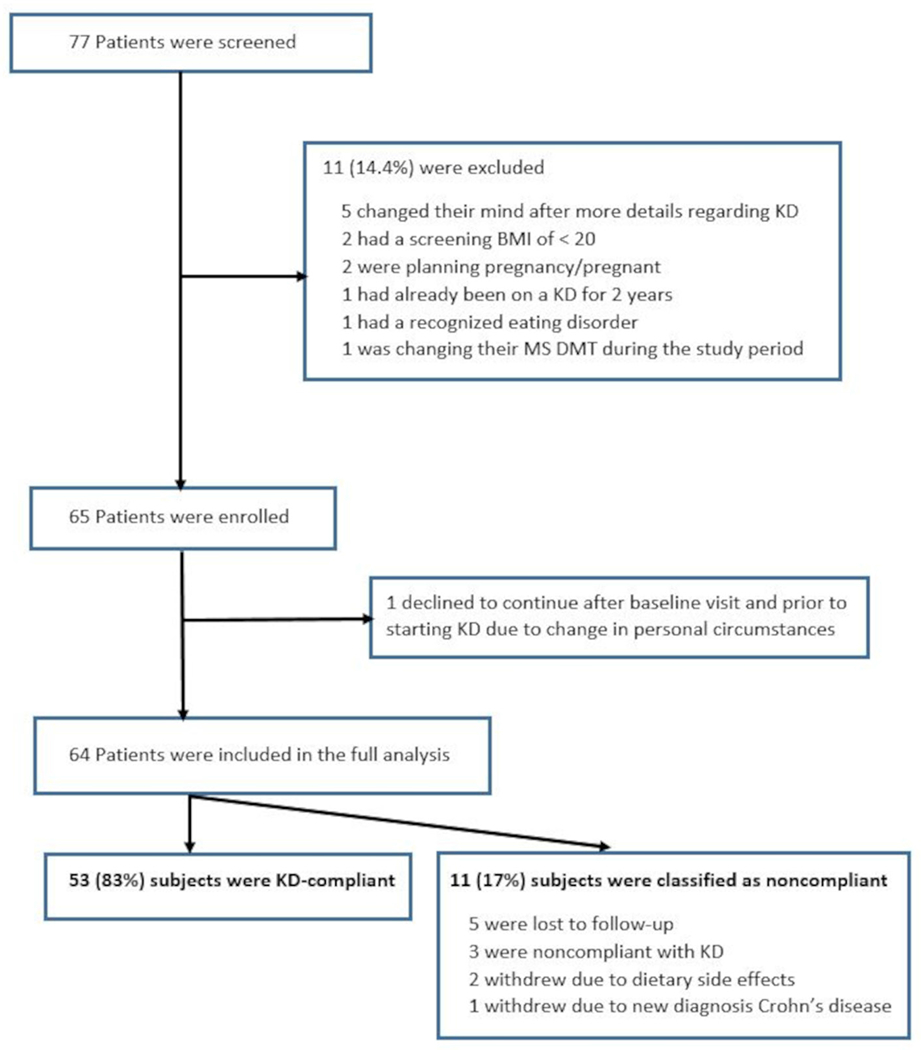
Subject screening, enrolment and compliance. BMI, body mass index; DMT, disease modifying therapy; KD, ketogenic diet; MS, multiple sclerosis.
Table 1.
Clinical demographics of the study population (n=64)
| Age, median years (range) | 40 (15–54) |
| Sex | |
| Female, n (%) | 55 (86) |
| Male, n (%) | 9 (14) |
| Race | |
| White, n (%) | 54 (84) |
| African-American, n (%) | 10 (16) |
| Ethnicity | |
| Hispanic, n (%) | 3 (5) |
| Body mass index category | |
| Normal, n (%) | 4 (6) |
| Overweight, n (%) | 20 (31) |
| Obese, n (%) | 40 (63) |
| MS disease duration, mean years (±SD) | 8.4 (5.5) |
| Time since last clinical relapse, mean years (±SD) | 4.4 (3.7) |
| Baseline EDSS score, median (range) | 2.3 (1.0–6.0) |
| Number of previous disease-modifying therapies attempted, mean (±SD) | 2.4 (1.3) |
| Current disease-modifying therapy, n (%) | |
| Natalizumab | 15 (23) |
| Fingolimod | 9 (14) |
| Dimethyl fumarate | 8 (12.5) |
| Interferon | 8 (12.5) |
| Glatiramer acetate | 8 (12.5) |
| Ocrelizumab/rituximab | 8 (12.5) |
| Teriflunomide | 5 (8) |
| Alemtuzumab | 2 (3) |
| None | 1 (2) |
| Time on current disease-modifying therapy, median months (range) | 24 (6–216) |
| Subjects with previous diet attempts, n (%) | 41 (64) |
EDSS, Expanded Disability Status Scale; MS, multiple sclerosis.
Nearly two-thirds of subjects had attempted a dietary intervention prior to enrolment, with 11 subjects attempting more than one type of diet. The most common diets attempted included those aimed at weight loss (eg, Weight Watchers) in 20 subjects (31%), KDs in 11 subjects (17%) and paleo diet in 11 subjects (17%). Less common attempts included low fat/Swank (n=4), vegan (n=2), intermittent fasting (n=2) and Mediterranean (n=1).
Tolerability and safety
From 64 subjects, 53 (83%) met adherence criteria for the 6-month intervention. The majority of non-adherent subjects were either lost to follow-up (n=5) or did not meet adherence criteria (n=3). Two subjects withdrew due to gastrointestinal side effects (eg, nausea, lack of appetite): one subject adhered for 4 months and the second subject adhered for 2 weeks prior to discontinuation. A single patient was diagnosed with Crohn’s disease 1 month after KDMAD start, manifesting with stomach pain, diarrhoea and fever. Of note, this subject reported intermittent diarrhoea prior to KDMAD that had been attributed to a recent cholecystectomy (figure 1).
Age, sex, BMI, prior diet attempts, MS duration and EDSS did not associate with likelihood of diet adherence. Subjects with at least 1 day of non-adherence in the first month of KDMAD were less likely to meet adherence criteria (OR −0.52, 95% CI: −0.95 to −0.09, p=0.018). In addition, white subjects were more likely to meet adherence criteria than black subjects (OR 1.62, 95% CI: 0.11 to 3.13, p=0.035).
Of the 56 subjects who completed the 6-month intervention, the most common side effects included constipation (43%), diarrhoea (18%), nausea (9%), weight gain (9%), fatigue (5%), worsened depression/anxiety (5%) and acne (5%). Menstrual irregularities, manifesting as a change in frequency (including shortened and lengthened time between menses) and/or amount (including decreased and increased) of menstrual bleeding, were reported by 13 of 48 women (27%). No subjects exhibited evidence of MS relapse.
Anthropometrics, patient-reported, clinical and laboratory outcome measures
Subjects demonstrated significant reductions in BMI, waist circumference, fat mass, fat-free mass and resting metabolic rate (table 2) on KDMAD. Fat mass percentage decreased (from 44% to 40% of total body mass), while the percentage of fat-free mass increased (56% to 60% of total body mass).
Table 2.
Anthropometric, patient-reported, and clinical outcome measures using an intention-to-treat analysis pre-KD and post-KD intervention
| Baseline (pre-diet) (n=64) | 3 months (on-diet) (n=59) | 6 months (on-diet) (n=57) | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean±SD | Δ Change from baseline | P value | Δ Change from baseline | P -value | |
| Anthropometric measures | |||||
|
| |||||
| Body Mass Index (BMI) | 33.2±7.0 | −2.9±1.5 | <0.001 | −3.4±2.2 | <0.001 |
|
| |||||
| Waist Circumference (cm) | 104.8±14.2 | −6.7±5.2 | <0.001 | −9.8±7.4 | <0.001 |
|
| |||||
| Bod Pod Assessment | |||||
|
| |||||
| Fat Mass (kg) | 41.3±16.1 | — | — | −7.7±5.4 | <0.001 |
|
| |||||
| Fat-Free Mass (kg) | 51.9±10.7 | — | — | −1.4±1.9 | <0.001 |
|
| |||||
| Resting Metabolic Rate (kcal/day) | 1510.8±306.2 | — | — | −67.8±60.1 | <0.001 |
|
| |||||
| Patient reported outcomes | |||||
|
| |||||
| Beck’s Depression Inventory | 8.5±5.3 | −3.3±3.8 | <0.001 | −3.8±4.5 | <0.001 |
|
| |||||
| Modified Fatigue Impact Scale | 33.3±18.1 | −11.6±12.7 | <0.001 | −14.4±14.9 | <0.001 |
|
| |||||
| MS Fatigue Severity Scale* | 21.9±8.8 | −2.7±5.4 | 0.015 | −3.4±5.8 | 0.002 |
|
| |||||
| MS Quality of Life-54* | |||||
|
| |||||
| Physical QoL | 66.9±16.3 | +9.2 ± 10.3 | <0.001 | +12.0 ± 14.0 | <0.001 |
|
| |||||
| Mental QoL | 71.3±16.6 | +8.5 ± 13.6 | 0.002 | +11.2 ± 18.3 | <0.001 |
|
| |||||
| Clinical outcome measures | |||||
|
| |||||
| EDSS | 2.3±0.9 | −0.4±0.6 | <0.001 | −0.5±0.6 | <0.001 |
|
| |||||
| MS Functional Composite | |||||
|
| |||||
| Timed 25-foot walk (sec) | 5.0±1.5 | — | — | −0.1±0.7 | 0.33 |
|
| |||||
| 9-Hole Peg Test (sec) | 21.5±3.6 | — | — | −1.1±1.5 | <0.001 |
|
| |||||
| PASAT (# correct) | 46.6±9.0 | — | — | +0.2 ± 4.9 | 0.815 |
|
| |||||
| SDMT (# correct) | 61.1±10.1 | — | — | +1.1 ± 5.6 | 0.18 |
|
| |||||
| 6 min walk (feet) | 1631±302 | — | — | +78 ± 137 | <0.001 |
|
| |||||
| Low Contrast Visual Acuity | |||||
|
| |||||
| 100 OU (# correct) | 56±9 | — | — | +0.6 ± 5 | 0.355 |
|
| |||||
| 2.5 OU (# correct) | 36±11 | — | — | +0.2 ± 6 | 0.856 |
|
| |||||
| 1.25 OU (# correct) | 27±12 | — | — | +1.2 ± 10 | 0.390 |
Results represent mean±SD as change from baseline to reported time point (eg, 3 months or 6 months on KD).
Bolded denotes a significant p-value.
These surveys were only completed on the last 45 subjects, such that at baseline n=44, 3 months n=40 and 6 months n=39.
EDSS, Expanded Disability Status Scale; KD, ketogenic diet; MS, multiple sclerosis; SDMT, Symbol Digit Modality Test.
Fatigue impact (MFIS), fatigue severity (MSFSS) and depression (BDI) scores were significantly reduced on KDMAD. We noted a significant increase in quality of life (QoL) scores for both physical and mental health on-diet (table 2). While all QoL subscores demonstrated significant improvements, the most prominent improvements were in physical health, energy, cognitive function and change in overall health (see online supplemental table 1).
Neurological disability, measured by EDSS, did not worsen on KDMAD—in fact, an overall reduction in EDSS (−0.5±0.6; p<0.001) was noted at study completion. Subjects also exhibited significant improvement in average Nine-Hole Peg Test speed (−1.1s±1.5, p<0.001); however, there were no significant changes on the Timed 25-Foot Walk, Paced Auditory Serial Addition Test (PASAT), or SDMT. Subjects exhibited improved walking speed/endurance on the 6MW, walking an average of 78 more ft within the allotted time compared with baseline (p<0.001) (table 2). Subjects did not exhibit significant changes in daily activity counts or time spent in LPA versus MVPA+VPA as measured by continuous accelerometry; although, compliance with wearing the accelerometer at the 6-month mark was significantly less compared with baseline (27±76 non-wear minutes/valid day vs 43±118 non-wear minutes/valid day, p=0.001) (online supplemental table 2).
Laboratory monitoring is presented in table 3. Carnitine deficiency is a potential side effect of KDs and was monitored regularly. At 3 months, 24% (14 of 59 subjects) exhibited a secondary carnitine deficiency and were subsequently supplemented. Thus, while subjects were found to have a significant decline in carnitine at 3 months, this was not apparent at 6 months, most likely due to supplementation. While the KD is considered to be a poor source of vitamin D, subjects exhibited a significant increase in vitamin D levels on-diet. We noted a significant reduction in fasting insulin and haemoglobin A1c at 3 and 6 months on-diet. Lipid profiles showed a significant reduction in triglycerides, with increases in low-density lipoprotein (LDL), cholesterol and high-density lipoprotein (HDL) on-diet.
Table 3.
Laboratory results using an intention-to-treat analysis pre-KD and post-KD intervention
| Baseline (n=64) | 3 months (n=59) | 6 months (n=57) | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean±SD | Δ Change from baseline | P value | Δ Change from baseline | P value | |
| 25-hydroxyvitamin D (ng/mL) | 44±17 | +11.5±16 | <0.001 | +12±17 | <0.001 |
|
| |||||
| Carnitine, free (nmol/mL) | 34±8 | −3.7±7 | 0.001 | −1.6±11 | 0.176 |
|
| |||||
| Insulin resistance | |||||
|
| |||||
| Insulin (uIU/mL) | 13.3±8.5 | −5.2±6.8 | <0.001 | −4.7±7.7 | 0.001 |
|
| |||||
| Haemoglobin A1c (%) | 5.4±0.6 | −0.3±0.4 | <0.001 | −0.3±0.5 | <0.001 |
|
| |||||
| Lipid profiles | |||||
|
| |||||
| Triglycerides (mg/dL) | 107±59 | −17±45 | 0.005 | −17±47 | 0.005 |
|
| |||||
| Low-density lipoprotein (LDL) (mg/dL) | 127±35 | +14±28 | <0.001 | +10±27 | 0.007 |
|
| |||||
| High-density lipoprotein (HDL) (mg/dL) | 55±13 | −0.4±9 | 0.763 | +3±10 | 0.03 |
|
| |||||
| LDL/HDL | 2.4±0.8 | +0.3±0.7 | 0.001 | +0.1±0.7 | 0.172 |
|
| |||||
| Cholesterol (mg/dL) | 200±40 | +11±32 | 0.007 | +10±31 | 0.021 |
|
| |||||
| Adipo-cytokines | |||||
|
| |||||
| Leptin (ng/mL) | 25.5±15.7 | −12.1±11.4 | <0.001 | −10.6±11.7 | <0.001 |
|
| |||||
| Adiponectin (μg/mL) | 11.4±7.8 | +0.7±3.8 | 0.162 | +1.7±3.9 | 0.002 |
Results represent mean±SD as change from baseline to reported time point (eg, 3 months or 6 months on KD).
KD, ketogenic diet.
Leptin, a pro-inflammatory adipokine, was reduced at 3 and 6 months on-diet (p<0.001). The changes in BMI and leptin over time were moderately correlated (r=0.52, n=56, p<0.001). We estimated the effect of change in BMI on change in leptin, demonstrating that for every one unit difference in BMI change associates with a 2.62 difference in leptin reduction from baseline (2.62, 95% CI: 1.86 to 3.38, p<0.001). To determine the role of diet (separate from BMI change), we ran a repeated measures analysis of change in leptin, while adjusting for change in BMI, and showed that change in leptin remained significant at 3 months (−4.6, 95% CI: −8.0 to −1.3, p=0.007) but not at 6 months on-diet (−2.0, 95% CI: −5.6 to +1.5, p=0.261). Thus, after accounting for change in BMI, there are still notable leptin decreases from baseline at 3 and 6 months, though these changes are more prominent early in the diet course.
Adiponectin, an anti-inflammatory adipokine, was increased at 6 months on KDMAD (+1.7±3.9, p=0.002). Similar to leptin, the changes in BMI and adiponectin over time were correlated (r=0.40, n=56, p=0.003). The effect of change in BMI on change in adiponectin was not significant (−0.16, 95% CI: −0.5 to 0.18, p=0.363). After accounting for changes in BMI, using a repeated measures analysis, adiponectin showed a non-significant trend to increase from baseline to 3 months (+0.27, 95% CI: −1.15 to 1.70, p=0.706) and 6 months (+1.51, 95% CI: −0.40 to 2.71, p=0.145).
DISCUSSION
We show that KDMAD is a well-tolerated dietary intervention in patients with relapsing MS that leads to weight loss, reduced fatigue and depression, and improved QoL. This study was not designed to demonstrate efficacy, which will require a larger randomised controlled trial.31 Future trial design will need to consider several important points, as there are challenges when studying diet in MS.32 The first is diet adherence. Uniquely, KDs provide objective evidence of adherence with home-based measures of ketosis in urine or blood. Though dietary interventions cannot be easily blinded, next-step trials should employ randomisation and focus on promising outcome metrics highlighted in this study—including patient-reported outcomes (fatigue, mood disorders, QoL) and clinical outcomes, such as walking speed and fine motor coordination. Our findings suggest that those with good adherence in the first month had higher odds of adhering, thus future trials should include study visits within the first month of intervention to reinforce adherence.
Overall, our results support the rationale for a large-scale study of a KD as a complementary treatment for MS. Our data do not, however, support widespread adoption of KDs as a therapeutic strategy for MS outside of a clinical trial. Our subjects were carefully monitored by a trained dietitian, key laboratory studies and regular neurological examinations. Theoretical risks to long-term adoption of an unmonitored KD include dyslipidaemia, nephrolithiasis and vitamin/mineral deficiencies.
By using strict adherence criteria, 83% of subjects successfully adhered to this diet for 6 months, which exceeds reports in epilepsy27 and weight loss.33 Body composition, disease severity and history of dieting do not correlate with adherence. White subjects were more likely to adhere compared with black subjects. Reasons for this are likely variable—unfortunately, these factors were not assessed given that three of four non-adherent black subjects were lost to follow-up and did not provide feedback regarding factors contributing to study discontinuation. Future diet studies should include qualitative interviews with subjects at multiple time points regarding specific supports and barriers to diet adherence.
One-quarter of subjects did not report any side effects on KDMAD. The majority who experienced side effects noted these during the first 2 weeks, with subsequent resolution. Constipation, the most common side effect, was typically managed with dietary and/or supplement manipulation. Menstrual irregularities were not uncommon and may represent unique influences of this diet on hormonal balance in women.
A distinct advantage of this study included the use of BOD POD in addition to the more rudimentary metrics of waist circumference and BMI. The KDMAD significantly reduced multiple metrics of body composition somewhat expected with weight loss, including body mass, waist circumference, fat mass and fat-free (ie, bone and muscle) mass. Importantly, the percentage of fat mass decreased (from 44% to 40% of total body mass), while the percentage of fat-free mass increased (56% to 60% of total body mass). Resting metabolic rate was significantly reduced at 6 months, attributable to the reduced caloric intake and reduced fat-free mass on KDMAD.
No subject exhibited symptoms of worsening MS—on the contrary, our study demonstrated improvements in multiple domains (figure 2)—including depression, fatigue impact and severity, and QoL. The magnitude of improvement in fatigue with KDMAD is comparable with improvements noted in MS clinical drug trials (eg, amantadine, modafinil and methylphenidate)34 and other diet interventions.4 6 The degree of improvement in QoL was marked across all domains assessed (see online supplemental table 1) and was substantially higher than changes noted in MS clinical drug trials.35–37
Figure 2.
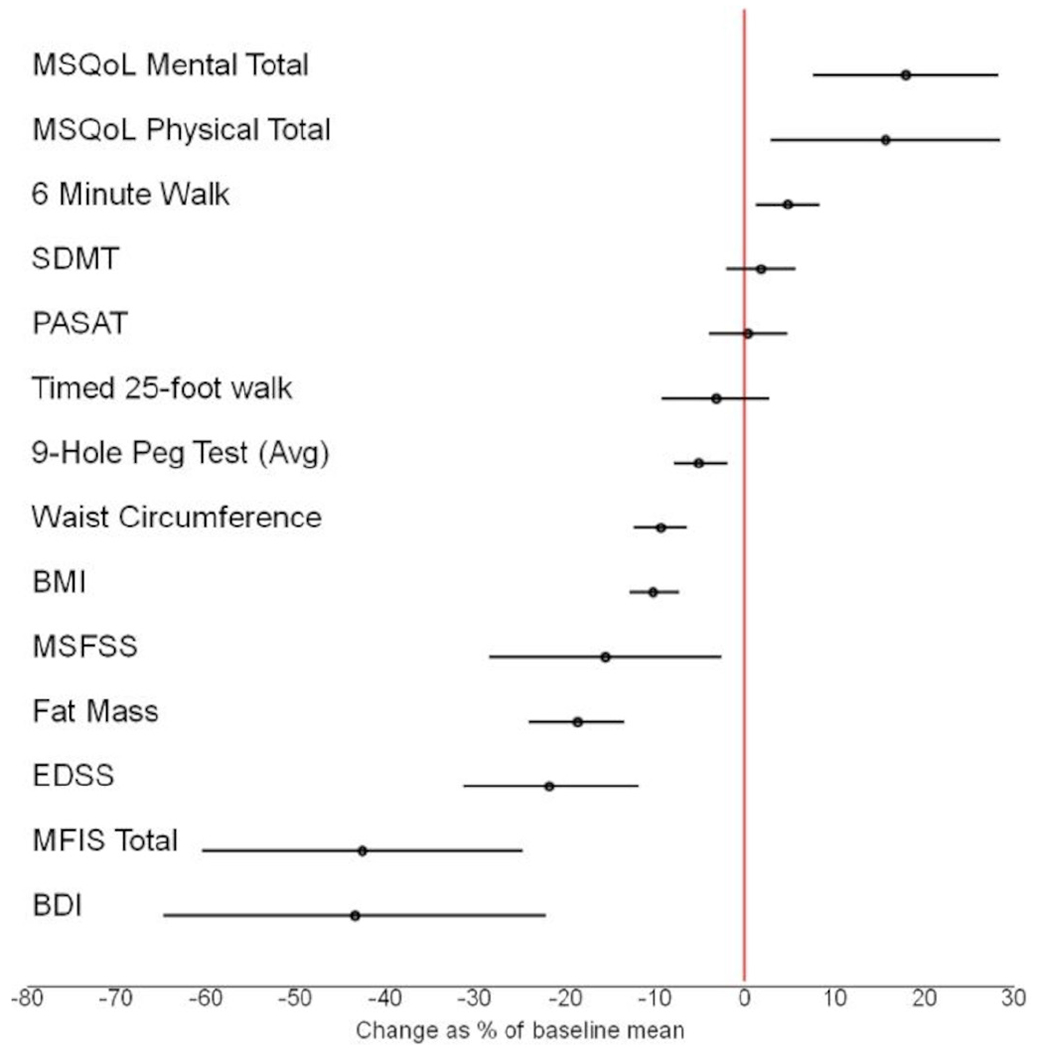
Change from baseline to 6 months in patient-reported and clinical outcomes with Bonferroni-adjusted 95% CIs, demonstrated as % change from baseline mean. BDI, Beck’s Depression Inventory; BMI, body mass index; EDSS, Expanded Disability Status Scale; MFIS, Modified Fatigue Impact Scale; MSFSS, Multiple Sclerosis Fatigue Severity Scale; MSQoL, Multiple Sclerosis Quality of Life; PASAT, Paced Auditory Serial Addition Test; SDMT, Symbol Digit Modality Test.
There was no evidence of worsening performance on clinical metrics on-diet—in fact, subjects showed stability and/or improvements in all objective modalities tested, with most prominent improvements noted in walking speed (6MW) and fine motor speed (Nine-Hole Peg Test) (figure 2). Despite improvements in walking speed, the KDMAD did not impact behavioural, real-world activity levels, measured by continuous accelerometry. This lack of change in physical activity could signify a behavioural hurdle that is not appreciably impacted by diet modification alone. Interpretation of this data is limited by the fact that compliance with accelerometer wear time was significantly lower at the 6-month visit.
Vitamin D levels increased on KDMAD, which may be secondary to two factors: first, subjects may have become more compliant with vitamin D while undergoing a diet intervention; and second, with a significant decline in fat mass, vitamin D may exhibit higher bioavailability. Consumption of the recommended multivitamin (with an average of 400–600 units of vitamin D) may also raise vitamin D levels to a small degree. Though LDL and total cholesterol increased at 6 months on-diet, the LDL to HDL ratio (a comparative metric for cardiac disease risk) was not significantly elevated.
Supporting preliminary findings from our pilot study, we show a significant decline in leptin on-diet. While this change is correlated with declining BMI, change in BMI alone does not fully account for this reduction. Our study also demonstrates a significant increase in adiponectin levels at 6 months, and these changes were correlated with change in BMI over time. After accounting for changes in BMI, there remained a trend toward increases in adiponectin from baseline. These results raise the possibility that one of the mechanisms of a KD may be adipokine release and regulation. Future work correlating these biomarkers directly with immune cell phenotypes and reactivities will be of interest.
Limitations of our study include the lack of a matched control group monitored on a ‘regular’ diet, which was not feasible as part of this phase II study. Prospective dietary studies in MS present unique challenges and the importance of phase II trials cannot be understated given the expense, planning and recruitment requirements for phase III trials. Thus, while the current study lacks controls, the findings herein are essential for next-step phase III trial design. Additional limitations include enrolment restricted to patients with clinically-stable relapsing MS, and thus our findings are not generalisable to a population with actively-relapsing or progressive MS.
This phase II trial demonstrates that the KDMAD is tolerable, safe, and promotes improvements in body composition, MS-related QoL, and significant reductions in adipose-related inflammation. Given our data, future research should aim to study KDs as a complementary therapeutic approach to the treatment of MS.
Supplementary Material
Key messages.
What is already known on this topic
Ketogenic diets have immunomodulatory properties that may benefit patients living with multiple sclerosis (MS). While animal models have substantiated these properties, data supporting the use of these diets in patients with MS remain limited.
What this study adds
This phase II study provides first-ever evidence that ketogenic diets are safe, tolerable and provide clinical benefits to persons living with relapsing MS.
How this study might affect research, practice or policy
The data herein justify the need for future research that aims to study ketogenic diets as a complementary therapeutic approach to the treatment of MS.
Funding
JNB’s work with the iTHRIV Scholars Program is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1TR003015 and KL2TR003016. A portion of this study was also funded via private foundational funding provided by the ZiMS Foundation.
Competing interests
JNB’s research is funded by the National Institutes of Health (NIH) and National Institute of Neurological Diseases and Stroke (NINDS) (grant number: K23NS116225) and by the iTHRIV Scholars Program via the National Center For Advancing Translational Sciences of the NIH under award numbers UL1TR003015 and KL2TR003016. Research support is also provided by the ZiMS Foundation. DL-G serves as a consultant for Functional Formularies. BB serves as a consultant to Novartis, Roche, UCB, Teva Neuroscience, Biogen and Sanofi. AGCB has served as a paid speaker for Nutricia North America. EW, SC, RC and MC have no competing interests. MDG has served on the DSMB for Anokion SMC and Immunic. She has received consulting fees from ADAMAS Pharmaceuticals, Biogen IDEC, Brainstorm Cell Therapeutics, EMD Serono, Genetec, Greenwich Biosciences, Horizons, Immunic, Merk, Novartis, Sanofi Genzyme and Vebrilio.
Footnotes
Publisher's Disclaimer: Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Patient consent for publication Not required.
Ethics approval This study involves human participants and was approved by the University of Virginia Institutional Review Board for Health Sciences Research (study ref ID: 20877). Subjects provided informed consent (and assent, if applicable) prior to study procedures.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/jnnp-2022-329074).
Data availability statement
Data are available upon reasonable request. Any data not published within the article are available, and the anonymised data will be shared by request from any qualified investigator.
REFERENCES
- 1.Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol 2016;7:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantarel BL, Waubant E, Chehoud C, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med 2015;63:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol 2015;17:344. [DOI] [PubMed] [Google Scholar]
- 4.Yadav V, Marracci G, Kim E, et al. Low-Fat, plant-based diet in multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord 2016;9:80–90. [DOI] [PubMed] [Google Scholar]
- 5.Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-Based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified Paleolithic diet: a Waitlist-Controlled, randomized pilot study. J Am Coll Nutr 2021;40:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahls TL, Titcomb TJ, Bisht B, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the waves randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin 2021;7:205521732110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald KC, Vizthum D, Henry-Barron B, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord 2018;23:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz Sand I, Benn EKT, Fabian M, et al. Randomized-controlled trial of a modified Mediterranean dietary program for multiple sclerosis: a pilot study. Mult Scler Relat Disord 2019;36:101403. [DOI] [PubMed] [Google Scholar]
- 9.Achanta LB, Rae CD. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res 2017;42:35–49. [DOI] [PubMed] [Google Scholar]
- 10.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007;48:43–58. [DOI] [PubMed] [Google Scholar]
- 11.Milder JB, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis 2010;40:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi IY, Lee C, Longo VD. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol Cell Endocrinol 2017;455:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi IY, Piccio L, Childress P, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 2016;15:2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DY, Hao J, Liu R, et al. Inflammation-Mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One 2012;7:e35476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Yang Y-Y, Zhou M-W, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett 2018;683:13–18. [DOI] [PubMed] [Google Scholar]
- 17.Monda V, Polito R, Lovino A, et al. Short-Term physiological effects of a very low-calorie ketogenic diet: effects on adiponectin levels and inflammatory states. Int J Mol Sci 2020;21. doi: 10.3390/ijms21093228. [Epub ahead of print: 02 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, et al. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res 2019;62:64–77. [DOI] [PubMed] [Google Scholar]
- 19.Cipryan L, Maffetone PB, Plews DJ, et al. Effects of a four-week very low-carbohydrate high-fat diet on biomarkers of inflammation: non-randomised parallel-group study. Nutr Health 2020;26:35–42. [DOI] [PubMed] [Google Scholar]
- 20.Brenton JN, Banwell B, Bergqvist AGC, et al. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2019;6:e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. [DOI] [PubMed] [Google Scholar]
- 22.Brenton JN, Banwell BL. Therapeutic approach to the management of pediatric demyelinating disease: multiple sclerosis and acute disseminated encephalomyelitis. Neurotherapeutics 2016;13:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenhouse HC, Schwarz JM. Immunoadolescence: neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 2016;70:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedström AK, Lima Bomfim I, Barcellos L, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014;82:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedström AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler 2012;18:1334–6. [DOI] [PubMed] [Google Scholar]
- 26.Body mass index: centers for disease control and prevention, 2021. Available: https://www.cdc.gov/healthyweight/assessing/bmi/index.html
- 27.Cervenka MC, Henry BJ, Felton EA, et al. Establishing an adult epilepsy diet center: experience, efficacy and challenges. Epilepsy Behav 2016;58:61–8. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Perera R, Engelhard MM, eds. A Generic Algorithm for Sleep-Wake Cycle Detection using Unlabeled Actigraphy Data. 2019 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), 2019. [Google Scholar]
- 29.Baird JF, Cederberg KLJ, Sikes EM, et al. Physical activity and walking performance across the lifespan among adults with multiple sclerosis. Mult Scler Relat Disord 2019;35:36–41. [DOI] [PubMed] [Google Scholar]
- 30.Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011;43:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahr LS, Bock M, Liebscher D, et al. Ketogenic diet and fasting diet as nutritional approaches in multiple sclerosis (NAMS): protocol of a randomized controlled study. Trials 2020;21:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald KC, Sand IK, Senders A, et al. Conducting dietary intervention trials in people with multiple sclerosis: lessons learned and a path forward. Mult Scler Relat Disord 2020;37:101478. [DOI] [PubMed] [Google Scholar]
- 33.Lemstra M, Bird Y, Nwankwo C, et al. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence 2016;10:1547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nourbakhsh B, Revirajan N, Morris B, et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol 2021;20:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol 2019;18:1009–20. [DOI] [PubMed] [Google Scholar]
- 36.Patti F, Pappalardo A, Montanari E, et al. Interferon-Beta-1A treatment has a positive effect on quality of life of relapsing-remitting multiple sclerosis: results from a longitudinal study. J Neurol Sci 2014;337:180–5. [DOI] [PubMed] [Google Scholar]
- 37.Patti F, Amato MP, Trojano M, et al. Quality of life, depression and fatigue in mildly disabled patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: 3-year results from the COGIMUS (cognitive impairment in multiple sclerosis) study. Mult Scler 2011;17:991–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Any data not published within the article are available, and the anonymised data will be shared by request from any qualified investigator.


