Abstract
Obesity is a risk factor for various types of cancer. Leptin, an adipocyte-derived hormone, may stimulate the proliferation of gastric cancer cells. However, the effect of leptin and underlying mechanism in gastric cancer remain unclear. In the present study, the role of leptin in gastric cancer was evaluated. The effect of leptin on the JAK-STAT and MEK signaling pathways was investigated in gastric cancer cells using wound-healing and cell invasion assays, immunoblotting and inhibition studies. Cancer-initiating cells derived from gastric cancer cells were used to investigate the effect of leptin on the maintenance of stemness and epithelial-mesenchymal transition (EMT) by immunoblotting. Clinicopathological characteristics including the serum leptin level and overall survival (OS) were analyzed in patients with (n=23) and without (n=23) obesity. Leptin induced the migration and invasion of gastric cancer cells by activating AKT and ERK and upregulating vascular endothelial growth factor (VEGF). Leptin increased the mRNA and protein levels of markers of stemness (CD44) and the EMT (Snail and N-cadherin). Pharmacological inhibitors of the JAK-STAT and MEK signaling pathways decreased leptin-induced migration and invasion, and the expression of VEGF. Obesity was associated with an elevated leptin level and body mass index was positively correlated with the leptin level (P=0.001 for both). The 5-year OS rate was not significantly different between the two groups (P=0.098). Leptin stimulates the migration and invasion of gastric cancer cells by activating the JAK-STAT and MEK pathways, and contributes to the maintenance of cancer stemness and metastatic potential. The present findings support an adverse effect of obesity in gastric cancer. Consequently, targeting of leptin-associated signaling pathways may have therapeutic potential for gastric cancer.
Keywords: gastric cancer, leptin, cell migration, cell invasion, cancer stemness
Introduction
Obesity is a major public health problem reaching pandemic proportions. The prevalence of adult obesity, defined as a body mass index (BMI) >30 kg/m2, tripled between 1975 and 2016, affecting 13% of the global adult population (>650 million individuals) (1). Obesity is a risk factor for several diseases, including cardiovascular disease and various types of cancer (2).
Gastric cancer can be caused by a variety of environmental and genetic factors (3). As well as smoking and a high-salt diet, obesity has been linked to gastric cancer (4,5). BMI is used to evaluate obesity in epidemiology studies. However, patients with the same BMI may have different body compositions. Therefore, BMI is not suitable for evaluating the impact of obesity on gastric cancer. One-third of women with a normal BMI have symptoms characteristic of metabolic obesity, while 30% of obese individuals can be classified as metabolically healthy (6,7). Therefore, an improved marker of adiposity is required to investigate the relationship between obesity and gastric cancer.
Leptin, a multifunctional hormone, is a marker of hyper-adiposity. It is primarily secreted by adipocytes, but also by tissues in the stomach and placenta, for instance (8,9). The main function of leptin, as the ligand of the leptin receptor, is hypothalamic-mediated regulation of appetite, which modulates food intake and energy expenditure (10,11). Obesity-induced tumorigenesis is associated with dysregulation of the adipokine signaling pathway, which includes leptin (7). Furthermore, the leptin receptor is important in carcinogenesis (12). Leptin-leptin receptor signaling promotes multiple processes involved in cancer progression, including cell proliferation, metastasis, angiogenesis and chemoresistance (13,14).
The mechanism by which leptin promotes cancer progression is best reported in the field of breast cancer. Binding of leptin to the leptin receptor activates various signaling pathways including JAK/STAT, MAPK and PI3K pathways, which are associated with cell proliferation (15). It has been reported that leptin is an essential factor for mammary stem cell survival and maintenance (16). In addition, leptin may further activate malignant cell growth through stimulation of angiogenesis and creation of vascular permeability (17,18).
Although gastric cardia cancer is reportedly associated with obesity, in contrast to breast cancer, the relationship between gastric cancer and leptin is unclear (2). The association between leptin and gastric cancer has been evaluated in vitro (19,20). The effect of leptin on cancer cell migration and invasion, and the expression levels of angiogenesis-related growth factors were investigated in vitro. The relationship between the serum leptin level and clinical profile of patients with gastric cancer was also explored.
Materials and methods
Patient selection
A total of 46 patients diagnosed with gastric adenocarcinoma, who underwent curative gastrectomy between 2005 and 2007, were identified in the database of our institution. The present study was approved (approval no. VCM07BR016) by the Institutional Review Board of St. Vincent's Hospital, Catholic University of Korea (Seoul, Republic of Korea). Written informed consent was provided by all patients. None of the patients had undergone prior surgery, chemotherapy, or radiation therapy. After surgery, clinical follow-up data were collected at the outpatient clinic. The cohort was initially classified based on BMI: normal, BMI 18.5–25 kg/m2 and obese, BMI ≥25 kg/m2. The clinicopathological characteristics of the cohort are listed in Table I.
Table I.
Clinicopathological characteristics of the study population.
| Characteristics | Normal (n=23) | Overweight (n=23) | P-value |
|---|---|---|---|
| Age, years | 58.8±10.1 | 63±9.8 | 0.163 |
| Sex | 0.765 | ||
| Male | 14 | 13 | |
| Female | 9 | 10 | |
| Weight, kg | 53.8±6.5 | 66.44±8.4 | 0.001 |
| Body mass index (kg/m2) | 20.3±1.4 | 25.83±3.4 | 0.001 |
| Histologic type | 0.767 | ||
| Differentiated | 11 (52.4%) | 12 (48%) | |
| Undifferentiated | 10 (47.6%) | 13 (52%) | |
| Tumor-node-metastasis stage | 0.005 | ||
| I | 14 (60.9%) | 6 (26.1%) | |
| II | 7 (30.4%) | 5 (21.7%) | |
| III | 2 (8.7%) | 12 (52.2%) | |
| Serum leptin level (pg/ml) | 4,236.1±8,314.3 | 8,626.5±8,700.7 | 0.001 |
Cell culture
The gastric cancer cell lines AGS and MKN74 were obtained from the Korean Cell Line Bank and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; both from Invitrogen; Thermo Fisher Scientific, Inc.), 1% penicillin and 1% streptomycin. The cells were incubated at 37°C in an atmosphere of 95% air and 5% CO2.
Proliferation assay
Cell proliferation assays were performed using the Cell Counting Kit-8 (Sigma-Aldrich; Merch KGaA) according to the manufacturer's protocol. Each cell line (5×103; 100 µl) was seeded into a well of a 96-well plate and cultured in 100 µl of RPMI-1640 supplemented with 10% FBS. After 24 h, seeded cells were treated with 0, 40, 80 and 120 ng/ml leptin into the culture medium. The cells were incubated for 2 h at 37°C with a total of 100 µl CCK-8 reagent. Absorbance was measured for each well at a wavelength of 450 nm using an auto-microplate reader.
Wound healing assay
Cell migration was evaluated by wound healing assay. AGS and MKN74 cells (100,000 cells per well) were seeded on 24-well plates containing 500 µl of RPMI-1640 medium. The cells were washed three times with phosphate-buffered saline (PBS) and a wound was generated by removing the cells from the center of the well using a sterile pipette tip. Detached cells were removed by washing with PBS. The cells were incubated for 24 to 48 h. Cell migration was studied by treating the cells with 4–80 ng/ml leptin, alone or following 30-min pre-incubation with AG490 (50 µl) or U0126 (20 µl; both from Sigma-Aldrich; Merck KGaA). Three images of the wound were obtained using a TMS inverted light microscope connected to a Coolpix 4500 camera (Nikon Corporation). Wound closure was quantified by measuring the area (in pixels) between the edges of the wound using the measurement tool in Adobe Photoshop®, with a grid superimposed on the image as a guide. Wound width was normalized to 100% at 0 h for each condition; data are percentages of wound closure.
In vitro cell invasion assay
Invasion assay was performed using 24-well Transwell plates with 8-µm isopore membranes (BD Biosciences). The upper side of the membrane was coated with Matrigel (50 µg/well) and the membranes were air-dried for 1-h incubation at 37°C. The lower side of the membranes was coated with 5 µg of fibronectin (BD Biosciences). To assess the effect of leptin (100 ng/ml for 24 h) on cell invasion, treated or untreated gastric cancer cells (2.5×104) in 200 µl of RPMI-1640 medium supplemented with 5% charcoal-treated FBS were placed in the upper chamber. The lower chamber was filled with 700 µl of RPMI-1640 medium, with 10% FBS as a chemoattractant. The invasion chamber was incubated for 24 h at 37°C in 5% CO2. Cells on the upper surface of the membrane were removed by gentle scrubbing using a cotton swab. Membranes were fixed at room temperature in 95% ethanol and 5% acetic acid for 30 min and stained for 30 min at room temperature with 0.4% crystal violet. The number of cells on the lower surface of the membrane in five randomly selected visual fields (magnification, ×400) was counted using a bright-field light microscope. Assays were performed in triplicate.
Cell line maintenance and isolation of cancer-initiating cells (CICs)
The cell lines were authenticated using the GeneMarker 10 Kit (Promega Corporation) and routinely screened for mycoplasma infection by PCR. A total of 24 h before treatment, the medium was exchanged for medium supplemented with 5% charcoal-treated PBS. CICs were isolated from AGS and MKN74 cells using stem-cell selection medium [Dulbecco's modified Eagle's medium (DMEM)/F12 medium; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with epidermal growth factor (20 ng/ml), fibroblast growth factor-2 (10 ng/ml), bovine serum albumin (0.4%) and insulin (5 µg/ml) without FBS under non-attachment conditions (Corning, Inc.). After 6 days, spheroids (40–70 µm in diameter) containing CICs were collected.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from gastric cancer cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was quantified by measuring the absorption at 260 nm and stored at −80°C. PrimeScript RT kit (Takara Biotechnology Co., Ltd.) and SYBR Select Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) were used for reverse transcription and qPCR. Briefly, RT-qPCR was performed under the following conditions: 30 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 1 min, followed by 10 min normalization to the β-actin (forward, 5′-CACCATTGGCAATGAGCGGTTC-3′ and reverse, 5′-AGGTCTTTGCGGATGTCCACGT-3′) mRNA level. The mRNA levels of Snail (forward, 5′-TGCCCTCAAGATGCACATCCGA-3′ and reverse, 5′-GGGACAGGAGAAGGGCTTCTC-3′), N-cadherin (forward, 5′-CCTCCAGAGTTTACTGCCATGAC-3′ and reverse, 5′-GTAGGATCTCCGCCACTGATTC-3′) and CD44 (forward, 5′-CCAGAAGGAACAGTGGTTTGGC-3′ and reverse, 5′-ACTGTCCTCTGGGCTTGGTGTT-3′) were measured by RT-qPCR. The quantitative analysis was performed using the 2−ΔΔcq method (21).
Enzyme-linked immunosorbent assay (ELISA)
Gastric cancer cells were seeded into plates at 3×106 cells/well with 4 ml of RPMI-1640 medium supplemented with 10% FBS. The cells were treated with 100 ng of leptin or 100 ng of leptin + AG490 (50 µmol/ml) for 24, 48, or 72 h. Using a VEGF-C (H) ELISA kit (cat. no. BMS297-2; Thermo Fisher Scientific, Inc.), performed according to the manufacturer's protocol, vascular endothelial growth factor (VEGF) was quantified in the supernatants of gastric cancer cells. A total of 200 µl of supernatant were added to each well. The sensitivity of the ELISA was 7.8 pg/ml. Absorbance was determined at 450 nm. Each test was performed in triplicate.
Western blot analysis
After leptin or control treatment, cells were harvested in cold PBS and the pellet was resuspended in lysis buffer [20 mM Tris-HCl (pH 7.4), 137 mM NaCl, 2 mM EDTA, 1% Triton X-100 and 10% glycerol] for 20 min at 4°C. The supernatant was then collected. Protein concentrations were measured in duplicate by Bradford assay. A total of 50–100 µg of protein was loaded in each lane, resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes. Following blocking (with 5% non-fat milk in TRIS-buffered saline with 0.1% Tween 20 at room temperature for 1 h), the membrane was incubated overnight at 4°C with primary antibodies against CD44 (1:1,000; cat. no. sc-7297), phosphor-Erk (1:1,000; cat. no. sc-7383), Erk (1:1,000; cat. no. sc-5294; all from Santa Cruz Biotechnology, Inc.), AKT (1:1,000; cat. no. ab191606), phosphor-AKT (1:1,000; cat. no. ab131443; both from Abcam), MMP-9 (1:1,000; cat. no. sc-515876; Santa Cruz Biotechnology, Inc.), SNAIL (1:1,000; cat. no. 3879; Cell Signaling Technology, Inc.), N-cadherin (1:1,000; cat. no. 13116), E-cadherin (1:500; cat. no. 14472) and β-actin (1:1,000; cat. no. A5441; all from Sigma-Aldrich; Merck KGaA). HRP-conjugated goat anti-mouse/rabbit IgG secondary antibodies (1:3,000; cat. no. 1662408; Bio-Rad Laboratories, Inc.) were subsequently applied for 1 h at room temperature. Bands were developed using the ECL Western Blot Analysis System (NEN, Western Lightning; PerkinElmer, Inc.). The relative content of each protein was detected by ImageQuant 10.1 TL software (GE Healthcare).
Statistical analysis
Data were analyzed using SPSS software (ver. 21.0; IBM Corp.). Continuous variables are expressed as the mean ± standard deviation (SD). Analysis of continuous variables was conducted by independent two-sample t-test or ANOVA with post-hoc analysis (Tukey's test). Categorical variables were compared with the chi-squared test. The relationship between two variables was analyzed using Pearson's correlation analysis. Survival curves were generated by the Kaplan-Meier method and analyzed by the log-rank test. P<0.05 was considered to indicate a statistically significant difference and all tests were two-sided unless otherwise indicated.
Results
BMI is associated with the serum leptin level in patients with gastric cancer
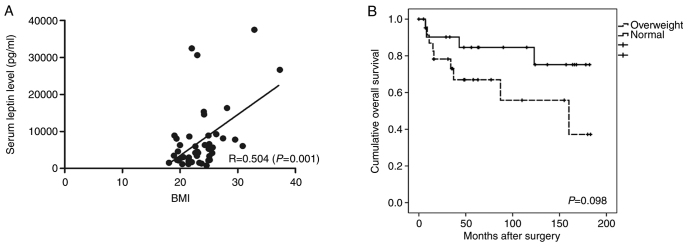
The clinicopathological data of the study population are presented in Table I. The average BMI was 20.3±1.4 and 25.83±3.4 kg/m2 in the normal and overweight groups, respectively (P=0.001). There were no significant differences in age, sex, or histologic type between the groups. In the normal group, the proportion of stage I patients was 60.9%, and in the overweight group, the proportion of stage III patients was 52.2%. There were significantly more patients with advanced disease in the overweight than normal group (P=0.005). The average serum leptin level was 4,236.1±8,314.3 and 8,626.5±8,700.7 pg/ml in the normal and overweight groups, respectively (P=0.001). There was a positive correlation between BMI and the serum leptin level (R=0.504, P=0.001; Fig. 1A). The 5-year overall survival (OS) rate was 58.4% in the overweight group and 82.6% in the normal group; the difference was not significant (Fig. 1B).
Figure 1.
(A) Scatter plot of the relationship between the serum leptin level and BMI. (B) Kaplan-Meier analysis comparing overall survival between the normal and overweight groups. BMI, body mass index.
Leptin induces gastric cancer cell proliferation, migration and invasion
To investigate the effect of leptin on gastric cancer cell migration, the leptin level in AGS and MKN74 cells was evaluated. RT-qPCR and western blot analysis revealed that leptin and leptin receptors were expressed in both cell lines (data not shown). Leptin was associated with the proliferation of gastric cancer cells (Fig. 2A and B). Particularly, the effect of leptin at 80 ng/ml in MKN74 cells was significantly different compared with the controls (P=0.034). In addition, leptin (4–80 ng/ml) significantly enhanced cell migration, with a maximal response at 80 ng/ml in AGS cells and 40 ng/ml in MKN74 cells compared with the controls (P<0.001 for both). The number of migratory AGS and MKN74 cells was 2.3-fold (P=0.01) and 1.8-fold higher, respectively, in the presence of 80 ng/ml leptin (Figs. 3A and S1A). The movement of leptin-treated cells (80 ng/ml) was inhibited by the JAK-STAT inhibitor AG490 and MEK inhibitor U0126. Moreover, leptin-induced migration was inhibited by 48% in AGS cells by 50 µM AG490 (P<0.001), and by 68% in MKN74 cells by 20 µM U0126 (P<0.001) (Figs. 3B and S1B).
Figure 2.
Effect of leptin on gastric cell proliferation, as revealed by Cell Counting Kit-8 assay. (A) AGS and MKN74 cells were treated with 0, 40, 80 and 120 ng/ml leptin for 24 h and proliferative cells were enumerated. Data were normalized to the 0 ng/ml leptin control. Using Student's t-test, 80 ng/ml of leptin in MKN74 showed significant proliferative effect compared with the control. (B) Effect of time on leptin-induced (80 ng/ml) AGS and MKN74 cell proliferation. For the MKN74 cell line, the condition at 48 h was significantly different compared with that at 24 h. *P=0.034 vs. control and **P=0.033 vs. 24 h.
Figure 3.
Effect of leptin on gastric cancer cell migration, as revealed by wound-healing assay. (A) AGS and MKN74 cells were treated with 0, 4, 40 and 80 ng/ml leptin for 24 h and migratory cells were enumerated (magnification, ×100). Data were normalized to the 0 ng/ml leptin control. *P<0.05 vs. 0 ng/ml control. (B) Effect of the JAK inhibitor AG490 and MEK inhibitor U0126 on leptin-induced (80 ng/ml, 12 h) AGS cell migration. *P<0.05 vs. 80 ng/ml control.
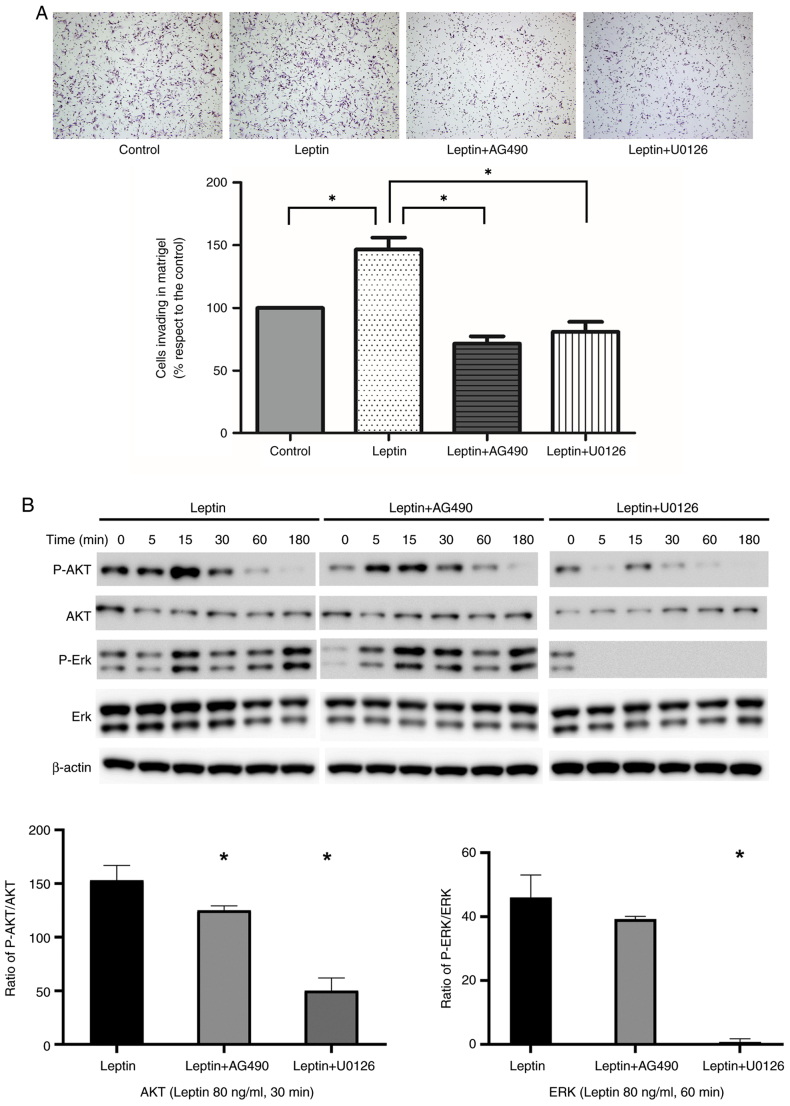
In a Matrigel Boyden chamber assay, leptin promoted AGS cell invasion of Matrigel (P<0.001). Invasion by leptin-treated cells was inhibited by AG490 and U0126 (P<0.001; Fig. 4A). Next, AGS cells were pre-incubated with JAK and MEK inhibitors. Western blotting and quantitative analysis suggested that the effect of leptin-induced phosphorylation of AKT and ERK proteins in AGS cells was significantly suppressed by AG491 or U0126. β-actin was used as the internal control (Fig. 4B). Similarly, the MMP-9 protein levels were slightly upregulated by leptin in AGS cell line and this effect was suppressed by AG490 or U0126 (Fig. S2).
Figure 4.
Effect of leptin on gastric cancer cell invasion in vitro. (A) Effect of leptin on AGS cell invasion, and of AG490 or U0126 on leptin-induced cell invasion. *P<0.05 vs. control. (B) Western blotting showed that the effect of leptin-induced phosphorylation of AKT and ERK was abolished by AG491 or U0126 treatment in AGS cells. Quantitative analysis of AKT and ERK phosphorylation by determining the ratio between total protein and phosphorylation levels from three separate experiments. Statistical analysis was performed using ANOVA and post-hoc analysis (Tukey's test). β-actin protein was used as the internal control. Data represent the mean ± SD from triplicate samples in three individual experiments. *P<0.05 vs. leptin.
Leptin promotes VEGF-C secretion by AGS and MKN74 cells
AGS and MKN74 cells did not express VEGF-C in the presence or absence of leptin (data not shown). The effect of leptin on the VEGF-C level in AGS cell supernatant was analyzed. Initially, both AGS and MKN74 cells expressed low levels of VEGF-C in response to leptin. Significant time-dependent effects were observed in AGS and MKN cells (P<0.001). ELISA revealed that leptin increased the VEGF-C levels in AGS and MKN74 cell supernatants compared with the control. Moreover, AG490 significantly decreased the VEGF-C levels in AGS and MKN74 cell supernatants compared with the leptin group (P=0.008; Fig. 5A and B).
Figure 5.
ELISA of the effect of leptin on the VEGF-C level in (A) AGS and (B) MKN74 cell supernatants. Leptin markedly increased the VEGR-C level in AGS and MKN74 cell supernatant compared with the control group. AG490 significantly decreased the VEGF-C level in AGS and MKN74 cell supernatants compared with the leptin group. *P<0.05 vs. control. VEGF, vascular endothelial growth factor.
Leptin induces stemness and epithelial-mesenchymal transition (EMT)
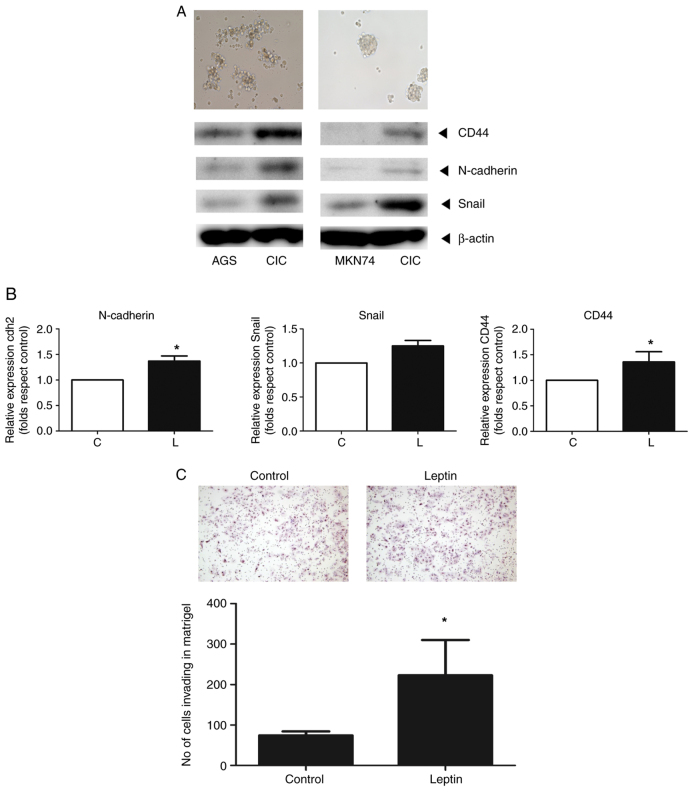
CICs were isolated from AGS and MKN74 gastric cells using stem cell selection medium. Western blotting showed that the protein levels of markers for CD44, N-cadherin and Snail increased in both cell types (Fig. 6A). Leptin increased the mRNA levels of markers of stemness (CD44) and EMT (Snail and N-cadherin) one- to two-fold compared with the controls, as determined by RT-qPCR (Fig. 6B). Finally, leptin increased the invasion of CICs 2.1-fold (Fig. 6C).
Figure 6.
(A) AGS spheroid formation (upper and left panel) and MKN74 spheroid formation (upper and right panel) were assessed in Matrigel Boyden chamber assays and the expression of stemness (CD44) and EMT markers (N-cadherin and snail) were measured in CICs and the gastric cancer cell lines (AGS and MKN74) by western analysis. (B) Effect of leptin on the RNA and protein levels of stemness (CD44) and EMT (N-cadherin, Snail) markers in CICs treated with leptin (80 ng/ml for 12 h), as revealed by reverse transcription-quantitative PCR and western blotting. (C) Effect of leptin on AGS spheroid formation and CIC invasion using Matrigel assay. *P<0.05 vs. control. CICs, cancer-initiating cells.
Discussion
According to a nationwide survey by the Korean Gastric Cancer Association, the rate of obese patients with gastric cancer increased from 28.7% in 2014 to 35.3% in 2019 (22). In the present study, leptin increased the invasion of AGS and MKN74 cells and activated the STAT and MEK signaling pathways, which are involved in cell growth and survival. Also, exogenous leptin modulates growth factor expression and cell migration. In CICs isolated from gastric cancer cells, leptin maintains stemness and EMT expression, and promotes spheroid formation and invasion.
In the gastrointestinal system, leptin receptors are expressed in the stomach and bowels, but leptin is produced only by parietal cells and chief cells in the stomach (9,23). Therefore, the stomach is unique in constitutively expressing both leptin and leptin receptors, and is capable of transducing autocrine leptin signals. Leptin is reportedly associated with more aggressive gastric cancers, which are associated with poorer survival (24,25). This suggests that obesity may be associated with the progression of gastric cancer. The serum leptin level, which is affected by adipose tissue mass, is elevated in obese patients and associated with an increased risk of several types of cancer (26–30). In the present study, the overweight group had a significantly higher serum leptin level. In a study of 63 patients with gastric cancer, the serum leptin level was higher in the high-than low-BMI group (20). In addition, the overweight group was more likely to have advanced disease, and had poorer OS. Therefore, obesity is associated with the prognosis of gastric cancer in a manner involving the serum leptin level.
Leptin and leptin receptor (Ob-R) were predominantly expressed in chief and parietal cells, but not in the surface epithelium in normal gastric mucosa adjacent to cancer tissue (31,32). Another study reported that the expression level of both leptin and Ob-R tended to increase as the depth of tumor invasion or TNM stage progressed (24). According to our previous study, the expression of leptin was significantly associated with TNM stage (33). Moreover, nodal and distant metastasis was frequently detected in leptin-strong and Ob-R positive tumors as compared with leptin-weak and Ob-R negative tumors (24,25). The present results were consistent with previous studies and suggested that leptin and Ob-R may function as an autocrine growth factor during the development and progression of gastric cancer. In the present study, the OS in the overweight group tended to be poorer than in the normal group. Therefore, there is a possibility that serum leptin level may have relationship with OS. However, since the sample size of the present study was small, it was not possible to perform subgroup analysis by adjusting the TNM stage, which is a limitation to the present study. Further larger cohort research with adjusting for TNM stage should be conducted in the future.
Metastasis is a hallmark of malignancy and a leading cause of cancer-related death (34). Cell migration and invasion are crucial for tumor progression and metastasis. These processes involve detachment of tumor cells from the primary site, migration thereof through primary tissue and intravasation into the blood or lymphatic systems, and invasion and proliferation in distant organs (35). In the present study, leptin induced cancer cell migration and invasion. Indeed, leptin reportedly enhances gastric cancer cell migration by increasing intercellular adhesion molecule-1 (ICAM-1) expression (36). In addition, leptin may exert effects via the JAK-STAT and MEK pathways, likely by phosphorylating AKT and ERK. The present study revealed that leptin may activate the JAK-STAT and MEK pathways, which is consistent with the previous findings that several signaling pathways activated by leptin have been identified in gastric cancer, that is, the JAK-STAT, ERK1/2 and MEK pathways, as well as the PI3K/AKT pathways. A previous study revealed that leptin upregulated ICAM-1 expression in eosinophils by the combined activation of the MAPK and JAK pathways (36–39). However, their changes were not examined in the present study. Further experiments will be conducted to test these possibilities. In vitro, leptin induced gastric cancer cell migration via the Rho/ROCK pathway, which regulates actin-myosin assembly and generates a traction force (36,40).
The AKT signaling pathway seems to be implicated in gastric cancer cell invasion. Membrane type 1-matrix metalloproteinase (MT1-MMP) is important for tumor invasion and MT1-MMP overexpression may be associated with gastric cancer cell invasion (41,42). The present study indicated that leptin affected the expression of MMP-9 protein and this effect was likely to have association with the JAK-STAT and MEK signaling pathways. Consistent with these results, previous studies reported that leptin triggered the generation and surface localization of MT1-MMP, likely in a manner involving the AKT pathway (43,44). Similarly, leptin binding to the Ob-R activates several signaling pathways. These findings suggested that leptin promotes cancer migration and invasion by activating signaling pathways; blockade of its action may have therapeutic potential.
Lymphatic metastasis of gastric cancer is an important prognostic factor, and is included in the staging system for gastric cancer. Lymphangiogenesis mediates cancer cell entry into the lymphatic system. VEGF-C is important in lymphangiogenesis, as is its receptor, which is primarily expressed on the lymphatic vessel endothelium (45). VEGF-C is associated with lymphatic metastasis in patients with early gastric cancer (46,47). The serum VEGF-C level is associated with poor OS and disease-specific survival, and thus has prognostic potential (45). In the present study, leptin induced VEGF-C secretion by gastric cancer cells via the JAK-STAT pathway in a time-dependent manner. Ramucirumab, a monoclonal antibody directed against VEGF receptor 2, is recommended as second-line palliative chemotherapy by the Korean Gastric Cancer Association (48). By considering the leptin-VEGF-C relationship, antiangiogenetic agents could be extended to adjuvant settings.
The EMT is an important step in metastasis (49,50). The EMT is the process by which epithelial cells lose their characteristic polarity and cell-cell adhesion ability, and acquire migratory and invasive characteristics (51). E-cadherin is an important component of adherent junctions and stabilizes cell-cell connections (52). N-cadherin is related to cell motility and migration. During the EMT, the E- to N-cadherin switch is a hallmark of cancer progression (53). SNAIL is a major EMT transcription factor, and is reportedly related to the downregulation of E-cadherin in colon cancer (54). CICs, a subgroup of cancer cells expressing EMT and stemness markers within tumors, likely adapt and respond to environmental stimuli (50). As in breast and ovarian cancers, it was found that leptin induces the expression of EMT markers (N-cadherin and Snail) and stemness markers (CD44) in CICs isolated from gastric cancer (50,55). In addition, leptin promoted spheroid formation and invasion of Matrigel by CICs. Therefore, leptin promotes the maintenance of an aggressive phenotype of gastric cancer. However, the flow cytometric assay was not examined in the present study to prove that leptin could maintain the stemness of gastric cancer cells, and to detect the proportion of CD44+ cells or CD133+ cells. Further experiments to elucidate more markers will be required to confirm the results of the present study.
There are several limitations to the present study. The expression of leptin or its receptor in gastric cancer and corresponding normal tissues, suggesting that leptin is produced locally and functions in a paracrine or autocrine manner, was not evaluated. In addition, further research to improve the reliability of the results that leptin affects the JAK-STAT and MEK signaling pathways is required in tissue or animals. Second, a retrospective cohort study was conducted to evaluate the prognostic significance of leptin and obesity, but certain clinicopathological characteristics were imbalanced between the groups. Third, the present study used a single-center design with small sample size and was therefore prone to inherent bias. Although a relationship was found between the serum leptin level and cancer progression, further research with a larger cohort is required to validate the association. Finally, the inhibition study was not conducted using CICs and further research about the leptin-associated signaling pathways in CICs is required.
In conclusion, a relationship between the serum leptin level and obesity and gastric cancer progression was identified, supporting an adverse effect of obesity in gastric cancer. It was also demonstrated that leptin promotes gastric cancer progression via the STAT and MEK signaling pathways, by inducing the migration and invasion of gastric cancer cells. Therefore, targeting leptin-associated signaling pathways could have therapeutic potential for gastric cancer.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by a research grant from St. Vincent's Hospital, Catholic University of Korea (grant no. SVHR201707).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KBP conducted investigation and wrote the original draft. EYK conceptualized the present study and conducted investigation. HC curated data, wrote, reviewed and edited the manuscript. KBP and KHJ confirm the authenticity of all the raw data. DJY conducted investigation. KHJ conceptualized and supervised the study, performed investigation, wrote, reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. VCM07BR016) by the Institutional Review Board of the College of Medicine, Catholic University of Korea (Seoul, Republic of Korea) and complied with its ethical standards. Written informed consent was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC), corp-author Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi M, Inoue M, Sawada N, Saito E, Abe SK, Hidaka A, Iwasaki M, Yamaji T, Shimazu T, Shibuya K, et al. Effect of body-mass index on the risk of gastric cancer: A population-based cohort study in A Japanese population. Cancer Epidemiol. 2019;63:101622. doi: 10.1016/j.canep.2019.101622. [DOI] [PubMed] [Google Scholar]
- 5.Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609–617. doi: 10.1093/annonc/mds244. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein MM, Brown KA, Iyengar NM. Targeting obesity-related dysfunction in hormonally driven cancers. Br J Cancer. 2021;125:495–509. doi: 10.1038/s41416-021-01393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: Leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 9.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 10.Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. Responses of leptin to short-term fasting and refeeding in humans: A link with ketogenesis but not ketones themselves. Diabetes. 1996;45:1511–1515. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 11.Surmacz E. Obesity hormone leptin: A new target in breast cancer? Breast Cancer Res. 2007;9:301. doi: 10.1186/bcr1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 13.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–2790. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato J, Jr, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591–602. doi: 10.1590/S0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- 16.Crean-Tate KK, Reizes O. Leptin regulation of cancer stem cells in breast and gynecologic cancer. Endocrinology. 2018;159:3069–3080. doi: 10.1210/en.2018-00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone I, Catalano S, Gelsomino L, Marsico S, Giordano C, Panza S, Bonofiglio D, Bossi G, Covington KR, Fuqua SA, Andò S. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012;72:1416–1427. doi: 10.1158/0008-5472.CAN-11-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KN, Choi HS, Yang SY, Park HK, Lee YY, Lee OY, Yoon BC, Hahm JS, Paik SS. The role of leptin in gastric cancer: Clinicopathologic features and molecular mechanisms. Biochem Biophys Res Commun. 2014;446:822–829. doi: 10.1016/j.bbrc.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Tas F, Karabulut S, Erturk K, Duranyildiz D. Clinical significance of serum leptin level in patients with gastric cancer. Eur Cytokine Netw. 2018;29:52–58. doi: 10.1684/ecn.2018.0408. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Information Committee of the Korean Gastric Cancer Association, corp-author. Korean gastric cancer association-led nationwide survey on surgically treated gastric cancers in 2019. J Gastric Cancer. 2021;21:221–235. doi: 10.5230/jgc.2021.21.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mix H, Widjaja A, Jandl O, Cornberg M, Kaul A, Göke M, Beil W, Kuske M, Brabant G, Manns MP, Wagner S. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47:481–486. doi: 10.1136/gut.47.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa M, Kitayama J, Nagawa H. Expression pattern of leptin and leptin receptor (OB-R) in human gastric cancer. World J Gastroenterol. 2006;12:5517–5522. doi: 10.3748/wjg.v12.i34.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Huang K, Zhu Z, Chen S, Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–1321. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 26.Stattin P, Lukanova A, Biessy C, Söderberg S, Palmqvist R, Kaaks R, Olsson T, Jellum E. Obesity and colon cancer: Does leptin provide a link? Int J Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 27.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 28.Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: A systematic review and meta-analysis. Obes Res Clin Pract. 2019;13:329–339. doi: 10.1016/j.orcp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Thomas T, Burguera B, Melton LJ, III, Atkinson EJ, O'Fallon WM, Riggs BL, Khosla S. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism. 2000;49:1278–1284. doi: 10.1053/meta.2000.9519. [DOI] [PubMed] [Google Scholar]
- 30.Mantzoros CS. The role of leptin in human obesity and disease: A review of current evidence. Ann Intern Med. 1999;130:671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 31.Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324–329. doi: 10.1136/gut.49.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider R, Bornstein SR, Chrousos GP, Boxberger S, Ehninger G, Breidert M. Leptin mediates a proliferative response in human gastric mucosa cells with functional receptor. Horm Metab Res. 2001;33:1–6. doi: 10.1055/s-2001-12617. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Jung H, Jun KH, Kim SK, Chin HM, Jung JH, Kim W, Jeon HM, Park CH, Park SM, et al. Correlation between the serum leptin level and the expression of leptin in stomach cancer patients. J Korean Gastric Cancer Assoc. 2008;8:176–181. doi: 10.5230/jkgca.2008.8.4.176. [DOI] [Google Scholar]
- 34.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Z, Fu S, Xu X, Yang Y, Du L, Li W, Kan S, Li Z, Zhang X, Wang L, et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer. 2014;110:1801–1810. doi: 10.1038/bjc.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji BC, Hsiao YP, Tsai CH, Chang SJ, Hsu SC, Liu HC, Huang YP, Lien JC, Chung JG. Cantharidin impairs cell migration and invasion of A375.S2 human melanoma cells by suppressing MMP-2 and −9 through PI3K/NF-κB signaling pathways. Anticancer Res. 2015;35:729–738. [PubMed] [Google Scholar]
- 38.Mavrommati I, Cisse O, Falasca M, Maffucci T. Novel roles for class II Phosphoinositide 3-Kinase C2β in signalling pathways involved in prostate cancer cell invasion. Sci Rep. 2016;6:23277. doi: 10.1038/srep23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: Implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37:2337–2348. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- 40.Ghasemi A, Saeidi J, Azimi-Nejad M, Hashemy SI. Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol (Dordr) 2019;42:243–260. doi: 10.1007/s13402-019-00428-0. [DOI] [PubMed] [Google Scholar]
- 41.Mimori K, Fukagawa T, Kosaka Y, Ishikawa K, Iwatsuki M, Yokobori T, Hirasaki S, Takatsuno Y, Sakashita H, Ishii H, et al. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bone marrow in gastric cancer cases. Ann Surg Oncol. 2008;15:2934–2942. doi: 10.1245/s10434-008-9916-z. [DOI] [PubMed] [Google Scholar]
- 42.Poincloux R, Lizárraga F, Chavrier P. Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 43.Dong Z, Xu X, Du L, Yang Y, Cheng H, Zhang X, Li Z, Wang L, Li J, Liu H, et al. Leptin-mediated regulation of MT1-MMP localization is KIF1B dependent and enhances gastric cancer cell invasion. Carcinogenesis. 2013;34:974–983. doi: 10.1093/carcin/bgt028. [DOI] [PubMed] [Google Scholar]
- 44.Jin G, Peng L, Zhang J, Qu L, Shou C. Cancer and embryo expression protein 65 promotes cancer cell growth and metastasis. Oncol Lett. 2015;9:1772–1778. doi: 10.3892/ol.2015.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrillo A, Laterza MM, Tirino G, Pompella L, Pappalardo A, Ventriglia J, Savastano B, Auricchio A, Orditura M, Ciardiello F, et al. Increased circulating levels of vascular endothelial growth factor C can predict outcome in resectable gastric cancer patients. J Gastrointest Oncol. 2019;10:314–323. doi: 10.21037/jgo.2018.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onogawa S, Kitadai Y, Amioka T, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Kuwai T, Tanaka S, Chayama K. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in early gastric carcinoma: Correlation with clinicopathological parameters. Cancer Lett. 2005;226:85–90. doi: 10.1016/j.canlet.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Arigami T, Natsugoe S, Uenosono Y, Yanagita S, Ehi K, Arima H, Mataki Y, Nakajo A, Ishigami S, Aikou T. Vascular endothelial growth factor-C and -D expression correlates with lymph node micrometastasis in pN0 early gastric cancer. J Surg Oncol. 2009;99:148–153. doi: 10.1002/jso.21228. [DOI] [PubMed] [Google Scholar]
- 48.Guideline Committee of the Korean Gastric Cancer Association (KGCA), corp-author Development Working Group & Review Panel: Korean practice guideline for gastric cancer 2018: An evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Kato S, Abarzua-Catalan L, Trigo C, Delpiano A, Sanhueza C, García K, Ibañez C, Hormazábal K, Diaz D, Brañes J, et al. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: An explanation for poor outcomes in obese women. Oncotarget. 2015;6:21100–21119. doi: 10.18632/oncotarget.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 54.Peña C, García JM, Silva J, García V, Rodríguez R, Alonso I, Millán I, Salas C, de Herreros AG, Muñoz A, Bonilla F. E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: Clinicopathological correlations. Hum Mol Genet. 2005;14:3361–3370. doi: 10.1093/hmg/ddi366. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, et al. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.