Abstract
Human epidermal growth factor receptor 2 (HER2) overexpression has been reported in various types of cancer, including breast, gastric, lung, colorectal and pancreatic cancer. A humanized anti-HER2 monoclonal antibody (mAb), trastuzumab, has been shown to improve survival of patients in HER2-positive breast and gastric cancer. An anti-HER2 mAb, H2Mab-77 (mouse IgG1, kappa) was previously developed. In the present study, a defucosylated version of mouse-dog chimeric anti-HER2 mAb (H77Bf) was generated. H77Bf possesses a high binding-affinity [a dissociation constant (KD): 7.5×10−10 M, as determined by flow cytometric analysis] for dog HER2-overexpressed CHO-K1 (CHO/dHER2) cells. H77Bf highly exerted antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) for CHO/dHER2 cells by canine mononuclear cells and complement, respectively. Moreover, administration of H77Bf significantly suppressed the development of CHO/dHER2 ×enograft tumor in mice compared with the control dog IgG. H77Bf also possesses a high binding-affinity (KD: 7.2×10−10 M) for a canine mammary gland tumor cell line (SNP), and showed high ADCC and CDC activities for SNP cells. Intraperitoneal administration of H77Bf in mouse xenograft models of SNP significantly suppressed the development of SNP xenograft tumors compared with the control dog IgG. These results indicated that H77Bf exerts antitumor activities against dHER2-positive canine cancers, and could be valuable treatment regimen for canine cancers.
Keywords: HER2, monoclonal antibody, ADCC, CDC, antitumor activity
Introduction
Human epidermal growth factor receptor 2 (HER2, also known as ERBB2) is a cell surface type I transmembrane glycoprotein that is highly expressed on various solid tumors and enable a broad repertoire of oncogenic signaling upon homo- and heterodimerization with HER/ERBB families. HER2 overexpression is observed in ~20-30% of human breast cancers, which are associated with poor prognosis and higher rates of recurrence (1). In 1998, trastuzumab became the first monoclonal antibody (mAb), which U.S. Food and Drug Administration (FDA) approved for treatment of HER2-positive breast cancers (2) and later in HER2-positive gastric cancers (3).
Trastuzumab was initially considered to inhibit HER2 signaling (4,5). Numerous studies have confirmed the inhibition of downstream phosphatidylinositol-3 kinase (PI3K)/Akt pathway, and the suppression of tumor cell proliferation (6–8). Concurrently, the HER2-selective tyrosine kinase inhibitors (TKIs) such as lapatinib, neratinib and tucatinib, were developed and exhibited a superior activity to suppress HER2 signaling (6,9,10). However, regardless of a weaker inhibitory activity to HER2 signaling, trastuzumab has exhibited greater clinical efficacy than TKIs. Trastuzumab has been the most effective therapy for HER2-positive breast cancer for more than 20 years (11). Clinically, this difference in efficacy suggests the involvement of immunologic engagement of antibody therapy, hardly observed in TKIs (12).
Trastuzumab possesses an Fc domain which allows for the direct engagement with Fcγ receptors (FcγRs) on various types of immune cells. The FcγR engagement allows for phagocytic engulfment of antibody-bound pathogens or cells, termed antibody-dependent cellular phagocytosis. The FcγR-mediated signaling activates dendritic cells, macrophages and neutrophils, which can alter adaptive immune responses through antigen presentation, cytokine production and chemotaxis. Furthermore, the FcγR engagement can stimulate natural killer (NK) cells which attack and lyse the target cells, termed antibody-dependent cellular cytotoxicity (ADCC) (13). Margetuximab contains several optimization mutations and exhibits improved FcγRIIIA engagement and ADCC activity compared with the parental Ab trastuzumab (14). Margetuximab was recently approved by FDA in heavily pretreated patients based on modest but significant improvement in progression-free survival (15,16). Moreover, the Fc domain can trigger the activation of complement family, and exert the complement-dependent cytotoxicity (CDC) (17,18).
With the increase in lifespan of both humans and dogs, the increased cancer incidence has been observed as well. Mammary neoplasia is the most frequently observed in dog tumors (19). Among them, ~50% are malignant. These spontaneous canine mammary tumors (CMT) share biological and histological characteristics with human breast carcinoma (20). Compared with murine model, CMT models have advantages as a naturally occurring models of human cancers (21). In canine tumors, the overexpression of dog HER2 (dHER2) has been reported not only in mammary carcinoma (22) but also osteosarcoma (23), bladder carcinoma (24), and anal sac gland carcinoma (25). Furthermore, in accordance with the American Society of Clinical Oncology and the College of American Pathologists guidelines for HER2 immunostaining, dHER2 has been revealed to be overexpressed in 32% of CMT (26), 81% of intestinal tumor, 42% of rectal carcinomas, and 28% of cutaneous squamous cell carcinomas (27). Additionally, a HER2-expressed recombinant Listeria vaccine administration resulted in the induction of anti-dHER2 immunity, which resulted in the reduced incidences of metastasis, and prolonged survival in a phase I study for canine osteosarcoma (28). These clinical outcomes promoted the evaluation of anti-dHER2 mAbs as a therapeutic modality for canine cancers.
Previously, an anti-HER2 mAb, H2Mab-77 (mouse IgG1, kappa), was developed (29). In the present study, a defucosylated mouse-dog chimeric anti-HER2 mAb (H77Bf) was produced. The present study aimed to investigate the ability of H77Bf to induce ADCC, CDC and antitumor efficacy in dHER2-expressing cells.
Materials and methods
Cell lines
A canine mammary gland tumor cell line, SNP, was purchased from the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer at Tohoku University (Miyagi, Japan) (30). CHO-K1 cells were purchased from the American Type Culture Collection. Dog HER2 (accession no. NM_001003217)-overexpressed CHO-K1 (CHO/dHER2) was established by transfection of pCAG/3×RIEDL-dHER2 into CHO-K1 cells as previously described (31). 3×RIEDL sequence represented three repeat of RIEDL amino acid sequence (32). RIEDL tag is an affinity tag that is used for the one-step membrane protein purification (32–36). CHO-K1, CHO/dHER2, and SNP were cultured in RPMI-1640 medium (Nacalai Tesque, Inc.), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin, 100 units/ml of penicillin, and 0.25 µg/ml amphotericin B (Nacalai Tesque, Inc.). The cell lines were maintained at 37°C in a humidified atmosphere under 5% CO2.
Animals
Animal experiments were performed following regulations and guidelines to minimize animal distress and suffering in the laboratory. Animal experiments for antitumor activity of H77Bf were approved (approval no. 2021-056) by the Institutional Committee for Experiments of the Institute of Microbial Chemistry (Numazu, Japan). Mice were maintained on an 11 h light/13 h dark cycle with food and water supplied ad libitum in a specific pathogen-free environment across the experimental period. Mice were monitored for weight and health every 2–5 days during the experiments. The loss of original body weight was determined to a point >25% and/or a maximum tumor size >3,000 mm3 as humane endpoints for euthanasia.
Antibodies
Anti-HER2 mAb H2Mab-77 was established as previously described (29). To generate H77B, we subcloned VH cDNA of H2Mab-77 and CH of dog IgGB into the pCAG-Ble vector (FUJIFILM Wako Pure Chemical Corporation). VL cDNA of H2Mab-77 and CL cDNA of dog kappa light chain were also subcloned into the pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation). The vector of H77B was transduced into BINDS-09 (FUT8-deficient ExpiCHO-S) cells using the ExpiCHO Expression System (Thermo Fisher Scientific, Inc.) (37–41). H77Bf was purified using Ab-Capcher (ProteNova Co., Ltd.). Dog IgG was purchased from Jackson ImmunoResearch Laboratories, Inc.
Flow cytometry
CHO-K1, CHO/dHER2, and SNP were harvested by 0.25% trypsin/1 mM ethylenediamine tetraacetic acid (EDTA; Nacalai Tesque, Inc.) treatment. After washing with blocking buffer [0.1% bovine serum albumin (BSA; Nacalai Tesque, Inc.) in phosphate-buffered saline (PBS)], cells were treated with H77Bf, or blocking buffer (control) for 30 min at 4°C. Then, cells were incubated in FITC-conjugated anti-dog IgG (cat. no. A18764; 1:1,000; Thermo Fisher Scientific, Inc.) for 30 min at 4°C. Fluorescence data were collected by the Cell Analyzer EC800 and analyzed by EC800 software ver. 1.3.6 (Sony Corp.).
Determination of binding affinity
CHO/dHER2 and SNP were suspended in serially diluted H77Bf (0.006–25 µg/ml) followed by FITC-conjugated anti-dog IgG (1:200). Fluorescence data were collected using the Cell Analyzer EC800. The dissociation constant (KD) was calculated by fitting binding isotherms to built-in one-site binding models in GraphPad Prism 8 (GraphPad Software, Inc.).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde-PBS for 10 min and quenched with 50 mM NH4Cl in PBS with 0.2 mM Ca2+ and 2 mM Mg2+. The cells were blocked with blocking buffer (PBS containing 0.2 mM Ca2+, 2 mM Mg2+ and 0.5% BSA) for 30 min and incubated with 10 µg/ml of H77Bf or blocking buffer for 1 h. The cells were further incubated with Alexa Fluor 488-conjugated anti-dog IgG (1:400; Jackson ImmunoResearch Laboratories, Inc.) and 0.3 µM of 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Inc.) for 45 min. The whole processes were performed at room temperature. Fluorescence images were acquired with a 40× objective on a BZ-X800 digital fluorescence microscope (Keyence Corporation).
ADCC of H77Bf
Canine mononuclear cells (MNCs) obtained from Yamaguchi University were resuspended in DMEM (Nacalai Tesque, Inc.) with 10% FBS and were used as effector cells (37,38,42). Target cells (CHO-K1, CHO/dHER2, and SNP) were labeled with 10 µg/ml Calcein AM (Thermo Fisher Scientific, Inc.) (31,39–41,43–53). The target cells (2×104 cells) were plated in 96-well plates and mixed with effector canine MNCs (effector/target cells ratio, 50), 100 µg/ml of H77Bf or control dog IgG. Following incubation for 4.5 h at 37°C, the Calcein release into the medium was analyzed using a microplate reader (Power Scan HT; BioTek Instruments, Inc.,) with an excitation wavelength (485 nm) and an emission wavelength (538 nm).
Cytolyticity (% lysis) was calculated as follows: % lysis=(E-S)/(M-S) ×100, where ‘E’ is the fluorescence in cultures of both effector and target cells, ‘S’ is the spontaneous fluorescence of only target cells, and ‘M’ is the maximum fluorescence following the treatment with a lysis buffer (10 mM Tris-HCl (pH 7.4), 10 mM of EDTA, and 0.5% Triton X-100).
CDC of H77Bf
Target cells (CHO-K1, CHO/dHER2, and SNP) were labeled with 10 µg/ml Calcein AM (31,39–41, 43–53). The target cells (2×104 cells) were plated in 96-well plates and mixed with rabbit complement (final dilution 1:10; Low-Tox-M Rabbit Complement; Cedarlane Laboratories,) and 100 µg/ml of control dog IgG or H77Bf. Following incubation for 4.5 h at 37°C, Calcein release into the medium was measured.
Antitumor activity of H77Bf in xenografts of CHO-K1, CHO/dHER2 and SNP cells
BALB/c nude mice (female, 5 weeks old, weighing 14–17 g) were purchased from Charles River Laboratories, Inc. CHO-K1, CHO/dHER2, or SNP cells (5×106 cells) were resuspended in DMEM and mixed with BD Matrigel Matrix Growth Factor Reduced (BD Biosciences) were subcutaneously injected into the left flank of mice.
On day 8 post-inoculation, 100 µg of H77Bf (n=8) or control dog IgG (n=8) in 100 µl PBS were intraperitoneally injected. On days 14 and 21, additional antibody inoculations were performed. Furthermore, on days 8, 14 and 21, canine MNCs were injected surrounding the tumors. The tumor volume was measured on days 7, 10, 14, 17, 21, 24 and 28 after the injection of cells. Tumor volumes were determined as previously described (31,37,39–41,50,54).
Statistical analyses
All data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was conducted with Welch's t test for ADCC, CDC, and tumor weight. ANOVA with Sidak's post hoc test were conducted for tumor volume and mouse weight. All calculations were performed using GraphPad Prism 8 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Flow cytometric analysis against CHO/dHER2 cells using H77Bf
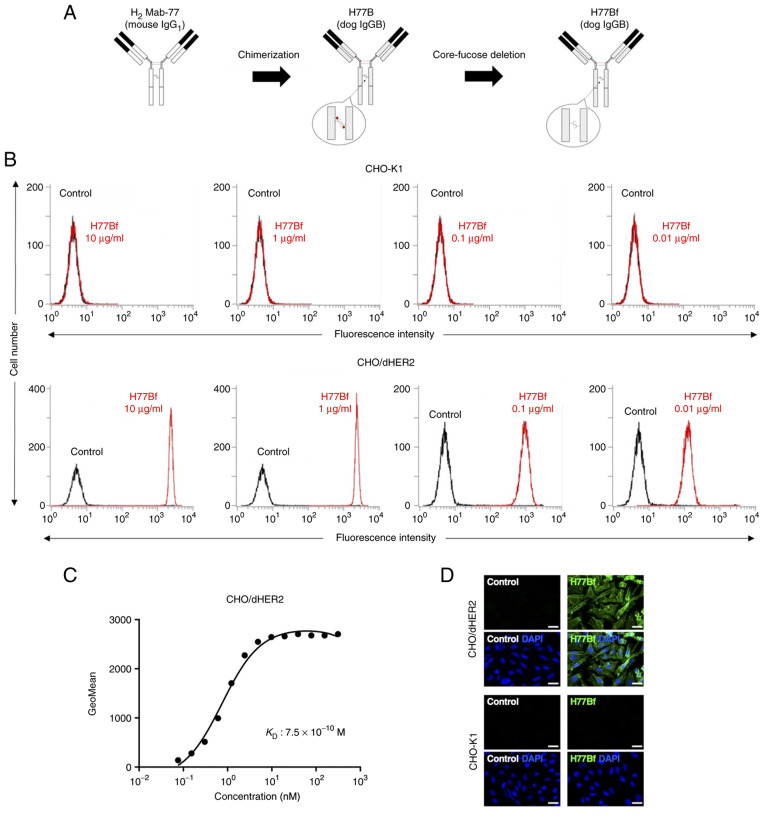
In our previous study, an anti-HER2 mAb (H2Mab-77) was established using cancer-specific mAb (CasMab) method (29). H2Mab-77 was revealed to be very useful for flow cytometry, western blotting and immunohistochemistry (IHC) (29). In the present study, a defucosylated mouse-dog chimeric anti-HER2 mAb (H77Bf) was produced by combining VH and VL of H2Mab-77 with CH and CL of dog IgG, respectively (Fig. 1A). H77Bf detected CHO/dHER2 cells dose-dependently, not parental CHO-K1 cells (Fig. 1B), indicating that H77Bf cross-reacted with dHER2.
Figure 1.
Flow cytometry using H77Bf. (A) Production of H77Bf (core-fucose-deficient dog IgGB) from H2Mab-77 (mouse IgG1). (B) CHO-K1 and CHO/dHER2 cells were treated with H77Bf or buffer control, followed by FITC-conjugated anti-dog IgG. (C) Determination of the binding affinity of H77Bf using flow cytometry for CHO/dHER2 cells. CHO/dHER2 cells were suspended in serially diluted H77Bf, followed by the addition of FITC-conjugated anti-dog IgG. Fluorescence data were analyzed using the EC800 Cell Analyzer. (D) Immunocytochemistry using H77Bf. CHO-K1 and CHO/dHER2 cells were incubated with buffer control or 10 µg/ml H77Bf for 1 h, followed by the incubation with Alexa Fluor 488-conjugated anti-dog IgG and DAPI for 45 min. Fluorescent images were acquired using a fluorescent microscope BZ-X800. Scale bars, 20 µm.
A kinetic analysis of the interactions of H77Bf with CHO/dHER2 cells was performed via flow cytometry. As revealed in Fig. 1C, the KD for the interaction of H77Bf with CHO/dHER2 cells was 7.5×10−10 M, suggesting that H77Bf exhibits high affinity for CHO/dHER2 cells.
Immunocytochemical analysis against CHO/dHER2 cells using H77Bf
It was examined whether H77Bf is applicable for immunocytochemistry. The H77Bf specificity was evaluated by using CHO/dHER2 and CHO-K1 cells. As revealed in Fig. 1D, H77Bf detected dHER2 on CHO/dHER2 cells, but not CHO-K1 cells. Buffer control showed no signal on both CHO/dHER2 and CHO-K1 cells. These results suggested that H77Bf recognizes exogenous dHER2 in immunocytochemistry.
H77Bf-mediated ADCC and CDC in CHO/dHER2 cells
It was investigated whether H77Bf was capable of mediating ADCC against CHO/dHER2 cells. H77Bf showed ADCC (31.8% cytotoxicity) against CHO/dHER2 cells more effectively than the control dog IgG (13.2% cytotoxicity; P<0.05). There was no difference between H77Bf and control dog IgG about ADCC against CHO-K1 (Fig. 2A).
Figure 2.
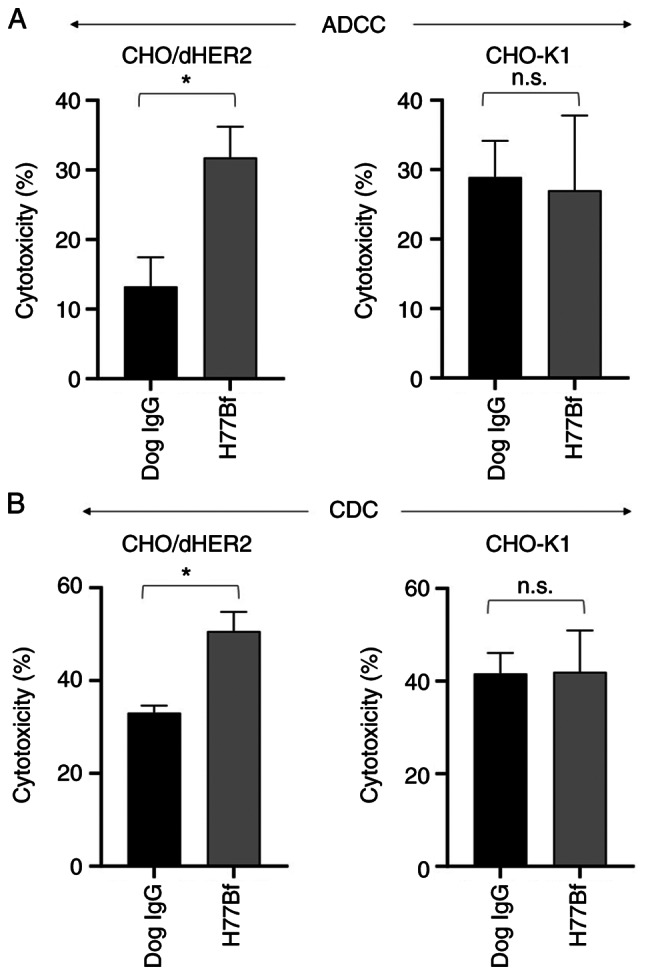
Evaluation of ADCC and CDC elicited by H77Bf. (A) ADCC elicited by H77Bf and control dog IgG targeting CHO/dHER2 and CHO-K1 cells. (B) CDC elicited by H77Bf and control dog IgG targeting CHO/dHER2 and CHO-K1 cells Values are presented as the mean ± SEM. (*P<0.05; Welch's t- test). ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; n.s., not significant.
It was then examined whether H77Bf could exert CDC against CHO/dHER2 cells. As revealed in Fig. 2B, H77Bf elicited a higher degree of CDC (50.7% cytotoxicity) in CHO/dHER2 cells compared with that elicited by control dog IgG (33.1% cytotoxicity; P<0.05). There was no difference between H77Bf and control dog IgG about CDC against CHO-K1 (Fig. 2B). These results demonstrated that H77Bf exhibited higher levels of ADCC and CDC against CHO/dHER2 cells.
Antitumor effects of H77Bf in the mouse xenografts of CHO/dHER2 cells
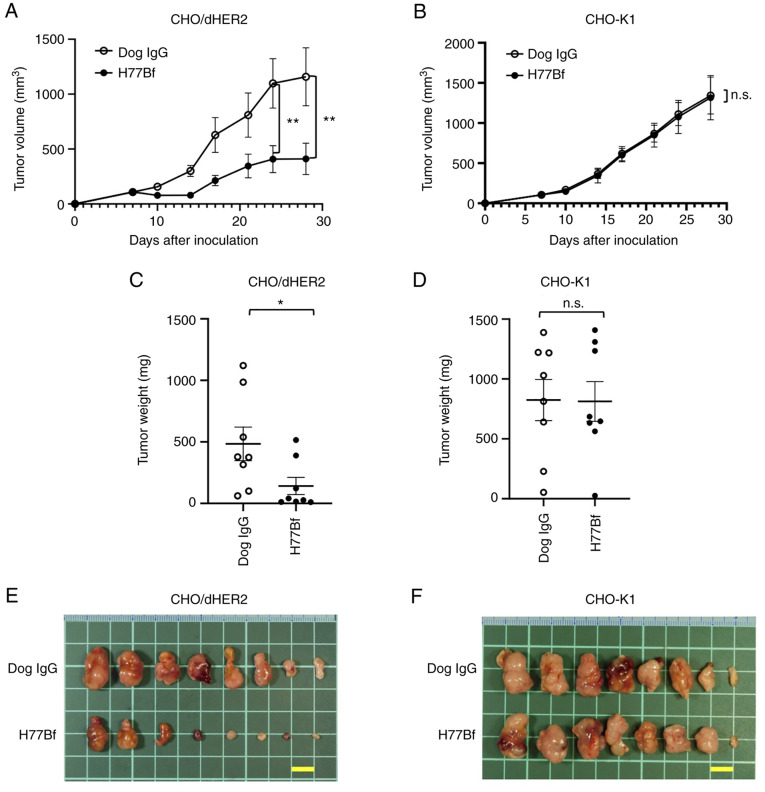
In the CHO/dHER2 ×enograft tumor, H77Bf and control dog IgG were intraperitoneally injected into mice on days 8, 14 and 21, following the CHO/dHER2 cells injection. On days 7, 10, 14, 17, 21, 24 and 28 after the injection, the tumor volume was measured. The H77Bf administration resulted in a significant reduction of tumors on days 24 (P<0.01) and 28 (P<0.01) compared with that of the control dog IgG (Fig. 3A). The H77Bf administration resulted in a 65% reduction of the volume compared with that of the control dog IgG on day 28 post-injection.
Figure 3.
Antitumor activity of H77Bf. (A and B) Evaluation of tumor volume in (A) CHO/dHER2 and (B) CHO-K1 ×enograft models. CHO/dHER2 and CHO-K1 cells (5×106 cells) were subcutaneously injected into mice. On day 8, 100 µg of H77Bf or control dog IgG were injected intraperitoneally into mice. Additional antibodies were injected on days 14 and 21. Mononuclear cells were also injected surrounding the tumors on days 8, 14 and 21. The tumor volume was measured on days 7, 10, 14, 17, 21, 24 and 28 after the injection. Values are presented as the mean ± SEM. **P<0.01 (ANOVA and Sidak's multiple comparisons test). (C and D) Tumor weight (day 28) was measured from excised xenografts of (C) CHO/dHER2 and (D) CHO-K1. Values are presented as the mean ± SEM. *P<0.05 (Welch's t-test). (E and F) Appearance of resected tumors of (E) CHO/dHER2 and (F) CHO-K1 ×enografts from the control dog IgG and H77Bf treated groups on day 28 (scale bar, 1 cm). n.s., not significant.
The weight of CHO/dHER2 tumors treated with H77Bf was significantly lower than that treated with control dog IgG (71% reduction; P<0.05; Fig. 3C). CHO/dHER2 tumors that were resected from mice on day 28 are demonstrated in Fig. 3E.
In the CHO-K1 ×enograft models, H77Bf and control dog IgG were injected intraperitoneally into mice on days 8, 14 and 21 after the injection of CHO-K1 cells. The tumor volume was measured on days 7, 10, 14, 17, 21, 24 and 28 after the injection of cells. No difference was observed between H77Bf and control dog IgG about CHO-K1 tumor volume (Fig. 3B) and CHO-K1 tumor weight (Fig. 3D). CHO-K1 tumors that were resected from mice on day 28 are demonstrated in Fig. 3F.
The body weights loss and skin disorder were not observed in CHO/dHER2 (Fig. 4A) and CHO-K1 (Fig. 4B) tumor-bearing mice. The mice on day 28 about CHO/dHER2 and CHO-K1 were shown in Fig. 4C and D, respectively.
Figure 4.
Body weights and appearance of the mice. (A and B) Body weights of mice implanted with (A) CHO/dHER2 and (B) CHO-K1 ×enografts on days 7, 10, 14, 17, 21, 24 and 28 (ANOVA and Sidak's multiple comparisons test). (C and D) Body appearance of (C) CHO/dHER2 and (D) CHO-K1-implanted mice on day 28 (scale bar, 1 cm). n.s., not significant.
Flow cytometry and immunocytochemical analysis against SNP cells using H77Bf
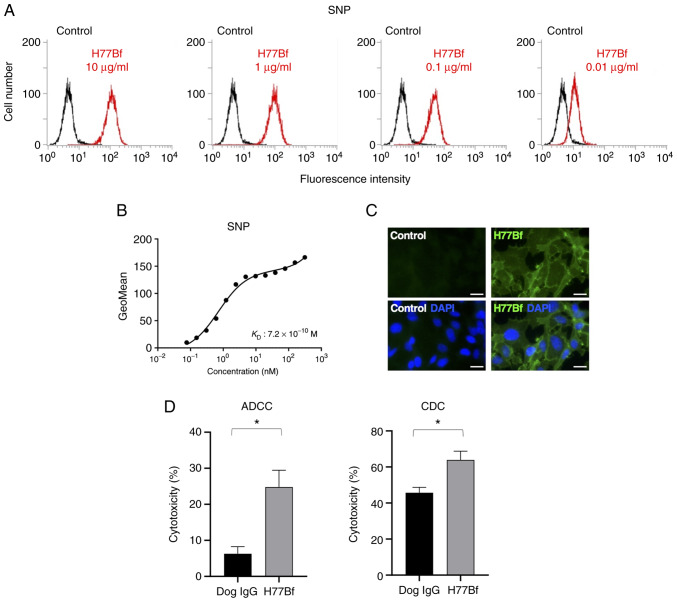
As demonstrated in Fig. 5A, H77Bf detected SNP cells dose-dependently. A kinetic analysis of the binding of H77Bf to SNP cells was performed via flow cytometry. The KD for the interaction of H77Bf with SNP cells was 7.2×10−10 M (Fig. 5B), suggesting that H77Bf shows high affinity for SNP cells.
Figure 5.
Flow cytometry, ADCC and CDC activity of H77Bf against canine mammary gland tumor cell line, SNP cells. (A) SNP cells were treated with H77Bf or buffer control, followed by FITC-conjugated anti-dog IgG. (B) Determination of the binding affinity of H77Bf for SNP cells using flow cytometry. SNP cells were suspended in 100 µl of serially diluted H77Bf, followed by the addition of FITC-conjugated anti-dog IgG. Fluorescence data were collected using the EC800 Cell Analyzer. (C) Immunocytochemistry using H77Bf. SNP cells were incubated with buffer control or 10 µg/ml H77Bf for 1 h, followed by the incubation with Alexa Fluor 488-conjugated anti-dog IgG and DAPI for 45 min. Fluorescent images were acquired using a fluorescent microscope BZ-X800 (scale bars, 20 µm). (D) ADCC and CDC elicited by H77Bf and control dog IgG targeting SNP cells. Values are presented as the mean ± SEM. *P<0.05; Welch's t-test). ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity.
Immunocytochemical analysis was then performed using H77Bf for SNP cells. As a result, H77Bf detected dHER2 on SNP cells (Fig. 5C). Buffer control detected no signal on SNP cells. These results indicated that H77Bf recognizes endogenous dHER2 in immunocytochemistry.
H77Bf-mediated ADCC and CDC in SNP cells
It was investigated whether H77Bf was capable of mediating ADCC against SNP cells. As revealed in Fig. 5D, H77Bf showed ADCC (24.8% cytotoxicity) against SNP cells more potently than did the control dog IgG (6.3% cytotoxicity; P<0.05). It was next investigated whether H77Bf exhibited CDC against SNP cells. H77Bf induced a higher degree of CDC (63.9% cytotoxicity) in SNP cells compared with that induced by control dog IgG (45.7% cytotoxicity; P<0.05) (Fig. 5D). These results demonstrated that H77Bf exhibited higher levels of ADCC and CDC against SNP cells.
Antitumor effects of H77Bf on SNP xenografts
In the SNP xenograft models, H77Bf and control dog IgG were injected intraperitoneally on days 8, 14 and 21, after the injection of SNP cells. The tumor volume was measured on days 7, 10, 14, 17, 21, 24 and 28 after the injection. The H77Bf administration resulted in a significant reduction in tumor growth on days 10 (P<0.01), 14 (P<0.01), 17 (P<0.01), 21 (P<0.01), 24 (P<0.01) and 28 (P<0.01) compared with that of the control dog IgG (Fig. 6A). The H77Bf administration resulted in a 47% reduction of tumor volume compared with that of the control dog IgG on day 28.
Figure 6.
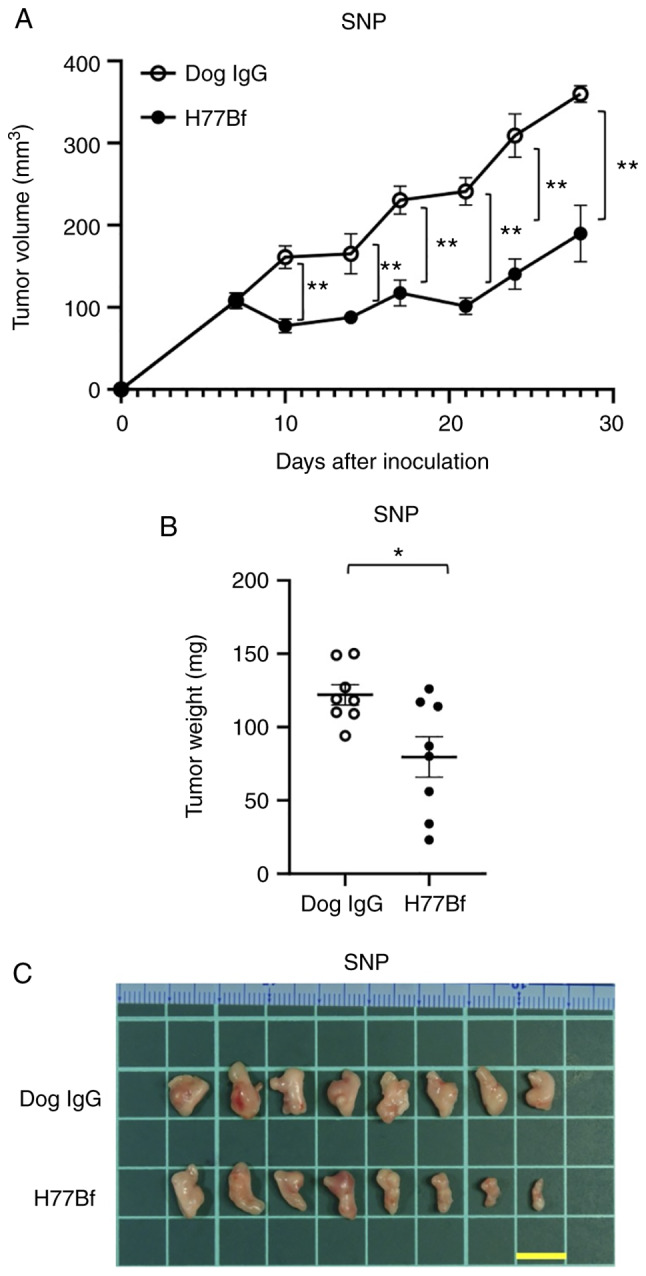
Antitumor activity of H77Bf against SNP xenograft. (A) Evaluation of tumor volume in SNP xenograft models. SNP cells (5×106 cells) were injected subcutaneously into mice. On day 8, 100 µg of H77Bf or control dog IgG in 100 µl PBS were injected intraperitoneally into mice. Additional antibodies were injected on days 14 and 21. Mononuclear cells were also injected surrounding the tumors on days 8, 14 and 21. The tumor volume was measured on days 7, 10, 14, 17, 21, 24 and 28 after the inoculation. Values are presented as the mean ± SEM. **P<0.01 (ANOVA and Sidak's multiple comparisons test). (B) Tumor weight (day 28) was measured from excised SNP xenografts. Values are presented as the mean ± SEM. *P<0.05 (Welch's t-test). (C) Appearance of resected SNP xenografts from the control dog IgG and H77Bf treated groups on day 28 (scale bar, 1 cm).
Tumors from the H77Bf-treated mice weighed significantly less than those from the control dog IgG-treated mice (35% reduction; P<0.05, Fig. 6B). Tumors that were resected from mice on day 28 are demonstrated in Fig. 6C.
The body weights loss and skin disorder were not observed in SNP tumor-bearing mice (Fig. 7A). The mice on day 28 about SNP xenograft were demonstrated in Fig. 7B.
Figure 7.
Body weights and appearance of the mice. (A) Body weights of mice implanted with SNP xenografts on days 7, 10, 14, 17, 21, 24 and 28 (ANOVA and Sidak's multiple comparisons test). (B) Body appearance of SNP-implanted mice on day 28 (scale bar, 1 cm). n.s., not significant.
Discussion
Human mAbs that exhibit cross-reactivity to dog have been investigated. It has been suggested that cetuximab (anti-EGFR) and trastuzumab (anti-HER2) can bind to certain canine cancer cell lines (55). The clinical relevance though is limited considering that those antibodies, such as trastuzumab, mostly work through ADCC (56). Furthermore, there is a problem that the humanized mAbs will induce an anti-human immune response in dogs. Therefore, the caninization of mAbs (only the complementarity determining regions are non-canine) is essential to develop antibody therapy for dog. Some caninized mAbs have received conditional approval by the United States Department of Agriculture for lymphoma (for example Blontress, targeting CD20; and Tactress, targeting CD52). However, no peer-reviewed clinical evidence of efficacy for the mAb has been published (57). In the present study, caninized mAb, H77Bf was developed from anti-HER2 mAb H2Mab-77. Among IgG subclasses (A, B, C and D) in dogs, the B and D subclasses were reported to be involved in ADCC (58). Therefore, B type dog IgG was converted and a defucosylated mAb was produced, which has been shown to exhibit more potent ADCC activity through binding to FcγRIIIa on NK cells (59). The cross-reactivity and binding affinity of H77Bf to CHO/dHER2 and SNP cells were first confirmed, and it was found that H77Bf possesses comparable high binding affinity to CHO/dHER2 (7.5×10−10 M) and SNP (7.2×10−10 M) cells, compared with human cancer A431 (2.1×10−9 M by H2Mab-77) and SK-BR-3 (7.3×10−9 M by H2Mab-77) cells, as previously reported (29). The quantitative analysis is considered to be essential to apply a human antibody to dog.
In vivo administration of H77Bf and canine MNC resulted in significant growth inhibition for CHO/dHER2 and SNP cells. These results provided evidences to support the suitability of H77Bf as a promising antibody therapy against canine cancers. The ADCC activity was also confirmed in vitro using canine MNCs, suggesting that ADCC activity could contribute to the antitumor activity of H77Bf. ADCC in humans is executed predominantly by NK cells through the FcγR that binds to the IgG1 or IgG3 subclass (60). The FcγR-like receptors have not been described on canine NK cells. Recently, a cell line-based assay to measure the ADCC of a canine therapeutic antibody was reported (61). The aforementioned study established a human NK cell line, NK-92 cells expressed with canine FcγR which can be used as effector cells. This system will contribute to the understanding of NK cell-mediated target cell lysis by canine therapeutic antibodies. Since the knowledge about canine NK cells is incomplete, further studies are needed to reveal the contribution of NK cells to ADCC in dogs. Furthermore, direct cytotoxic mechanisms by the complement system in dogs is also to be determined.
Drug-conjugated mAbs rely on direct cytotoxicity of the payloads through endocytosis of receptor-bound mAbs-drug conjugate (62,63). Trastuzumab deruxtecan (T-DXd, DS-8201) is a HER2-targeting antibody conjugated with a novel DNA topoisomerase I inhibitor (64). T-DXd showed promising clinical outcomes in patients with metastatic breast cancer, who had received multiple anti-HER2-targeting regimens (65). Currently, the clinical efficacy and safety of T-DXd have been evaluated in various clinical trials. T-DXd have been approved in not only HER2-positive breast cancer (65–67), but also HER2-mutant lung cancer (66). A mouse-canine chimeric mAb against dog podoplanin (68–70) (P38B) conjugated with emtansine as the payload (P38B-DM1) was previously generated and challenged for tumor therapy. P38B-DM1 showed cytotoxicity to podoplanin-expressing cells and exhibited higher antitumor activity than P38B in the xenograft model (71). Therefore, H77B-drug conjugate is one more option to treat dHER2-positive CMT. Recently, FDA-approved human immune checkpoint inhibitor against PD-1 and PD-L1 are used in canine tumor treatment (72–74); the combination of immune checkpoint inhibitors with other antibody-drugs is expected to be more effective. H77Bf could contribute to the development of canine cancer treatment, which can be feedback for human cancer treatment.
IHC has played a critical role as a diagnostic tool for the identification of neoplasms with conventional histopathology. In human breast cancer pathology, IHC is routinely used to assist with the prognosis and to determine the specific treatment (e.g. trastuzumab) for patients. Although IHC is not routinely used in CMTs, an increasing number of studies have been looking for reliable diagnostic and/or prognostic IHC biomarkers including dHER2 (21). A positive correlation between dHER2 in serum and tissue expression (by IHC) was reported (26). There is also a positive correlation between dHER2 expression and tumor mitotic index, high histological grade and size (75). However, not all studies have confirmed this, and no difference between dHER2 expression in non-neoplastic and neoplastic lesions was observed (76). Furthermore, in contrast to HER2-positive breast cancer in human, dHER2 amplification and HER2-enrichment subtype are not observed through whole-exome and transcriptome analyses of 191 spontaneous CMTs (77). Therefore, the standardization of dHER2 IHC is essential since those IHC analyses were performed by different Abs. Our established H2Mab-77 mAb is available for IHC (29), and its caninized mAb H77Bf exerts the antitumor activity against dHER2 positive cells, which could contribute to both diagnosis and therapy for dHER2-positive canine tumors.
Acknowledgements
The authors would like to thank Ms. Miyuki Yanaka, Mr. Takuro Nakamura, Mr. Yu Komatsu and Ms. Saori Handa (Department of Antibody Drug Development, Tohoku University Graduate School of Medicine) for technical assistance of in vitro experiments, and Mr. Shun-ichi Ohba and Ms. Akiko Harakawa [Institute of Microbial Chemistry (BIKAKEN), Numazu, Microbial Chemistry Research Foundation] for technical assistance of animal experiments.
Glossary
Abbreviations
- HER2
human epidermal growth factor receptor 2
- mAb
monoclonal antibody
- ADCC
antibody-dependent cellular cytotoxicity
- CDC
complement-dependent cytotoxicity
- FDA
Food and Drug Administration
- PI3K
phosphatidylinositol-3 kinase
- TKI
tyrosine kinase inhibitor
- FcγR
Fcγ, receptor
- NK
natural killer
- CMT
canine mammary tumor
- RPMI
Roswell Park Memorial Institute
- PBS
phosphate-buffered saline
- K D
dissociation constant
- DAPI
4′,6-diamidino-2-phenylindole
- MNC
mononuclear cell
- SEM
standard error of the mean
- IHC
immunohistochemistry
- T-DXd
Trastuzumab deruxtecan
Funding Statement
The present study was supported in part by Japan Agency for Medical Research and Development (AMED; grant nos: JP22ama121008, JP21am0401013, JP22bm1004001, JP22ck0106730 and JP21am0101078).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
TO, TT, MS and TA performed the experiments. MKK, MK and YK designed the experiments. TM prepared canine MNCs. TA, HS, TY and YK analyzed the data. HS and YK wrote the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The animal study protocol was approved (approval no. 2021-056) by the Institutional Committee for Experiments of the Institute of Microbial Chemistry (Numazu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 4.Moasser MM, Krop IE. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncol. 2015;1:1154–1161. doi: 10.1001/jamaoncol.2015.2286. [DOI] [PubMed] [Google Scholar]
- 5.Moasser MM. Two dimensions in targeting HER2. J Clin Oncol. 2014;32:2074–2077. doi: 10.1200/JCO.2014.55.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le XF, Pruefer F, Bast RC., Jr HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle. 2005;4:87–95. doi: 10.4161/cc.4.1.1360. [DOI] [PubMed] [Google Scholar]
- 8.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 9.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 10.Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 11.Maadi H, Soheilifar MH, Choi WS, Moshtaghian A, Wang Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers (Basel) 2021;13:3540. doi: 10.3390/cancers13143540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao LC, Force J, Hartman ZC. Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer Res. 2021;81:4641–4651. doi: 10.1158/0008-5472.CAN-21-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musolino A, Gradishar WJ, Rugo HS, Nordstrom JL, Rock EP, Arnaldez F, Pegram MD. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J Immunother Cancer. 2022;10:e003171. doi: 10.1136/jitc-2021-003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13:R123. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAndrew NP. Updates on targeting human epidermal growth factor receptor 2-positive breast cancer: What's to know in 2021. Curr Opin Obstet Gynecol. 2022;34:41–45. doi: 10.1097/GCO.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 16.Rugo HS, Im SA, Cardoso F, Cortés J, Curigliano G, Musolino A, Pegram MD, Wright GS, Saura C, Escrivá-de-Romaní S, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-Positive advanced breast cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golay J, Taylor RP. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies (Basel) 2020;9:58. doi: 10.3390/antib9040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: Untangling an intricate relationship. Nat Rev Immunol. 2018;18:5–18. doi: 10.1038/nri.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salas Y, Márquez A, Diaz D, Romero L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: A growing animal health problem. PLoS One. 2015;10:e0127381. doi: 10.1371/journal.pone.0127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray M, Meehan J, Martínez-Pérez C, Kay C, Turnbull AK, Morrison LR, Pang LY, Argyle D. Naturally-occurring canine mammary tumors as a translational model for human breast cancer. Front Oncol. 2020;10:617. doi: 10.3389/fonc.2020.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaszak I, Ruszczak A, Kanafa S, Kacprzak K, Król M, Jurka P. Current biomarkers of canine mammary tumors. Acta Vet Scand. 2018;60:66. doi: 10.1186/s13028-018-0417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: Application of the human classification. Virchows Arch. 2008;453:123–132. doi: 10.1007/s00428-008-0644-3. [DOI] [PubMed] [Google Scholar]
- 23.Flint AF, U'Ren L, Legare ME, Withrow SJ, Dernell W, Hanneman WH. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41:291–296. doi: 10.1354/vp.41-3-291. [DOI] [PubMed] [Google Scholar]
- 24.Millanta F, Impellizeri J, McSherry L, Rocchigiani G, Aurisicchio L, Lubas G. Overexpression of HER-2 via immunohistochemistry in canine urinary bladder transitional cell carcinoma-A marker of malignancy and possible therapeutic target. Vet Comp Oncol. 2018;16:297–300. doi: 10.1111/vco.12345. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto S, Kato D, Kamoto S, Yamamoto K, Tsuboi M, Shinada M, Ikeda N, Tanaka Y, Yoshitake R, Eto S, et al. Detection of human epidermal growth factor receptor 2 overexpression in canine anal sac gland carcinoma. J Vet Med Sci. 2019;81:1034–1039. doi: 10.1292/jvms.19-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos LC, Silva JO, Santos FS, Araújo MR, Lavalle GE, Ferreira E, Cassali GD. Prognostic significance of tissue and serum HER2 and MUC1 in canine mammary cancer. J Vet Diagn Invest. 2015;27:531–535. doi: 10.1177/1040638715592445. [DOI] [PubMed] [Google Scholar]
- 27.Brunetti B, Bacci B, Sarli G, Pancioni E, Muscatello LV. Immunohistochemical screening of HER2 in canine carcinomas: A preliminary study. Animals (Basel) 2021;11:1006. doi: 10.3390/ani11041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M, Paterson Y. Immunotherapy with a HER2-targeting listeria induces HER2-Specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 29.Itai S, Fujii Y, Kaneko MK, Yamada S, Nakamura T, Yanaka M, Saidoh N, Chang YW, Handa S, Takahashi M, et al. H2Mab-77 is a sensitive and specific Anti-HER2 monoclonal antibody against breast cancer. Monoclon Antib Immunodiagn Immunother. 2017;36:143–148. doi: 10.1089/mab.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osaki T, Sunden Y, Sugiyama A, Azuma K, Murahata Y, Tsuka T, Ito N, Imagawa T, Okamoto Y. Establishment of a canine mammary gland tumor cell line and characterization of its miRNA expression. J Vet Sci. 2016;17:385–390. doi: 10.4142/jvs.2016.17.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tateyama N, Asano T, Ohishi T, Takei J, Hosono H, Nanamiya R, Tanaka T, Sano M, Saito M, Kawada M, et al. An Anti-HER2 monoclonal antibody H2Mab-41 exerts antitumor activities in mouse xenograft model using dog HER2-overexpressed cells. Monoclon Antib Immunodiagn Immunother. 2021;40:184–190. doi: 10.1089/mab.2021.0022. [DOI] [PubMed] [Google Scholar]
- 32.Asano T, Kaneko MK, Kato Y. RIEDL tag: A novel pentapeptide tagging system for transmembrane protein purification. Biochem Biophys Rep. 2020;23:100780. doi: 10.1016/j.bbrep.2020.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asano T, Kaneko MK, Kato Y. Development of a novel epitope mapping system: RIEDL insertion for epitope mapping method. Monoclon Antib Immunodiagn Immunother. 2021;40:162–167. doi: 10.1089/mab.2021.0023. [DOI] [PubMed] [Google Scholar]
- 34.Asano T, Kaneko MK, Takei J, Tateyama N, Kato Y. Epitope mapping of the Anti-CD44 monoclonal antibody (C44Mab-46) using the REMAP Method. Monoclon Antib Immunodiagn Immunother. 2021;40:156–161. doi: 10.1089/mab.2021.0012. [DOI] [PubMed] [Google Scholar]
- 35.Nanamiya R, Sano M, Asano T, Yanaka M, Nakamura T, Saito M, Tanaka T, Hosono H, Tateyama N, Kaneko MK, Kato Y. Epitope mapping of an anti-human epidermal growth factor receptor monoclonal antibody (EMab-51) using the RIEDL insertion for epitope mapping method. Monoclon Antib Immunodiagn Immunother. 2021;40:149–155. doi: 10.1089/mab.2021.0010. [DOI] [PubMed] [Google Scholar]
- 36.Sano M, Kaneko MK, Aasano T, Kato Y. Epitope mapping of an antihuman EGFR monoclonal antibody (EMab-134) Using the REMAP method. Monoclon Antib Immunodiagn Immunother. 2021;40:191–195. doi: 10.1089/mab.2021.0014. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Ohishi T, Kaneko MK, Takei J, Mizuno T, Kawada M, Saito M, Suzuki H, Kato Y. Defucosylated mouse-dog chimeric Anti-EGFR antibody exerts antitumor activities in mouse xenograft models of canine tumors. Cells. 2021;10:3599. doi: 10.3390/cells10123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno T, Kato Y, Kaneko MK, Sakai Y, Shiga T, Kato M, Tsukui T, Takemoto H, Tokimasa A, Baba K, et al. Generation of a canine anti-canine CD20 antibody for canine lymphoma treatment. Sci Rep. 2020;10:11476. doi: 10.1038/s41598-020-68470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takei J, Kaneko MK, Ohishi T, Hosono H, Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al. A defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep. 2020;44:1949–1960. doi: 10.3892/or.2020.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takei J, Ohishi T, Kaneko MK, Harada H, Kawada M, Kato Y. A defucosylated anti-PD-L1 monoclonal antibody 13-mG2a-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Biochem Biophys Rep. 2020;24:100801. doi: 10.1016/j.bbrep.2020.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tateyama N, Nanamiya R, Ohishi T, Takei J, Nakamura T, Yanaka M, Hosono H, Saito M, Asano T, Tanaka T, et al. Defucosylated anti-epidermal growth factor receptor monoclonal antibody 134-mG2a-f exerts antitumor activities in mouse xenograft models of dog epidermal growth factor receptor-overexpressed cells. Monoclon Antib Immunodiagn Immunother. 2021;40:177–183. doi: 10.1089/mab.2021.0022. [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Mizuno T, Yamada S, Nakamura T, Itai S, Yanaka M, Sano M, Kaneko MK. Establishment of P38Bf, a core-fucose-deficient mouse-canine chimeric antibody against dog podoplanin. Monoclon Antib Immunodiagn Immunother. 2018;37:218–223. doi: 10.1089/mab.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asano T, Ohishi T, Takei J, Nakamura T, Nanamiya R, Hosono H, Tanaka T, Sano M, Harada H, Kawada M, et al. AntiHER3 monoclonal antibody exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol Rep. 2021;46:173. doi: 10.3892/or.2021.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka T, Ohishi T, Asano T, Takei J, Nanamiya R, Hosono H, Sano M, Harada H, Kawada M, Kaneko MK, Kato Y. An antiTROP2 monoclonal antibody TrMab6 exerts antitumor activity in breast cancer mouse xenograft models. Oncol Rep. 2021;46:132. doi: 10.3892/or.2021.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosono H, Ohishi T, Takei J, Asano T, Sayama Y, Kawada M, Kaneko MK, Kato Y. The anti-epithelial cell adhesion molecule (EpCAM) monoclonal antibody EpMab-16 exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol Lett. 2020;20:383. doi: 10.3892/ol.2020.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneko MK, Ohishi T, Takei J, Sano M, Nakamura T, Hosono H, Yanaka M, Asano T, Sayama Y, Harada H, et al. AntiEpCAM monoclonal antibody exerts antitumor activity against oral squamous cell carcinomas. Oncol Rep. 2020;44:2517–2526. doi: 10.3892/or.2020.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneko MK, Ohishi T, Nakamura T, Inoue H, Takei J, Sano M, Asano T, Sayama Y, Hosono H, Suzuki H, et al. Development of core-fucose-deficient humanized and chimeric anti-human podoplanin antibodies. Monoclon Antib Immunodiagn Immunother. 2020;39:167–174. doi: 10.1089/mab.2020.0019. [DOI] [PubMed] [Google Scholar]
- 48.Hosono H, Takei J, Ohishi T, Sano M, Asano T, Sayama Y, Nakamura T, Yanaka M, Kawada M, Harada H, et al. AntiEGFR monoclonal antibody 134mG2a exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Int J Mol Med. 2020;46:1443–1452. doi: 10.3892/ijmm.2020.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohishi T, Kato Y, Kaneko MK, Ohba SI, Inoue H, Harakawa A, Kawada M. Anti-metastatic activity of an anti-EGFR monoclonal antibody against metastatic colorectal cancer with KRAS p.G13D mutation. Int J Mol Sci. 2020;21:6037. doi: 10.3390/ijms21176037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takei J, Kaneko MK, Ohishi T, Kawada M, Harada H, Kato Y. H2Mab-19, an anti-human epidermal growth factor receptor 2 monoclonal antibody exerts antitumor activity in mouse oral cancer xenografts. Exp Ther Med. 2020;20:846–853. doi: 10.3892/etm.2020.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato Y, Ohishi T, Takei J, Nakamura T, Sano M, Asano T, Sayama Y, Hosono H, Kawada M, Kaneko MK. An anti-human epidermal growth factor receptor 2 monoclonal antibody H2Mab-19 exerts antitumor activity in mouse colon cancer xenografts. Monoclon Antib Immunodiagn Immunother. 2020;39:123–128. doi: 10.1089/mab.2020.0009. [DOI] [PubMed] [Google Scholar]
- 52.Takei J, Kaneko MK, Ohishi T, Kawada M, Harada H, Kato Y. A novel anti-EGFR monoclonal antibody (EMab-17) exerts antitumor activity against oral squamous cell carcinomas via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Oncol Lett. 2020;19:2809–2816. doi: 10.3892/ol.2020.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itai S, Ohishi T, Kaneko MK, Yamada S, Abe S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y, et al. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget. 2018;9:22480–22497. doi: 10.18632/oncotarget.25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato Y, Ohishi T, Yamada S, Itai S, Takei J, Sano M, Nakamura T, Harada H, Kawada M, Kaneko MK. Anti-Human epidermal growth factor receptor 2 monoclonal antibody H2Mab-41 exerts antitumor activity in a mouse xenograft model of colon cancer. Monoclon Antib Immunodiagn Immunother. 2019;38:157–161. doi: 10.1089/mab.2019.0017. [DOI] [PubMed] [Google Scholar]
- 55.Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, Wrba F, Horvat R, Thalhammer JG, Willmann M, Jensen-Jarolim E. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. 2012;50:200–209. doi: 10.1016/j.molimm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins DM, O'Donovan N, McGowan PM, O'Sullivan F, Duffy MJ, Crown J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. 2012;23:1788–1795. doi: 10.1093/annonc/mdr484. [DOI] [PubMed] [Google Scholar]
- 57.Klingemann H. Immunotherapy for dogs: Still running behind humans. Front Immunol. 2021;12:665784. doi: 10.3389/fimmu.2021.665784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergeron LM, McCandless EE, Dunham S, Dunkle B, Zhu Y, Shelly J, Lightle S, Gonzales A, Bainbridge G. Comparative functional characterization of canine IgG subclasses. Vet Immunol Immunopathol. 2014;157:31–41. doi: 10.1016/j.vetimm.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 60.Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009;100:1566–1572. doi: 10.1111/j.1349-7006.2009.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno T, Takeda Y, Tsukui T, Igase M. Development of a cell line-based assay to measure the antibody-dependent cellular cytotoxicity of a canine therapeutic antibody. Vet Immunol Immunopathol. 2021;240:110315. doi: 10.1016/j.vetimm.2021.110315. [DOI] [PubMed] [Google Scholar]
- 62.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 63.Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies (Basel) 2020;9:34. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K, Tsurutani J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. 2017;141:1682–1689. doi: 10.1002/ijc.30870. [DOI] [PubMed] [Google Scholar]
- 65.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al. Trastuzumab deruxtecan in previously treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 68.Kaneko MK, Honma R, Ogasawara S, Fujii Y, Nakamura T, Saidoh N, Takagi M, Kagawa Y, Konnai S, Kato Y. PMab-38 recognizes canine podoplanin of squamous cell carcinomas. Monoclon Antib Immunodiagn Immunother. 2016;35:263–266. doi: 10.1089/mab.2016.0036. [DOI] [PubMed] [Google Scholar]
- 69.Ito A, Ohta M, Kato Y, Inada S, Kato T, Nakata S, Yatabe Y, Goto M, Kaneda N, Kurita K, et al. A real-time near-infrared fluorescence imaging method for the detection of oral cancers in mice using an indocyanine green-labeled podoplanin antibody. Technol Cancer Res Treat. 2018;17:1533033818767936. doi: 10.1177/1533033818767936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kato Y, Ohishi T, Kawada M, Maekawa N, Konnai S, Itai S, Yamada S, Kaneko MK. The mouse-canine chimeric anti-dog podoplanin antibody P38B exerts antitumor activity in mouse xenograft models. Biochem Biophys Rep. 2019;17:23–26. doi: 10.1016/j.bbrep.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato Y, Ito Y, Ohishi T, Kawada M, Nakamura T, Sayama Y, Sano M, Asano T, Yanaka M, Okamoto S, et al. Antibody-drug conjugates using mouse-canine chimeric anti-dog podoplanin antibody exerts antitumor activity in a mouse xenograft model. Monoclon Antib Immunodiagn Immunother. 2020;39:37–44. doi: 10.1089/mab.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pantelyushin S, Ranninger E, Guerrera D, Hutter G, Maake C, Markkanen E, Bettschart-Wolfensberger R, Rohrer Bley C, Läubli H, Vom Berg J. Cross-reactivity and functionality of approved human immune checkpoint blockers in dogs. Cancers (Basel) 2021;13:785. doi: 10.3390/cancers13040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maekawa N, Konnai S, Nishimura M, Kagawa Y, Takagi S, Hosoya K, Ohta H, Kim S, Okagawa T, Izumi Y, et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis Oncol. 2021;5:10. doi: 10.1038/s41698-021-00147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klingemann H. Immunotherapy for dogs: Running behind humans. Front Immunol. 2018;9:133. doi: 10.3389/fimmu.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muhammadnejad A, Keyhani E, Mortazavi P, Behjati F, Haghdoost IS. Overexpression of her-2/neu in malignant mammary tumors; translation of clinicopathological features from dog to human. Asian Pac J Cancer Prev. 2012;13:6415–6421. doi: 10.7314/APJCP.2012.13.12.6415. [DOI] [PubMed] [Google Scholar]
- 76.Ressel L, Puleio R, Loria GR, Vannozzi I, Millanta F, Caracappa S, Poli A. HER-2 expression in canine morphologically normal, hyperplastic and neoplastic mammary tissues and its correlation with the clinical outcome. Res Vet Sci. 2013;94:299–305. doi: 10.1016/j.rvsc.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 77.Kim TM, Yang IS, Seung BJ, Lee S, Kim D, Ha YJ, Seo MK, Kim KK, Kim HS, Cheong JH, et al. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat Commun. 2020;11:3616. doi: 10.1038/s41467-020-17458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.