Abstract
Advanced adenomas represent a subset of colorectal polyps that are known to confer an increased risk of colorectal neoplasia to the affected individual and their first-degree relatives (FDRs). Accordingly, professional guidelines suggest earlier and more intensive screening for FDRs of those with advanced adenomas similar to FDRs of those with colorectal cancer (CRC). Although the risk to family members is less clear among patients with advanced serrated polyps, they are often considered in the same category. Unfortunately, there is a growing concern that patients, endoscopists, and primary care providers are unaware of the familial risk associated with these polyps, leaving a wide gap in screening these high-risk individuals. Herein, we propose a standardized language around advanced colorectal polyps and present a detailed review of the literature on associated familial risk. We outline the challenges to implementing the current screening recommendations and suggest approaches to overcome these limitations, including a proposed new colonoscopy quality metric to capture communication of familial CRC risk. Improving screening in these high-risk groups has the potential to substantially reduce the burden of CRC.
INTRODUCTION
The marked decrease in colorectal cancer (CRC) incidence and mortality in the United States over the past 2 decades has been largely attributed to increased uptake of CRC screening (1). Despite this progress, an estimated 147,950 new cases of CRC and 53,200 deaths from CRC will occur in 2020, making it the second most common cause of cancer death in the United States (2). CRC disease burden remains high, in part, because the screening programs fall short of reaching all eligible individuals. National efforts are largely geared toward screening average-risk persons but often fail to target substantial subsets of the population who are at increased risk (3–5). One such high-risk group is the first-degree relatives (FDRs) of individuals with CRC and advanced adenomas (AAs).
FDRs of patients with CRC have greater than a 2-fold higher risk of developing CRC. Risk increases with 2 or more affected FDRs and as the age of the patient with CRC decreases (6–12). Accordingly, screening is recommended by age 40 or 10 years younger than the relative’s CRC diagnosis. FDRs of patients with AAs have a similarly increased risk and the same early screening recommendations apply. AAs are a distinct category of colorectal polyps defined based on size (tubular adenoma ≥ 1 cm) or histology (any adenoma with villous histology or high-grade dysplasia). They confer a higher risk of future colorectal neoplasia for the affected individual and their FDRs, thus warranting earlier and more frequent screening (13–18). All major guidelines recommend starting CRC screening by the age of 40 years in FDRs of individuals with one or more AAs (see Table 1) (19–21).
Table 1.
Colorectal Cancer Screening Guidelines for Individuals with a Family History of Advanced Colorectal Polyp
| Family history | Age to initiate screening | Preferred test, interval | |
|---|---|---|---|
|
|
|||
| Banff Consensus Group (CAG/AGA) 20 | Documented advanced adenoma in ≥ 1 FDR (any age) | Age 40–50, or 10y younger than age at diagnosis of FDR* | Colonoscopy every 5–10y or FIT every 1–2 y |
|
| |||
| United States Multi Society Task Force (US-MSTF) 19 | Documented Advanced adenoma in 1 FDR <60y or in 2 FDRs (any age) | Age 40, or 10y younger than age of diagnosis of FDR* | Colonoscopy every 5y# |
|
| |||
| Advanced adenoma in 1 FDR ≥60y | Age 40 | Same as average-risk persons (colonoscopy every 10y or FIT annually) | |
|
| |||
| Documented Advanced serrated lesion in ≥1 FDR^ | According to recommendations for family history of documented advanced adenoma | ||
|
| |||
| National Comprehensive Cancer Network (NCCN) 65 | Confirmed Advanced adenoma or advanced serrated lesion in 1 FDR (any age) | Age 40, or at age of diagnosis of advanced adenoma in FDR* | Colonoscopy every 5–10y |
y: years, CAG: Canadian Association of Gastroenterology, AGA: American Gastroenterological Association, US-MSTF: United States Multi Society Task Force, NCCN: National Comprehensive Cancer Network, FDR: first degree relative, ASL: advanced serrated lesion, FIT: fecal immunochemical test
whichever is earlier
If colonoscopy is declined, annual FIT should be offered
Advanced Serrated Lesion- weak recommendation, very low quality evidence
American Cancer Society (ACS) 2018 Guidelines:103 Only for average-risk adults, no screening guidelines specifically for people at increased or high risk of colorectal cancer, recommend referring to US MSTF guidelines.
Patients with AA have poor knowledge of the increased risk of CRC to themselves and their family members. In a study of 137 patients with advanced polyps, only 29% were aware that their polyp was precancerous and only 40% were aware that their FDRs may be at increased risk of CRC (22). Although this lack of risk recognition is undoubtedly multifactorial and includes both patient-related factors (limited health literacy and lack of contact with family members) and system-related factors (electronic medical records and privacy laws), it is also driven by a breakdown in communication between endoscopists and their patients (23,24). In the same study, patients reported that their endoscopists are their top source for information about CRC risk and recommendations, although only 7% of patients were provided recommendations for family members from their endoscopists (22). Identification and proper screening of FDRs of patients with AAs offers a major opportunity to expand screening and potentially decrease the CRC burden.
This review will focus on AAs as a CRC risk factor. Herein, we (i) describe the prevalence, history, and evolution of AAs and provide a current working definition of the term advanced serrated polyp (ASP) and advanced colorectal polyp (ACP), (ii) summarize the published data on the impact of AAs on the risk of CRC (for the proband and their FDRs) and guideline recommendations, (iii) highlight the barriers to screening this cohort of FDRs, and (iv) propose quality metrics for endoscopists related to communication for families at high risk for CRC.
PART I
Prevalence of AAs
The population prevalence of AAs by age and subtype of the polyp can be estimated from the CRC screening trials (see Figure 1). The overall prevalence is 3.5%–10.5%, with higher rates seen in men with advancing age and symptomatic patients (25–28). Regarding racial differences, a recent meta-analysis of 9 studies found no significant differences in the prevalence of AAs between whites and blacks (29).
Figure 1.
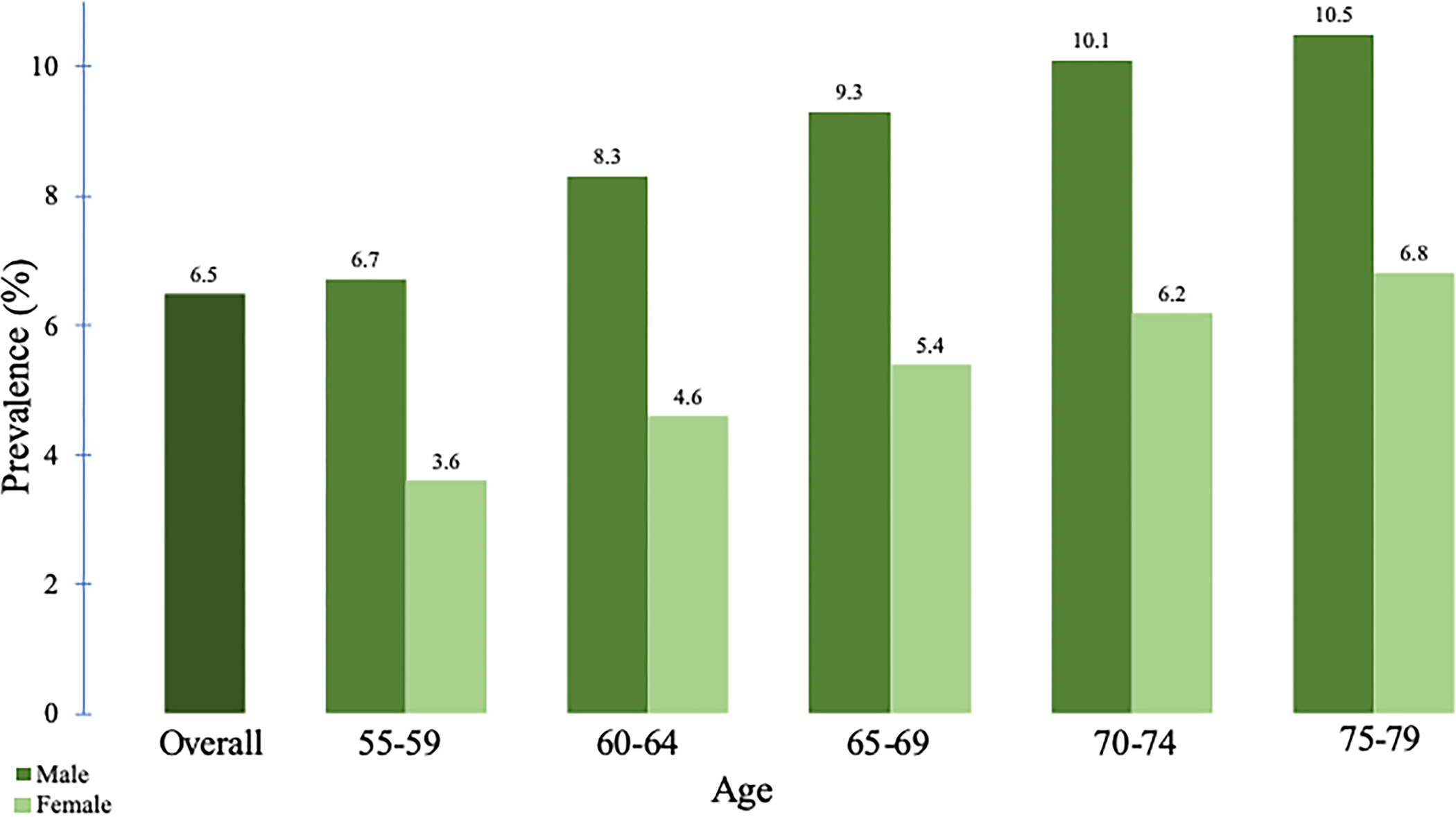
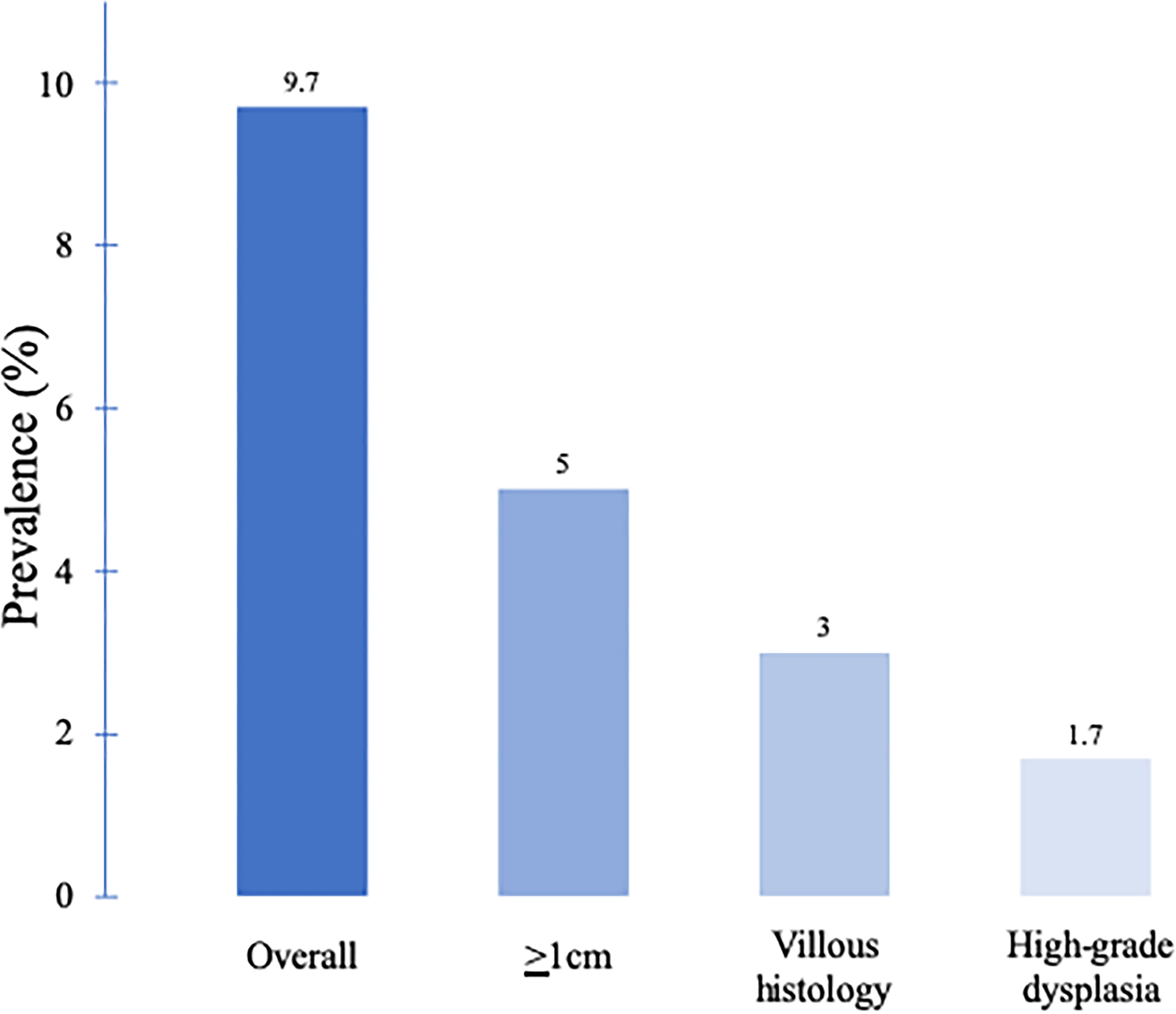
Prevalence of advanced adenoma (AA) by age and sex. (a) The prevalence of AA is higher in men compared with that of in women and increases with increasing age (26). (b) The prevalence of AA varies according to the polyp subtype (27).
The history and current definition of the AA and ASP
The concept of AA as a more aggressive colorectal lesion was first introduced in the original polyp screening trials (30) and remains present in the current guidelines (19–21). Winawer et al. classified large adenomas, adenomas with high-grade dysplasia or infiltrating cancer, as clinically relevant adenomas with increased malignant potential (30–32). The term high-grade dysplasia replaced the previously used terms carcinoma in situ, intramucosal carcinoma, and focal carcinoma (33). The National Polyp Study landmark article in 1993 subsequently defined the adenoma with advanced pathological features. (34) This was based on a study where individuals who had removal of a rectosigmoid large (≥1 cm) or villous adenoma had a 3 times higher risk of CRC and nearly 6 times higher likelihood if multiple (≥3) adenomas were present, as compared to those who had low risk (<1 cm) tubular adenomas (35). This increased risk was replicated in additional studies (36).
The guidelines published in 2000 written by combined societies including the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the American Association for the Study of Liver Diseases introduced the term AA to refer to polyps that are large (≥1 cm), contain villous histology, or high-grade dysplasia (37). This term AA is still used today, and the components have not changed much in the past 20 years.
The term high-risk adenoma (HRA) was introduced in the 2006 United States Multi-Society Task Force (US-MSTF) surveillance guidelines (38). Although the features are largely similar, HRA is distinct from AA in that it also includes in its definition the presence of ≥3 adenomas. It is important to note that this qualifier HRA is intended only for surveillance recommendations; the finding of ≥3 adenomas is not known to have risk implications for FDRs based on the current literature.
More recently, the terminology has expanded to include ASPs, defined as any sessile serrated adenoma/polyp (SSP) ≥1 cm, SSP with any grade of cytological dysplasia, or traditional serrated adenoma (TSA) ≥1 cm. Large hyperplastic polyp (HP) ≥1 cm has not been traditionally included in this definition, although previous studies have combined HPs with other serrated polyps (39,40). Risk to FDRs is presumed to be increased, and some guidelines recommend similar screening in FDRs as with conventional AAs (19,21). However, limited data exist related to the magnitude of risk among FDRs with ASPs. In this review, data and recommendations for AA and ASP are largely kept separate, but when the approach is similar, we use the term ACP to refer to both AA and ASP (Table 2).
Table 2.
Colorectal Polyp Definitions
| Type of colorectal polyp | Definition | |
|---|---|---|
|
| ||
| Advanced adenoma | AA | Large tubular adenoma ≥1cm, or any adenoma with villous histology or high-grade dysplasia |
|
| ||
| Advanced serrated polyp | ASP | Large sessile serrated polyp ≥1cm, or SSP with any grade of cytological dysplasia, or traditional serrated adenoma ≥1cm or large hyperplastic polyp ≥1cm diagnosed proximal to the splenic flexure and/or with endoscopic features suggestive of SSP* |
|
| ||
| Advanced colorectal polyp | ACP | Advanced adenoma or advanced serrated polyp (AA+ASP) |
|
| ||
| High-risk adenoma | HRA | Advanced adenoma, or the presence of ≥3 adenomas |
Flat or sessile shape, indistinct border, mucus cap, rim of debris, cloud-like surface, lacy vessel pattern45
Challenges in defining AAs and ASPs
The definition of ACP is problematic because the assessment of size and histology may be subjective. It is well known that polyp measurement is fraught with inaccuracy and the size is generally overestimated with significant clustering around the 1-cm and 2-cm marks (41). These limitations have prompted many to question the accuracy of historical data regarding the polyp size and risk estimates which may challenge the validity of guideline recommendations (42). Nonetheless, these standard, albeit potentially inaccurate, estimates have been useful in stratifying risk among patients with colorectal polyps.
Accurate histologic diagnosis is also critical. In general, interobserver reliability is generally good among pathologists for adenomatous vs nonadenomatous polyps. However, for categorizing adenomas as nonadvanced or advanced, interobserver agreement is only moderate between general and expert pathologists (kappa 0.56 [0.44–0.67]) and between expert pathologists (kappa 0.64 [0.43–0.85]) (43). Other studies show even worse agreement of diagnosis for colorectal polyps according to the histologic type (kappa = 0.46) and degree of dysplasia (kappa = 0.26) (44). These issues are compounded for serrated polyps in part because those histologic criteria are more recent and less widely recognized than that for adenoma types (45). Several studies have shown that polyps that were previously called hyperplastic were frequently classified as SSP when re-examined later by expert pathologists or when the specimen was reoriented (46–49), and there is still substantial variability in the rate of diagnosis of serrated polyps among individual pathologists (50–52).
PART II
Risk of CRC in individuals with a positive family history, but no hereditary syndrome
In the present review, we focus on individuals with a positive family history of AA in the absence of an identified hereditary cancer syndrome. However, if at any time the family history pattern suggests an inherited syndrome, additional workup including genetic counseling and testing should be considered. Hereditary causes account for about 5%–10% of CRC, and the National Comprehensive Cancer Network (NCCN) recommends the genetic evaluation in any patient younger than 50 years with CRC or with an FDR with CRC at younger than 50 years (53). In addition, when ASPs are encountered, the provider should always keep in mind the serrated polyposis syndrome, defined as at least 5 serrated polyps proximal to the rectum, all ≥5 mm, with 2 or more that are ≥10 mm, or more than 20 serrated polyps of any size distributed throughout the large bowel with at least 5 proximal to the rectum (54). However, the risk estimates and management for familial CRC syndromes and family history of CRC are beyond the scope of this study and are summarized elsewhere (55). Instead, we focus on a distinct high-risk group, which is individuals with a family history of neoplastic polyps.
Risk of CRC in individuals with an FDR with a non-AA
In 1996, the National Polyp Study observed that siblings and parents of patients with adenomatous polyps are at increased risk for CRC (Relative Risk 1.78, 95% confidence interval [CI] 1.18–2.67) (56). A series of case control studies reporting that individuals with adenomas (or AAs) were more likely to have an FDR with CRC than those without adenomas supported these data (7). This prompted gastrointestinal societies to treat individuals with an FDR with any adenomatous polyp as higher risk than the general population, warranting earlier screening and at shorter intervals (57–60).
Based on the inherent limitations of case control studies, including selection and information bias (i.e., self-reported polyp family history), and the implications of screening everyone with an FDR with any adenoma (61–63), the American College of Gastroenterology focused their 2009 recommendation for earlier screening to only those with an FDR with an AA (64). The US-MSTF and the NCCN followed suit (65,66). Nearly 10 years later, new robust data demonstrated that indeed a family history of non-AA does not significantly increase the risk of advanced colorectal neoplasia (67).
Risk of CRC in individuals with an FDR with AA
Several studies demonstrated increased risk of CRC in individuals with an FDR with AA according to the polyp subtype (Table 3) (10,68). Overall, the FDRs of patients with AAs have 1.7–3.9 times the risk of CRC compared with those without family histories (14,69). These risk estimates are fairly similar to the increased risk associated with having an FDR with CRC (70). A French case control study recruited FDRs of 306 index cases with large tubular adenomas ≥1 cm (advanced based on the size). The resulting case group of 168 FDRs was matched on age, sex, and geographical area to 2 controls randomly selected from patients undergoing screening colonoscopy (controls considered high risk for colorectal neoplasia were excluded) (14). FDRs with a positive family history of AA were more likely to develop a composite endpoint of CRC and/or large adenomas compared with controls (8.4% vs 4.2%, odds ratio [OR] 2.27, 95% CI 1.01–5.09). The odds were higher when the index case was younger than 60 years (OR 3.82, 95% CI 0.92–15.87), men (OR 4.01, 95% CI 1.45–11.09), or had a large distal adenoma (OR 3.14, 95% CI 1.27–7.73). Only 55% of the eligible FDRs in this study actually had a colonoscopy, and therefore, the study was underpowered to determine the outcome of CRC alone. However, the absolute prevalence of CRC was 3-fold higher in relatives compared with controls.
Table 3.
Level of risk for family member of patients with advanced colorectal polyps
| Pathology in Proband | Risk in First Degree Relative, OR/RR (95% CI) |
|
|---|---|---|
| Advanced Adenoma | Colorectal Cancer | |
|
| ||
| • Advanced adenoma | *6.05 (2.74–13.36)104 | -- |
|
| ||
| ○ Tubular adenoma ≥1 cm | 8.59 (3.4–21.45)104 **2.27 (1.01–5.09)14 |
3.9 (0.89–17.01)14 |
| ○ Adenoma with villous histology |
***1.65 (1.28–2.14)69 6.28 (2.02–19.53)104 |
1.68 (1.29–2.18)69 |
| ○ Adenoma with high-grade dysplasia | 19.98 (2.03–197)104 | -- |
|
| ||
| • Advanced serrated polyp | -- | -- |
in siblings
for composite endpoint of large adenoma and/or CRC
adenoma with villous histology only (not advanced by size or high grade dysplasia)
SSP ≥1 cm, SSP with dysplasia, TSA ≥1 cm
limited available data
Another case control study within a cohort of 126,936 Utah residents undergoing colonoscopy observed that FDRs of those with villous adenomas compared with no adenoma had a higher risk of CRC (RR 1.65 (1.28–2.14)) (69). The analysis did not include large adenomas or adenomas with high-grade dysplasia. Recall bias was minimized in this study by using the colonoscopy reports and validated genealogy records to confirm familial relationships.
Risk of CRC in individuals with an FDR with ASPs
The relatively recent recognition of the importance of serrated polyps as precursors to about 15%–30% of CRCs has substantially enriched the discussion of ACPs (71). ASPs are defined by the US-MSTF as SSP ≥ 1 cm, SSP with any grade of cytological dysplasia, or TSA ≥ 1 cm (19). SSP with cytological dysplasia represents a small subset of SSPs that develop discrete foci of dysplasia and are believed to transform quickly to malignancy (72). TSAs ≥ 1 cm are considered advanced by size and have associated recommendations for earlier screening in FDRs (US-MSTF and NCCN) (19,21). However, for the purposes of proband surveillance, TSA of any size is considered a high-risk lesion with a recommended 3-year interval for repeat colonoscopy (73). Smaller TSAs < 1 cm may confer increased risk, although data are limited. In 1 study, TSA at baseline was associated with increased risk of metachronous advanced neoplasia compared with conventional adenoma; however, in multivariate analysis, the size of TSA on index examination was not a significant predictor of advanced neoplasia on surveillance (74).
ASPs are associated with increased risk of synchronous and metachronous advanced neoplasia, and large SSPs ≥ 1 cm are associated with a 3.5-fold higher personal risk of future CRC (39,75–78). The magnitude of risk to family members is still not defined based on the most recent literature. A large data set from Korea found that having an FDR with CRC was a risk factor for any SSP (OR 3.14, 95% CI 1.57–6.27), although it was not specific to ASP (79). Egoavil et al. (80) demonstrated an increased risk of CRC in FDRs of individuals with multiple serrated polyps, but who did not meet the criteria for serrated polyposis syndrome (standardized incidence ratio 2.79, 95% CI 2.10–3.63) (81).
Based on the currently available data, it is unclear as to whether to include large HPs ≥ 1 cm in this definition of ASP. Many studies combine large HP with large SSP (39,40) which makes it difficult to know whether individuals with large HP alone have increased risk for future colorectal neoplasia separate from the risk associated with large SSP. This grouping is usually performed to account for the difficulty in differentiating HP from SSP histologically, which can occur up to 25% of the time (82). An alternate strategy is to consider large HPs that are located proximal to the splenic flexure in the definition of ASP, which would account for the challenges in histopathologic diagnosis and the known epidemiology of SSPs. We propose including large SSPs and TSAs as ASPs. Until further data become available to quantify the specific risk associated with large HPs, we recommend considering including HP ≥ 1 cm diagnosed proximal to the splenic flexure and those with typical endoscopic features associated with SSPs (flat or sessile shape, indistinct border, mucus cap, rim of debris, cloud-like surface, and lacy vessel pattern) (45) as advanced lesions, accepting that data are limited.
Currently, it is unclear whether individuals with an FDR with an ASP are at increased risk for CRC because data in this area are lacking. Nonetheless, the increased risk to FDRs from conventional AA is extrapolated with a recommendation by some guidelines for earlier and more frequent screening (19,21). The US-MSTF acknowledges that this is a weak recommendation with very low quality evidence, and the NCCN lists this as a category 2A recommendation. It may be reasonable to consider ASP as higher-risk lesions for family members, but it may be too premature to equate the risk to that of conventional AA.
PART III
Barriers to screening FDRs of individuals with ACPs
Although screening rates among those with positive family histories are higher than in the general population (at the same age), they still remain low at less than 50% for individuals (aged 40–49 years) with an FDR with CRC (70,83). Limited data are available for the screening rates among FDRs of patients with ACPs, but it is likely lower (84).
Achieving adequate screening among FDRs of patients with ACP could be achieved by outreach to FDRs of patients with ACPs or by the routine collection of the family history of polyps during clinical care. There are, however, substantial provider, patient, and system level barriers to be considered (Figure 2). Challenges at each level include the following: (i) gaps in knowledge exist—both patient knowledge of their diagnosis and provider knowledge of the recommendations, (ii) ineffective and inopportune timing of communication postcolonoscopy, (iii) inaccurate and incomplete family history data, and (iv) lack of effective tools and resources that facilitate awareness and compliance among patients and their FDRs. It is also possible that differences in the guidelines may lead to confusion about insurance coverage.
Figure 2.
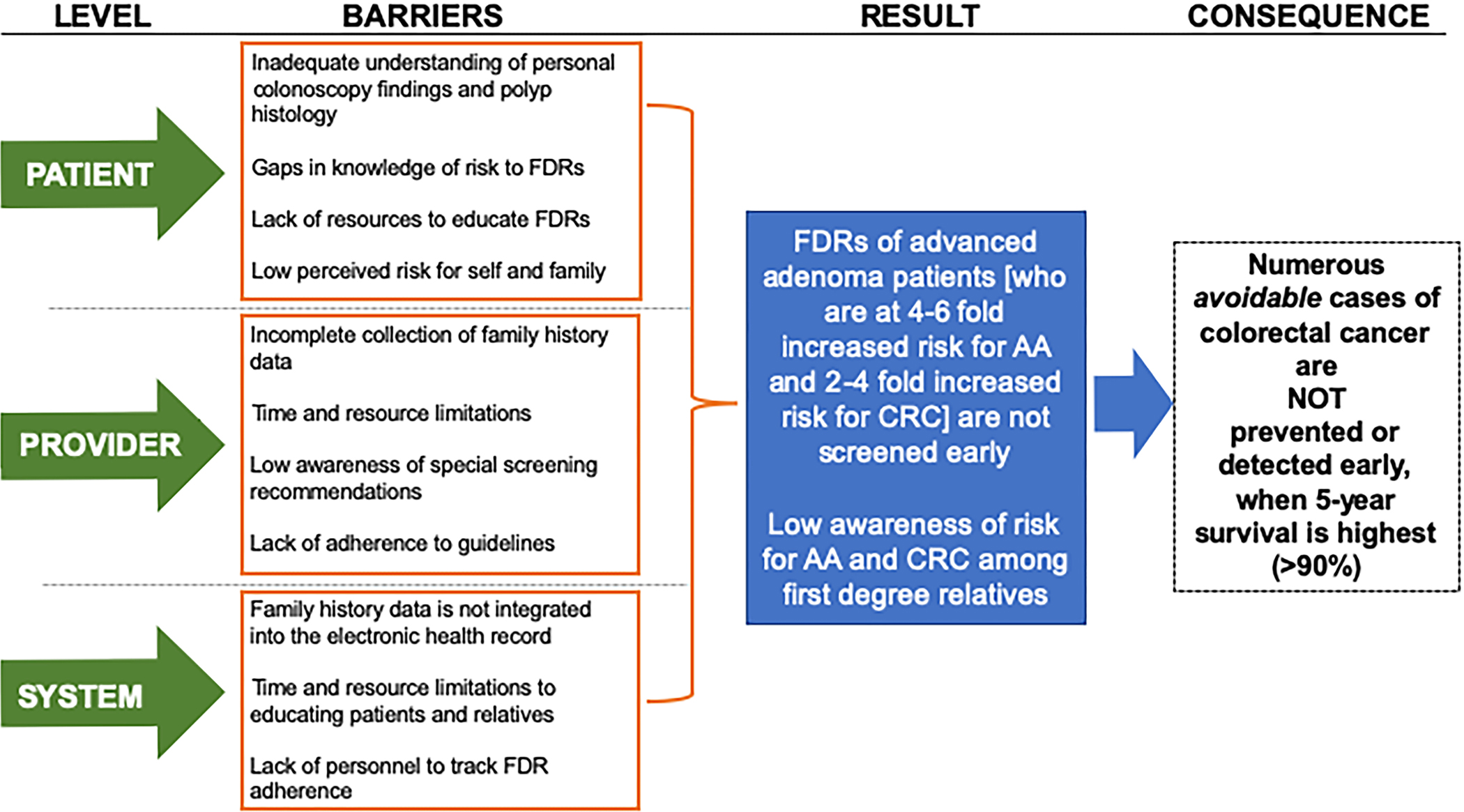
Proposed patient, provider, and system level barriers to screening first-degree relatives of patients with advanced colorectal polyp. AA, advanced adenoma; CRC, colorectal cancer; FDR, first-degree relative.
Barriers to outreach to FDRs through patients with ACPs
Most patients have an inadequate understanding of their colonoscopy and polypectomy results which is disadvantageous because risk perception is related to the screening behavior (85,86). Surveys show that patients typically do not know the size, number, or histologic classification of their colon polyps, despite being informed verbally or provided with a report of the endoscopy and pathology results (87,88). It is thus to be expected that the patients’ FDRs and the relatives’ providers are unaware of the increased CRC risk. There are also a host of patient-related factors that contribute to the communication complexity. Health literacy is low, and contact with family members may be inconsistent and an unreliable channel to convey early screening recommendations (89). In addition, patients are frequently unable to provide complete detailed family history reporting.
These patient-level gaps are compounded by similar provider-level gaps. Physicians are often not equipped with time or resources to provide postprocedural communication regarding early screening recommendations. This is combined with the inopportune time of approaching patients postanesthesia, limited knowledge by primary care providers or endoscopists on published recommendations, and the fact that many gastroenterologists do not adhere to the guidelines (often may even intentionally disagree), resulting in the overuse and underuse of colonsocopy (90–95). Although most endoscopy units routinely convey information to patients about their surveillance recommendations, there is rarely a system for effectively communicating the implications of the results for the patient’s FDRs. There is a need for better systems and effective strategies to reach high-risk family members of patients with ACPs (96,97).
Barriers to the collection of a family history of ACPs
Limited data exist regarding the collection of ACP family history, but it is likely more poorly collected than CRC family history and undoubtedly shares similar barriers. Although accurate recording of family history in medical records is the most important strategy for identifying hereditary cancer syndromes, it is lacking in approximately half of primary care patient medical records (98) and up to 40% of patients undergoing outpatient colonoscopy (99). A major challenge is the lack of tools for the systematic collection of these data efficiently or effectively integrated within the clinical workflow. Additional obstacles are associated with patient confidentiality which prevents the providers communicating directly with their patient’s relatives. Health Insurance Portability and Accountability Act privacy laws make it nearly impossible for a gastroenterologist who has actionable information (positive finding of an ACP) to inform the patient’s FDRs and successfully complete early screening in these high-risk individuals. Recognizing individuals at high risk has helped reduce CRC incidence and mortality by half in Lynch syndrome (100), but identification of patients at increased risk based on an FDR with ACPs would require a major change in the approach to collecting family history. Given the effectiveness of screening FDRs of individuals with CRC, it is reasonable to expect a similar benefit from more intensive screening in this population.
PART IV
Tools, resources, and needs
Few studies have focused on the communication about CRC risk and screening outside of disclosing genetic test results in families with a known inherited predisposition. There is, therefore, a critical need for effective communication and educational efforts for both patients and providers. Only recently have the resources been developed to improve awareness among gastroenterologists and primary care physicians about the early screening guidelines recommended for FDRs of patients with ACP (101). The National Colorectal Cancer Roundtable provides free, downloadable template letters according to specific polyp subtype that communicate the colonoscopy and pathology results, associated risk, and recommendations for patients and FDRs (102). A focused effort to improve communication of the ACP results and specific recommendations for family members is a critical first step to reaching more eligible individuals for earlier screening. Further studies are needed to better understand how or whether patients with AA communicate risk to unaffected family members and whether these individuals know that they are at risk. Identifying the most effective and feasible methods to communicate with FDRs will also be critical. With the many technological advances available for reaching and teaching patients, it is time to sharpen our understanding of the best methods to engage patients and their family members.
Proposal of endoscopic reporting quality metrics to document family risk based on the advanced polyp findings
There is an enormous opportunity for the endoscopist to improve the clinical care not only for patients with ACPs, but for their FDRs. As the provider who diagnoses and manages advanced polyps, the endoscopist is uniquely positioned to ensure that patients and their family members are well informed about their risk and screening recommendations based on the advanced polyp findings. Given that professional guidelines support more intensive screening for family members of those with advanced polyps, standardized communication of CRC risk and screening recommendations for family members can be proposed as quality metrics (103).
A quality metric must be measurable and simple. We propose 2 quality metrics for endoscopists who diagnose and manage advanced colorectal neoplasia (CRC and/or advanced polyps) (Table 4). The major underlying goal is to notify the patients with advanced neoplasia that their family members may be at increased risk and may require more intensive screening. This can be accomplished by including a family recommendation in the colonoscopy report and/or pathology notification. For example, if the patient is found to have an AA, we recommend communicating that (i) FDRs may have an increased risk of CRC based on the patient’s polyp finding, (ii) patient should share these polyp findings with FDRs and inform them of this increased risk, (iii) FDRs should talk to their health care provider about appropriate age and method to initiate screening, and (iv) guidelines suggest that FDRs may require earlier and/or more frequent screening. We also suggest that the endoscopist notify the referring provider. This would enable the primary care doctor to also encourage the patient to reach out to family members and encourage them to speak with their providers about CRC screening. This could be included in the letter usually generated to the referring provider. This communication can be standardized within an endoscopy practice and applied to all patients diagnosed with advanced neoplasia.
Table 4.
Proposed quality metrics for communication of increased familial risk in patients diagnosed with advanced colorectal neoplasia
| Proposed Quality Metrics | Threshold | Type of Quality Metric |
|---|---|---|
| 1. When a patient is found to have an advanced colorectal polyp* or colorectal cancer, the endoscopist should document notification to the referring provider that first-degree relatives of the patient may have an increased risk of colorectal cancer | ≥90% | Process Measure |
| 2. When a patient is found to have an advanced colorectal polyp* or colorectal cancer, the endoscopist should document notification to the patient that first-degree relatives may have an increased risk of colorectal cancer and should be notified and encouraged to talk to their health care provider about appropriate age and method to initiate screening | ≥90% | Process measure |
Advanced colorectal polyp (ACP): tubular adenoma ≥1cm in size, adenoma with villous histology or high-grade dysplasia, sessile serrated polyp ≥1cm in size, sessile serrated polyp with any degree of cytologic dysplasia, traditional serrated adenoma ≥1 cm, hyperplastic polyp≥1cm diagnosed proximal to the splenic flexure with endoscopic features suggestive of SSP (flat or sessile shape, indistinct border, mucus cap, rim of debris, cloud-like surface, lacy vessel pattern)45
Endoscopy practices can track the overall and individual endoscopist adherence to familial risk communication for patients diagnosed with advanced colorectal neoplasia in the endoscopy suite. We propose that practices aim for ≥90% adherence. These proposed quality metrics can empower and support endoscopists to open the channels of communication with their patients and patients with their FDRs.
CONCLUSIONS
It is widely accepted that individuals with an FDR with CRC are at increased risk of colorectal neoplasia and warrant earlier and more frequent screening. This review emphasizes that individuals with an FDR with AA have a similarly increased risk of CRC (1.7–3.9 times higher) and earlier and more frequent screening strategies are recommended by professional gastrointestinal and oncologic society guidelines. Although there are numerous barriers to identifying and screening all of these high-risk individuals based on the positive family history of AA, improving the communication between patients, providers, and family members is a critical first step. One way to promote these conversations is to incorporate familial risk communication into colonoscopy quality metrics. The actionable findings after colonoscopy have important implications for patients, and through them their relatives, that may help decrease the burden of CRC.
Financial support:
J.M.K. received funding from the National Institutes of Health (NIH) T32-DK007038.
Footnotes
CONFLICTS OF INTEREST
Guarantor of the article: Jennifer M. Kolb, MD, MS.
Potential competing interests: J.M.K., S.G.P., D.A.L., and D.J.A. have nothing to disclose. C.L.M. is a consultant for Pfizer.
REFERENCES
- 1.Ansa BE, Coughlin SS, Alema-Mensah E, et al. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J Clin Med 2018;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 3.National Colorectal Cancer Roundtable. 80% by 2018 (http://nccrt.org/what-we-do/80-percent-by-2018/). Accessed November 1, 2019.
- 4.Singal AG, Gupta S, Skinner CS, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion. JAMA 2017;318:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 2016;122:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and the risk of colorectal cancer. N Engl J Med 1994;331: 1669–74. [DOI] [PubMed] [Google Scholar]
- 7.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 2001;96:2992–3003. [DOI] [PubMed] [Google Scholar]
- 8.Wong MCS, Chan CH, Lin J, et al. Lower relative contribution of positive family history to colorectal cancer risk with increasing age: A systematic review and meta-analysis of 9.28 million individuals. Am J Gastroenterol 2018;113:1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St John DJ, McDermott FT, Hopper JL, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med 1993;118: 785–90. [DOI] [PubMed] [Google Scholar]
- 10.Quintero E, Carrillo M, Leoz ML, et al. Risk of advanced neoplasia in first-degree relatives with colorectal cancer: A large multicenter cross-sectional study. PLoS Med 2016;13:e1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur J Cancer 2006;42:216–27. [DOI] [PubMed] [Google Scholar]
- 12.Baglietto L, Jenkins MA, Severi G, et al. Measures of familial aggregation depend on definition of family history: Meta-analysis for colorectal cancer. J Clin Epidemiol 2006;59:114–24. [DOI] [PubMed] [Google Scholar]
- 13.Click B, Pinsky PF, Hickey T, et al. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA 2018;319: 2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottet V, Pariente A, Nalet B, et al. Colonoscopic screening of first-degree relatives of patients with large adenomas: Increased risk of colorectal tumors. Gastroenterology 2007;133:1086–92. [DOI] [PubMed] [Google Scholar]
- 15.Leung K, Pinsky P, Laiyemo AO, et al. Ongoing colorectal cancer risk despite surveillance colonoscopy: The polyp prevention trial continued follow-up study. Gastrointest Endosc 2010;71:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133: 1077–85. [DOI] [PubMed] [Google Scholar]
- 17.Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: A population-based cohort study. Gut 2012;61: 1180–6. [DOI] [PubMed] [Google Scholar]
- 18.Toll AD, Fabius D, Hyslop T, et al. Prognostic significance of high-grade dysplasia in colorectal adenomas. Colorectal Dis 2011;13:370–3. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153: 307–23. [DOI] [PubMed] [Google Scholar]
- 20.Leddin D, Lieberman DA, Tse F, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 2018;155: 1325–47.e3. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network. Colon Cancer. Version 2, 2019. (https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf). Accessed November 1, 2019.
- 22.Patel SG, Ahnen DJ, Gumidyala A, et al. Poor knowledge of personal and familial colorectal cancer risk and screening recommendations associated with advanced colorectal polyps. Dig Dis Sci [Epub ahead of print March 6, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroy PC III, Barrison AF, Ling BS, et al. Family history and colorectal cancer screening: A survey of physician knowledge and practice patterns. Am J Gastroenterol 2002;97:1031–6. [DOI] [PubMed] [Google Scholar]
- 24.Barrison AF, Smith C, Oviedo J, et al. Colorectal cancer screening and familial risk: A survey of internal medicine residents’ knowledge and practice patterns. Am J Gastroenterol 2003;98:1410–6. [DOI] [PubMed] [Google Scholar]
- 25.Hong W, Dong L, Stock S, et al. Prevalence and characteristics of colonic adenoma in mainland China. Cancer Manag Res 2018;10:2743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner H, Altenhofen L, Stock C, et al. Incidence of colorectal adenomas: Birth cohort analysis among 4.3 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev 2014;23: 1920–7. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343:162–8. [DOI] [PubMed] [Google Scholar]
- 28.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectalcancer screening for detection of advanced neoplasia. N Engl J Med 2006;355:1863–72. [DOI] [PubMed] [Google Scholar]
- 29.Imperiale TF, Abhyankar PR, Stump TE, et al. Prevalence of advanced, precancerous colorectal neoplasms in black and white populations: A systematic review and meta-analysis. Gastroenterology 2018;155: 1776–86.e1. [DOI] [PubMed] [Google Scholar]
- 30.Winawer SJ, Zauber AG, O’Brien MJ, et al. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer 1992;70:1236–45. [DOI] [PubMed] [Google Scholar]
- 31.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251–70. [DOI] [PubMed] [Google Scholar]
- 32.Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg 1979;190:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond JH. Polyp guideline: Diagnosis, treatment, and surveillance for patients with nonfamilial colorectal polyps. The Practice Parameters Committee of the American College of Gastroenterology. Ann Intern Med 1993;119:836–43. [DOI] [PubMed] [Google Scholar]
- 34.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993;328:901–6. [DOI] [PubMed] [Google Scholar]
- 35.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326: 658–62. [DOI] [PubMed] [Google Scholar]
- 36.Konishi F, Morson BC. Pathology of colorectal adenomas: A colonoscopic survey. J Clin Pathol 1982;35:830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bond JH. Polyp guideline: Diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 2000;95: 3053–63. [DOI] [PubMed] [Google Scholar]
- 38.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85. [DOI] [PubMed] [Google Scholar]
- 39.He X, Hang D, Wu K, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 2020;158:852–61.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holme Ø, Bretthauer M, Eide TJ, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2015;64:929–36. [DOI] [PubMed] [Google Scholar]
- 41.Anderson BW, Smyrk TC, Anderson KS, et al. Endoscopic overestimation of colorectal polyp size. Gastrointest Endosc 2016;83: 201–8. [DOI] [PubMed] [Google Scholar]
- 42.Sakata S, Klein K, Stevenson ARL, et al. Measurement bias of polyp size at colonoscopy. Dis Colon Rectum 2017;60:987–91. [DOI] [PubMed] [Google Scholar]
- 43.van Putten PG, Hol L, van Dekken H, et al. Inter-observer variation in the histological diagnosis of polyps in colorectal cancer screening. Histopathology 2011;58:974–81. [DOI] [PubMed] [Google Scholar]
- 44.Yoon H, Martin A, Benamouzig R, et al. [Inter-observer agreement on histological diagnosis of colorectal polyps: The APACC study]. Gastroenterol Clin Biol 2002;26:220–4. French. [PubMed] [Google Scholar]
- 45.Kolb JM, Soetikno RM, Rao AK, et al. Detection, diagnosis, and resection of sessile serrated adenomas and polyps. Gastroenterology 2017;153: 646–8. [DOI] [PubMed] [Google Scholar]
- 46.Khalid O, Radaideh S, Cummings OW, et al. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J Gastroenterol 2009;15:3767–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: Is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice? Virchows Arch 2007;450:613–8. [DOI] [PubMed] [Google Scholar]
- 48.Kolb JM, Morales SJ, Rouse NA, et al. Does better specimen orientation and a simplified grading system promote more reliable histologic interpretation of serrated colon polyps in the community practice setting? Results of a nationwide study. J Clin Gastroenterol 2016;50: 233–8. [DOI] [PubMed] [Google Scholar]
- 49.Tinmouth J, Henry P, Hsieh E, et al. Sessile serrated polyps at screening colonoscopy: Have they been under diagnosed? Am J Gastroenterol 2014;109:1698–704. [DOI] [PubMed] [Google Scholar]
- 50.Glatz K, Pritt B, Glatz D, et al. A multinational, internet-based assessment of observer variability in the diagnosis of serrated colorectal polyps. Am J Clin Pathol 2007;127:938–45. [DOI] [PubMed] [Google Scholar]
- 51.Payne SR, Church TR, Wandell M, et al. Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol 2014;12:1119–26. [DOI] [PubMed] [Google Scholar]
- 52.Abdeljawad K, Vemulapalli KC, Kahi CJ, et al. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc 2015;81: 517–24. [DOI] [PubMed] [Google Scholar]
- 53.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal. Version 3, 2019. (https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf). Accessed November 1, 2019.
- 54.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolb JM, Ahnen DJ, Samadder NJ. Evidenced-based screening strategies for a positive family history. Gastrointest Endosc Clinic N Am. 2020;30: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winawer SJ, Zauber AG, Gerdes H, et al. Risk of colorectal cancer in the families of patients with adenomatous polyps. National Polyp Study Workgroup. N Engl J Med 1996;334:82–7. [DOI] [PubMed] [Google Scholar]
- 57.Ko C, Hyman NH; Standards Committee of The American Society of Colon and Rectal Surgeons, et al. Practice parameter for the detection of colorectal neoplasms: An interim report (revised). Dis Colon Rectum 2006;49:299–301. [DOI] [PubMed] [Google Scholar]
- 58.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: Colorectal cancer screening and surveillance. Gastrointest Endosc 2006;63:546–57. [DOI] [PubMed] [Google Scholar]
- 59.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline fromthe American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–95. [DOI] [PubMed] [Google Scholar]
- 60.World Gastroenterology Organisation/International Digestive Cancer Alliance Practice Guidelines: Colorectal Cancer Screening. 2007. Produced by the World Gastroenterology Organisation (WGO) Guidelines and Publications Committee. [Google Scholar]
- 61.Imperiale TF, Ransohoff DF. Risk for colorectal cancer in persons with a family history of adenomatous polyps: A systematic review. Ann Intern Med 2012;156:703–9. [DOI] [PubMed] [Google Scholar]
- 62.Nakama H, Zhang B, Fukazawa K, et al. Family history of colorectal adenomatous polyps as a risk factor for colorectal cancer. Eur J Cancer 2000;36:2111–4. [DOI] [PubMed] [Google Scholar]
- 63.Austin GL, Goldstein JI, Peters SL, et al. Are colorectal cancer screening recommendations for first-degree relatives of patients with adenomas too aggressive? Clin Gastroenterol Hepatol 2011;9:308–13. [DOI] [PubMed] [Google Scholar]
- 64.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–50. [DOI] [PubMed] [Google Scholar]
- 65.National Comprehensive Cancer Network. Colon Cancer. Version 2, 2017. (https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf). Accessed November 1, 2019.
- 66.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 67.Ng SC, Kyaw MH, Suen BY, et al. Prospective colonoscopic study to investigate risk of colorectal neoplasms in first-degree relatives of patients with non-advanced adenomas. Gut 2020;69:304–10. [DOI] [PubMed] [Google Scholar]
- 68.Lynch KL, Ahnen DJ, Byers T, et al. First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin Gastroenterol Hepatol 2003;1:96–102. [DOI] [PubMed] [Google Scholar]
- 69.Tuohy TM, Rowe KG, Mineau GP, et al. Risk of colorectal cancer and adenomas in the families of patients with adenomas: A population-based study in Utah. Cancer 2014;120:35–42. [DOI] [PubMed] [Google Scholar]
- 70.Lowery JT, Ahnen DJ, Schroy PC, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer 2016;122:2633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noffsinger AE. Serrated polyps and colorectal cancer: New pathway to malignancy. Annu Rev Pathol 2009;4:343–64. [DOI] [PubMed] [Google Scholar]
- 72.Burgess NG, Pellise M, Nanda KS, et al. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut 2016;65:437–46. [DOI] [PubMed] [Google Scholar]
- 73.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020; 158:1131–53.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon JY, Kim HT, Hong SP, et al. High-risk metachronous polyps are more frequent in patients with traditional serrated adenomas than in patients with conventional adenomas: A multicenter prospective study. Gastrointest Endosc 2015;82:1087–93.e3. [DOI] [PubMed] [Google Scholar]
- 75.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010;139:1497–502. [DOI] [PubMed] [Google Scholar]
- 76.Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702. [DOI] [PubMed] [Google Scholar]
- 77.Hiraoka S, Kato J, Fujiki S, et al. The presence of large serrated polyps increases risk for colorectalcancer. Gastroenterology 2010;139:1503–10, 1510.e1–3. [DOI] [PubMed] [Google Scholar]
- 78.Anderson JC, Butterly LF, Robinson CM, et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: Data from the New Hampshire colonoscopy registry. Gastroenterology 2018;154:117–27.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pyo JH, Ha SY, Hong SN, et al. Identification of risk factors for sessile and traditional serrated adenomas of the colon by using big data analysis. J Gastroenterol Hepatol 2018;33:1039–46. [DOI] [PubMed] [Google Scholar]
- 80.Egoavil C, Juarez M, Guarinos C, et al. Increased Risk of Colorectal Cancer in Patients With Multiple Serrated Polyps and Their First-Degree Relatives. Gastroenterology 2017;153:106–112 e2. [DOI] [PubMed] [Google Scholar]
- 81.Snover DC, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, et al. , eds. WHO classification of tumours of the digestive system. Vol 3. 4th ed. World Health Organization: Lyon, France, 2010, pp, 160–165. [Google Scholar]
- 82.Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016;150:895–902.e5. [DOI] [PubMed] [Google Scholar]
- 83.Tsai MH, Xirasagar S, Li YJ, et al. Colonoscopy screening among US adults aged 40 or older with a family history of colorectal cancer. Prev Chronic Dis 2015;12:E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cottet V, Pariente A, Nalet B, et al. Low compliance with colonoscopic screening in first-degree relatives of patients with large adenomas. Aliment Pharmacol Ther 2006;24:101–9. [DOI] [PubMed] [Google Scholar]
- 85.Atkinson TM, Salz T, Touza KK, et al. Does colorectal cancer risk perception predict screening behavior? A systematic review and metaanalysis. J Behav Med 2015;38:837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SE, Pérez-Stable EJ, Wong S, et al. Association between cancer risk perception and screening behavior among diverse women. Arch Intern Med 2008;168:728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumaravel V, Heald B, Lopez R, et al. Patients do not recall important details about polyps, required for colorectal cancer prevention. Clin Gastroenterol Hepatol 2013;11:543–7.e1–2. [DOI] [PubMed] [Google Scholar]
- 88.Brock AS, Wallace K, Romagnuolo J, et al. Patients’ short-term knowledge of personal polyp history inadequate despite systematic notification of results after polypectomy. South Med J 2013;106:285–9. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi LC, Wardle J, von Wagner C. Limited health literacy is a barrier to colorectal cancer screening in England: Evidence from the English longitudinal study of ageing. Prev Med 2014;61:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson MR, Grubber J, Grambow SC, et al. Physician non-adherence to colonoscopy interval guidelines in the Veterans Affairs Healthcare System. Gastroenterology 2015;149:938–51. [DOI] [PubMed] [Google Scholar]
- 91.Murphy CC, Sandler RS, Grubber JM, et al. Underuse and overuse of colonoscopy for repeat screening and surveillance in the Veterans Health Administration. Clin Gastroenterol Hepatol 2016;14:436–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murphy CC, Lewis CL, Golin CE, et al. Underuse of surveillance colonoscopy in patients at increased risk of colorectal cancer. Am J Gastroenterol 2015;110:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saini SD, Nayak RS, Kuhn L, et al. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: Results of a national survey. J Clin Gastroenterol 2009;43:554–8. [DOI] [PubMed] [Google Scholar]
- 94.Shah TU, Voils CI, McNeil R, et al. Understanding gastroenterologist adherence to polyp surveillance guidelines. Am J Gastroenterol 2012; 107:1283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yabroff KR, Klabunde CN, Yuan G, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med 2011;26:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel SG, Lowery JT, Gatof D, et al. Practical opportunities to improve early detection and prevention of colorectal cancer (CRC) in members of high-risk families. Dig Dis Sci 2015;60:748–61. [DOI] [PubMed] [Google Scholar]
- 97.Martinez ME, Marshall JR. Environmental and life style issues in colorectal cancer. In: Levin B, Kelsen DP, Daly JM, Kern SE, Tepper JE (eds). Gastrointestinal Oncology: Principles and Practices. Lippincott Williams and Wilkins: Philadelphia, PA, 2002, pp 665–83. [Google Scholar]
- 98.Courtney RJ, Paul CL, Carey ML, et al. A population-based cross-sectional study of colorectal cancer screening practices of first-degree relatives of colorectal cancer patients. BMC Cancer 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kessels K, Eisinger JD, Letteboer TG, et al. Sending family history questionnaires to patients before a colonoscopy improves genetic counseling for hereditary colorectal cancer. J Dig Dis 2017;18:343–8. [DOI] [PubMed] [Google Scholar]
- 100.Singh H, Schiesser R, Anand G, et al. Underdiagnosis of Lynch syndrome involves more than family history criteria. Clin Gastroenterol Hepatol 2010;8:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molmenti CL, Kolb JM, Karlitz JJ. Advanced colorectal polyps on colonoscopy: A Trigger for earlier screening of family members. Am J Gastroenterol 2020;115:311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Molmenti CLSP Ahnen DJ, Karlitz J, et al. ; National Colorectal Cancer Advanced Adenoma Working Group, American Cancer Society. Advanced Colorectal Polyp: GI Brief. American Cancer Society, 2019. (https://nccrt.org/resource/advanced-colorectal-polyp-brief/). [Google Scholar]
- 103.Cohen J, Pike IM. Defining and measuring quality in endoscopy. Gastrointest Endosc 2015;81:1–2. [DOI] [PubMed] [Google Scholar]
- 104.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 105.Ng SC, Lau JY, Chan FK, et al. Risk of advanced adenomas in siblings of individuals with advanced adenomas: A cross-sectional study. Gastroenterology 2016;150:608–16; quiz e16–7. [DOI] [PubMed] [Google Scholar]


