Abstract
Patients with higher-risk myelodysplastic syndromes (MDS) refractory to hypomethylating agents (HMAs) have limited therapeutic options and an expected overall survival (OS) of 3–5 months. Eltanexor is an investigational oral selective inhibitor of nuclear export with low central nervous system penetrance and an acceptable tolerability profile. Preclinical studies suggest that myeloid malignancies are sensitive to nuclear export inhibition. Eltanexor exhibited efficacy in hematologic models, supporting exploration in a clinical trial. This phase 1/2 study (NCT02649790) assessed single-agent activity of eltanexor in patients with higher-risk MDS and 5–19% myeloblasts. Two starting doses of eltanexor were evaluated: 20 mg (n = 15), 10 mg (n = 5), both administered on days 1–5 each week of a 28-day cycle. Twenty patients with primary HMA-refractory MDS, with a median age of 77 years (range 62–89), and a median of two prior treatment regimens (range 1–4) were enrolled. Of these, 15 were evaluated for efficacy and 20 for safety. The overall response rate (ORR) was 53.3%, with seven patients (46.7%) achieving marrow complete remission (mCR) and one additional patient achieving hematologic improvement (HI). In the 10 mg group, three patients (60%) reached mCR and two (40%) stable disease (SD), while for 20 mg, four patients (40%) had mCR and two (20%) SD. A total of three patients (20%) had HI and became transfusion independent ≥ 8 weeks. Median OS for the efficacy-evaluable patients (n = 15) was 9.86 months (7.98, NE). Overall, the most frequently reported treatment-related adverse events were nausea (45%), diarrhea (35%), decreased appetite (35%), fatigue and neutropenia (both 30%). Single-agent oral eltanexor was active, safe, and well tolerated in patients with higher-risk, primary HMA-refractory MDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01319-y.
To the Editor:
Higher-risk myelodysplastic syndrome (MDS) carries a significant risk of progression to acute myeloid leukemia (AML) and decreased survival [1]. Hypomethylating agents (HMAs) are first-line therapies for this population, demonstrating tolerability and improved overall survival (OS) [2–5]. However, HMAs are not curative for MDS, and thus, treatment of refractory or relapsed higher-risk MDS patients after HMA therapy represents a profound unmet medical need, with no approved therapeutics and median OS (mOS) of 4–5.6 months [6, 7].
Selective inhibitor of nuclear export (SINE) compounds are a class of novel and specific antagonists of the karyopherin exportin 1 protein (XPO1). Eltanexor is a reversible, oral SINE compound, which in non-clinical AML mouse studies showed robust single-agent activity, with improved tolerability and overall survival [8, 9]. We therefore analyzed eltanexor treatment in a phase 1 cohort of patients with higher-risk, HMA-refractory MDS.
Twenty patients with MDS were treated with single-agent eltanexor at a starting dose of 20 mg (n = 15) or 10 mg (n = 5) on days 1–5 of each week of each 28-day cycle (Additional file 1: Figure S1). The majority of patients were aged ≥ 75 years with de novo MDS, and all patients had MDS primary refractory to HMAs (Additional file 1: Table S1).
Of the 20 patients, seven (35%) had marrow complete remission (mCR), including two patients that also achieved hematologic improvement (HI). One (5%) additional patient had HI and stable disease (SD), and four patients (20%) had SD. Overall, the disease control rate (DCR) was 60% (95% CI 36.1–80.9), and the overall response rate (ORR) was 40% (95% CI 19.1–64.0). Of the 20 patients enrolled, 15 patients were evaluable for efficacy, as five 20 mg patients discontinued the study and were thus not evaluable for disease response; ORR for the efficacy-evaluable patients was 53.3% (95% CI 26.6–78.7; Table 1).
Table 1.
Efficacy in evaluable patients
| Eltanexor (10 mg) N = 5 |
Eltanexor (20 mg) N = 10 |
Total N = 15 |
|
|---|---|---|---|
| ORR, n (%) | 3 (60) | 5 (50) | 8 (53.3) |
| MCR, n (%) | 3 (60) | 4 (40) | 7 (46.7) |
| HI, n (%) | 1 (20) | 2 (20) | 3 (20) |
| HI with MCR | 1 (20) | 1 (10) | 2 (13.3) |
| HI with SD | 0 | 1 (10) | 1 (6.7) |
| SD, n (%) | 2 (40) | 2 (20) | 4 (26.7) |
| PD, n (%) | 0 | 3 (30) | 3 (20) |
| Treatment duration, weeks | 15 | 12 | 13 |
| Median time to response, weeks | 8.1 | 9.1 | 8.4 |
| Median duration of response, weeks (95% CI) |
7.86 (NE, NE) |
25.29 (13.12, NE) |
19.21 |
ORR overall response rate; MCR marrow complete remission; HI hematologic improvement; SD stable disease; PD progressive disease; CI confidence interval; NE not evaluable
Three patients (20%) had HI and became transfusion independent ≥ 8 weeks. Among patients on eltanexor at the 20 mg dose, four patients (40%) reached mCR, including one patient who reached mCR with HI, one patient (10%) had HI and SD, and two (20%) had SD. Of patients on the 10 mg dose, three patients (60%) reached mCR, including one patient who had mCR with HI, and two patients had SD (40%).
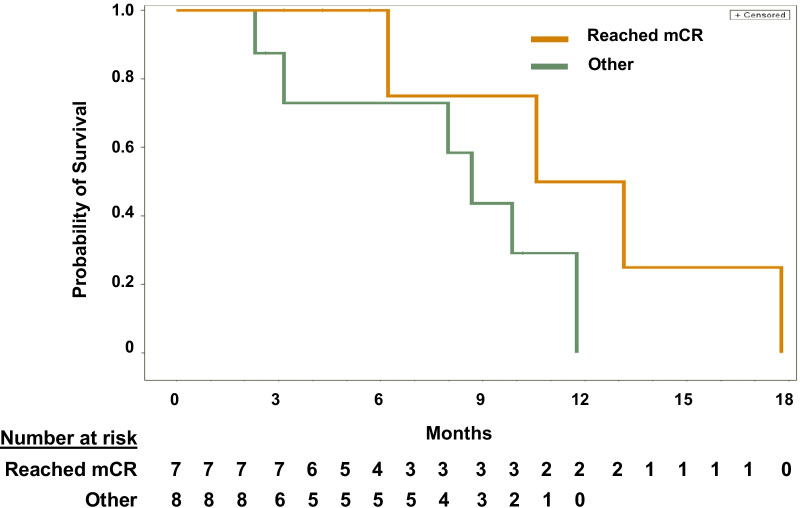
After 3–4 cycles of treatment, levels of normal neutrophils and platelets improved, while blasts decreased, and patients had a median reduction in bone marrow blasts from baseline of -78.2% (range −86% to 33%; Additional file 1: Figure S2). mOS for the entire efficacy-evaluable cohort was 9.86 months. OS for patients who reached mCR was longer than for those who did not reach mCR, with a median of 11.86 vs 8.67 months, respectively (HR = 0.28, p = 0.11; Fig. 1). Additionally, mOS for patients with mCR was longer than mOS for patients with progressive disease (PD; 3.15 months [HR = 0.23, p = 0.08]), and patients with SD had longer OS than patients with PD (9.86 vs 3.15 months, HR = 0.38, p = 0.09).
Fig. 1.
Overall Survival. The median OS for all patients was 9.86 months. OS was higher in mCR patients (n = 7) than patients who did not reach mCR (n = 8): median 11.86 vs 8.67 months (HR = 0.28, p = 0.11), and longer than OS for patients with PD (n = 3, mOS = 3.15 months, HR = 0.23, p = 0.08). mCR = marrow complete remission; OS = overall survival; HR = hazard ratio; PD = progressive disease
None of the patients experienced dose-limiting toxicities (DLTs). The most frequently occurring adverse events (AEs) of any grade were non-hematologic and occurred more often in the 20 mg treatment group than the 10 mg group (Additional file 1: Table S2). Serious AEs (grade 3/4) were infrequent, and there were no grade 5 events. Of the 20 patients initially treated with eltanexor, seven patients (35.0%) required a dose delay, and eight patients (40.0%) required a dose reduction to 10 mg due to adverse events.
Novel agents to treat relapsed/refractory MDS patients are currently in development, though recent phase 3 trials to develop novel treatments for higher-risk MDS patients were unsuccessful, failing to meet the primary endpoints of improving OS [10, 11]. All patients in this study had MDS primary refractory to HMAs, 19/20 were considered higher risk by IPSS scoring, and 25% of patients had poor risk cytogenetics. Despite these factors, the ORR was still 53.3% in efficacy-evaluable patients, demonstrating an encouraging initial response to eltanexor monotherapy. Consistent with the selectivity of eltanexor for leukemic cells, responses included both a substantial reduction in blasts and commensurate increases in normal neutrophils and platelets, even for patients who achieved SD rather than a response [12]. A limitation of this report is the small patient population.
Overall, eltanexor represents a mechanistically novel, oral agent with good tolerability and robust single-agent activity conferring improved survival relative to historical controls, supporting the continued development of this novel agent.
Supplementary Information
Additional file 1. Methods, supplementary tables S1 and S2, and supplementary figures S1 and S2.
Acknowledgements
The authors would like to acknowledge the helpful discussion and review by Eric Sbar.
JetPub Scientific Communications, LLC, funded by Karyopharm Therapeutics, provided drafts and editorial assistance to the authors during preparation of this manuscript.
Abbreviations
- AE
Adverse event
- AML
Acute myeloid leukemia
- CI
Confidence intervals
- DLT
Dose-limiting toxicity
- DOR
Duration of response
- HI
Hematologic improvement
- HMA
Hypomethylating agent
- HR
Hazard ratio
- IPSS
International Prognostic Scoring System
- mCR
Marrow complete response
- MDS
Myelodysplastic syndromes
- mOS
Median overall survival
- NE
Not evaluable
- ORR
Overall response rate
- OS
Overall survival
- PD
Progressive disease
- SD
Stable disease
- SINE
Selective inhibitor of nuclear export
- XPO1
Exportin 1
Author contributions
SL, SM, JK, KC, AD, FY, and EB collected the data. JS, SS, MK, and BB contributed to the study design. FY analyzed the data. All authors interpreted the data. All authors edited, reviewed manuscript drafts, and approved the final version.
Funding
This study was supported by Karyopharm Therapeutics Inc.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients prior to enrollment by the ethics committee at each respective institution.
Consent for publication
Not applicable.
Competing interests
SL reports consultancy for Abbvie, AstraZeneca, BMS, Innate, Pin Therapeutics, and employment at Janssen Research and Development. SM reports research funding from Incyte, Karyopharm, and Taiho. JK, KC, AD, FY, and EB are all current employees of Karyopharm Therapeutics Inc. MK and SS are stockholders of Karyopharm Therapeutics Inc. JS, MK, and SS are former employees of Karyopharm Therapeutics Inc. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bewersdorf JP, Carraway H, Prebet T. Emerging treatment options for patients with high-risk myelodysplastic syndrome. Ther Adv Hematol. 2020;11:204062072095500. doi: 10.1177/2040620720955006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, Issa JPJ, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 3.Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the european organisation for rese. J Clin Oncol. 2011;29:1987–1996. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 4.Gurion R, Vidal L, Gafter-Gvili A, Yeshurun YB, Raanani P, Shpilberg O. 5-Azacitidine prolongs overall survival in patients with myelodysplastic syndrome - a systematic review and meta-analysis. Haematologica. 2010;95:303–310. doi: 10.3324/haematol.2009.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenaux P, Mufti GJ, Santini V, et al. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional care regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110:817–817. doi: 10.1182/blood.V110.11.817.817. [DOI] [Google Scholar]
- 6.Prébet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adès L. High-risk MDS after HMAs. HemaSphere. 2019;3:138–140. doi: 10.1097/HS9.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hing ZA, Fung HYJ, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016;30:2364–2372. doi: 10.1038/leu.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.XPOVIO highlights of prescribing information. www.fda.gov/medwatch. (Accessed Feb 1, 2021).
- 10.Garcia-Manero G, Fenaux P, Al-Kali A, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:496–508. doi: 10.1016/S1470-2045(16)00009-7. [DOI] [PubMed] [Google Scholar]
- 11.Astex Pharmaceuticals Inc. Astex and Otsuka announce results of phase 3 ASTRAL-2 and ASTRAL-3 studies of guadecitabine (SGI-110) in patients with previously treated acute myeloid leukemia (AML) and myelodysplastic syndromes or chronic myelomonocytic leukemia (MDS/CMML) – Astex. 2020. https://astx.com/astex-and-otsuka-announce-results-of-phase-3-astral-2-and-astral-3-studies-of-guadecitabine-sgi-110-in-patients-with-previously-treated-acute-myeloid-leukemia-aml-and-myelodysplastic-syndromes-or/ (Accessed Jan 27, 2022).
- 12.Etchin J, Berezovskaya A, Conway AS, et al. KPT-8602, a second-generation inhibitor of XPO1-mediated nuclear export, is well tolerated and highly active against AML blasts and leukemia-initiating cells. Leukemia. 2017;31:143–150. doi: 10.1038/leu.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Methods, supplementary tables S1 and S2, and supplementary figures S1 and S2.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.