Abstract
Background
This study was conducted to compare the number of cases of non-airborne/droplet-transmitted notifiable infectious disease (NID) before and after COVID-19 pandemic.
Methods
This study used an open database - National Notifiable Diseases Surveillance System to collect the epidemiological data of NIDs. Ten fecal-oral-, six vector-borne-, four direct-contact, and four sexually-transmitted NIDs between pandemic period (defined as from January 2020 to December 2021) and the pre-pandemic period (defined as the period from January 2018 to December 2019) were included for the analysis.
Results
Overall, the annual case number of these 24 non-airborne/droplet-transmitted NIDs was 19,186, 19,101, 19,567, and 19,863 in 2018, 2019, 2020 and 2021, respectively. The overall case number in the pandemic period was higher than those in pre-pandemic period (39,430 vs 38,287) and the monthly case number was significantly higher in pandemic period than pre-pandemic period (1643 vs 1595, p < 0.05). However, the lower case number in the pandemic period than those in pre-pandemic period was observed in overall ten fecal-oral-transmitted NIDs (1278 vs 1775), six vector-borne-NIDs (922 vs 2210), and four direct-contact transmitted NIDs (196 vs 344). In contrast, the case number of sexually-transmitted NIDs in the pandemic period was higher than those in pre-pandemic period (37,034 vs 33,958), particularly for gonorrhea (14,463 vs 8732).
Conclusions
Most of the fecal-oral-, vector-borne, and direct-contact transmitted NIDs had declined during pandemic in Taiwan. In contrast, gonorrhea had large increase, and other NPIs were needed.
Abbreviations: NID, notifiable infectious disease; NPI, non-pharmaceutical intervention
Keywords: COVID-19, Fecal-oral, Vector-borne, Contact, Sexually-transmitted, Notifiable infectious disease, Taiwan
1. Introduction
It has been more than two years after the first outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), in Wuhan, China and there have been more than 485 million confirmed cases of coronavirus disease 2019 (COVID-19) in the whole world [1], [2]. In addition to the development of effective treatments against COVID-19, many non-pharmaceutical interventions (NPIs), such as wearing mask, social distance and avoiding crowd gathering were introduced to prevent the spread or transmission of SARS-CoV-2 through respiratory droplets [3], [4], [5]. Although the implementation of these NPIs was developed to contain COVID-19, many studies had showed it also help decrease the activity of other airborne/droplet-transmitted infectious diseases, such as influenza, invasive pneumococcal diseases (IPD), and measles [6], [7], [8], [9], [10], [11]. In contrast, the effect of NPIs on the epidemiology of non-airborne/droplet-transmitted infectious diseases was rarely reported and most of them were conducted during the first wave of COVID-19 [12], [13], [14], [15], [16], [17]. To better understand the long-term effect of COVID-19 on the activity of non-airborne/droplet-transmitted infectious diseases, this study was conducted to compare the case number of non-airborne/droplet-transmitted notifiable infectious disease (NID) between pandemic period and the pre-pandemic period.
2. Methods
This study used an open database - National Notifiable Diseases Surveillance System. Through this open database (https://nidss.cdc.gov.tw/nndss/disease?id=025), we can freely collect the epidemiological data of NIDs. This database was established to conduct surveillance investigation of important infectious disease for health authority to make preventive policy for the development and transmission of infectious diseases in Taiwan. The infectious disease was considered as NID, if the regular, frequent, and timely information regarding individual cases is necessary. In this study, we investigate the epidemiology of non-airborne/droplet-transmitted NIDs between 2018 and 2021. In contrast, the airborne/droplet-transmitted NIDs and the NIDs with zero cases through the whole study period were excluded.
In this study, we collected the annual case number of fecal-oral-, vector-borne-, direct-contact, and sexually-transmitted NIDs during the study period. In addition to overall case number, we collected the number of domestic and imported cases. To compare the change of epidemiology of NIDs after COVID-19 pandemic, the study period was divided into pre-pandemic period (from January 2018 to December 2019) and pandemic period (from January 2020 to December 2021). To present the magnitude of change, we calculated the percentage (%) of change using the difference of case number between pandemic period and pre-pandemic period divided by the case number in pre-pandemic period. Poisson distribution model was conducted to define change between the monthly cases during the pre-pandemic (2018–2019) and pandemic period (2020–2021) cases.
3. Results
In this study, we extracted the data of ten fecal-oral transmitted NIDs including enterohaemorrhagic E. coli infection, typhoid fever, paratyphoid fever, acute flaccid paralysis and poliomyelitis, amoebiasis, listeriosis, acute hepatitis A infection, toxoplasmosis, shigellosis, and acute hepatitis E infection, six vector-borne-NIDs, including Chikungunya fever, malaria, dengue fever, Zika virus infection, scrub typhus, and Japanese encephalitis, four direct-contact transmitted NIDs including enterovirus infection with severe complications, Hansen’s disease, Leptospirosis, and tetanus, and four sexually-transmitted NID including AIDS, HIV infection, syphilis and gonorrhea. Overall, the annual case number of these 24 non-airborne/droplet-transmitted NIDs was 19,186, 19,101, 19,567, and 19,863 in 2018, 2019, 2020 and 2021, respectively. The overall case number in the pandemic period was higher than those in pre-pandemic period (39,430 vs 38,287) and the monthly case number was significantly higher in pandemic period than pre-pandemic period (p < 0.05).
3.1. Fecal-oral transmitted NIDs
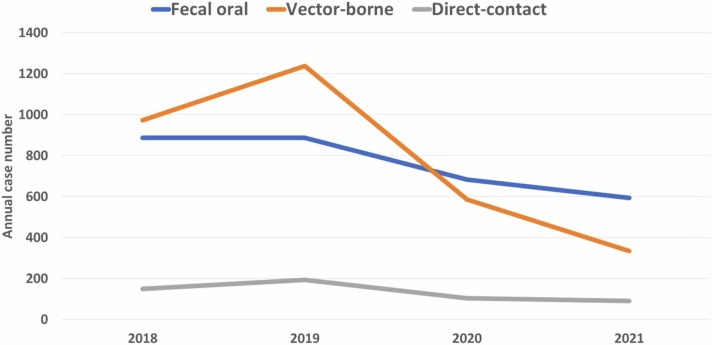
Overall, the annual case number of ten fecal-oral transmitted NID was 887, 888, 684, and 594 in 2018, 2019, 2020 and 2021, respectively ( Fig. 1). The overall case number in the pandemic period was lower than those in pre-pandemic period (1278 vs 1775) and the monthly case number was significantly lower in pandemic period than pre-pandemic period (p < 0.05). The similar trend was observed in domestic cases (1052 vs 1187) and imported cases (226 vs 588). Table 1 summarized the annual case number of each fecal-oral transmitted NID during the study period. The case number of amoebiasis had largest reduction of 249 cases from pre-pandemic period to pandemic period, followed by acute flaccid paralysis and poliomyelitis (−69), shigellosis (−52) and acute hepatitis A infection (−52). Additionally, enterohaemorrhagic E. coli infection, paratyphoid fever, typhoid fever and acute flaccid paralysis and poliomyelitis had more than 50% of reduction from pre-pandemic period to the pandemic period. However, the case number of toxoplasmosis remained unchanged between pre-pandemic and pandemic period. Four domestic diseases – typhoid fever, acute hepatitis A infection, shigellosis, and toxoplasmosis had increased case number during pandemic period than pre-pandemic period. In contrast, six diseases – enterohaemorrhagic E. coli infection, paratyphoid fever, acute flaccid paralysis and poliomyelitis, amoebiasis, acute hepatitis E infection and listeriosis had decreased domestic case number during pandemic period than pre-pandemic period. Finally, all imported cases had lower cases number during pandemic period than pre-pandemic period.
Fig. 1.
Annual case of overall fecal-oral-, vector-, and direct-contract transmitted notifiable infectious disease from 2018 to 2022.
Table 1.
The case number of fecal-oral-transmitted notifiable infectious diseases.
| Disease | All |
Domestic |
Imported |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No of cases |
Change (%) | No of cases |
Change (%) | No of cases |
Change (%) | ||||
| Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | ||||
| Enterohaemorrhagic E. coli infection | 1 | 0 | -100.0 | 1 | 0 | -100.0 | 0 | 0 | – |
| Paratyphoid fever | 17 | 1 | -94.1 | 4 | 1 | -75.0 | 13 | 0 | -100.0 |
| Typhoid fever | 38 | 13 | -65.8 | 8 | 9 | 12.5 | 30 | 4 | -86.7 |
| Acute flaccid paralysis and poliomyelitis | 130 | 61 | -53.1 | 129 | 61 | -52.7 | 1 | 0 | -100.0 |
| Amoebiasis | 687 | 438 | -36.2 | 321 | 248 | -22.7 | 366 | 190 | -48.1 |
| Acute hepatitis E infection | 17 | 12 | -29.4 | 13 | 12 | -7.7 | 4 | 0 | -100.0 |
| Acute hepatitis A infection | 195 | 148 | -24.1 | 136 | 140 | 2.9 | 59 | 8 | -86.4 |
| Shigellosis | 324 | 272 | -16.0 | 216 | 248 | 14.8 | 108 | 24 | -77.8 |
| Listeriosis | 332 | 299 | -9.9 | 330 | 299 | -9.4 | 2 | 0 | -100.0 |
| Toxoplasmosis | 34 | 34 | 0.0 | 29 | 34 | 17.2 | 5 | 0 | -100.0 |
3.2. Vector-borne NIDs
The annual case number of six vector-borne NIDs was 973, 1237, 587 and 335 in 2018, 2019, 2020 and 2021, respectively (Fig. 1). The overall case number in the pandemic period was lower than those in pre-pandemic period (922 vs 2210) and the monthly case number was significantly lower in pandemic period than pre-pandemic period (p < 0.05). The similar trend was observed in domestic cases (836 vs 1186) and imported cases (86 vs 1024). Table 2 summarized the annual case number of each vector-borne NID during the study period. The case number of dengue fever had largest reduction of 1024 from pre-pandemic period to pandemic, followed by scrub typhus (−121) and Chikungunya fever (−119). Additionally, Chikungunya fever, dengue, malaria and Zika virus infection had more than 50% of reduction from pre-pandemic period to the pandemic period. The lower case numbers in pandemic period than those in pre-pandemic period were consistently observed in terms of both domestic and imported cases.
Table 2.
The case number of vector-transmitted notifiable infectious diseases.
| Disease | All |
Domestic |
Imported |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No of cases |
Change (%) | No of cases |
Change (%) | No of cases |
Change (%) | ||||
| Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | ||||
| Chikungunya fever | 123 | 4 | -96.7 | 21 | 0 | -100.0 | 102 | 4 | -96.1 |
| Dengue fever | 1173 | 149 | -87.3 | 283 | 73 | -74.2 | 890 | 76 | -91.5 |
| Malaria | 14 | 4 | -71.4 | 0 | 0 | – | 14 | 4 | -71.4 |
| Zika virus | 7 | 2 | -71.4 | 0 | 0 | – | 7 | 2 | -71.4 |
| Japanese encephalitis | 58 | 49 | -15.5 | 56 | 49 | -12.5 | 2 | 0 | -100.0 |
| Scrub typhus | 835 | 714 | -14.5 | 826 | 714 | -13.6 | 9 | 0 | -100.0 |
3.3. Direct-contact transmitted NIDs
The annual case number of four direct-contact transmitted NIDs was 150, 194, 105 and 91 in 2018, 2019, 2020 and 2021, respectively (Fig. 1). The overall case number in the pandemic period was lower than those in pre-pandemic period (196 vs 344) and the monthly case number was significantly lower in pandemic period than pre-pandemic period (p < 0.05). The similar trend was observed in domestic cases (189 vs 330) and imported cases (7 vs 14). Table 3 summarized the annual case number of each direct-contact transmitted NID during the study period. The case number of enterovirus infection with severe complication had largest reduction of 99 from pre-pandemic period to pandemic, followed by leptospirosis (−41), Hansen’s disease (−7) and tetanus (−1). The lower case numbers of each NID in pandemic period than those in pre-pandemic period were observed in both domestic cases and imported cases.
Table 3.
The case number of direct-contact transmitted notifiable infectious diseases.
| Disease | All |
Domestic |
Imported |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No of cases |
Change (%) | No of cases |
Change (%) | No of cases |
Change (%) | ||||
| Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | ||||
| Enterovirus infection with severe complication | 105 | 6 | -94.3 | 104 | 6 | -94.2 | 1 | 0 | -100.0 |
| Hansen's disease | 17 | 10 | -41.2 | 6 | 4 | -33.3 | 11 | 6 | -45.5 |
| Leptospirosis | 207 | 166 | -19.8 | 205 | 166 | -19.0 | 2 | 0 | -100.0 |
| Tetanus | 15 | 14 | -6.7 | 15 | 13 | -13.3 | 0 | 1 | – |
3.4. Sexually transmitted NIDs
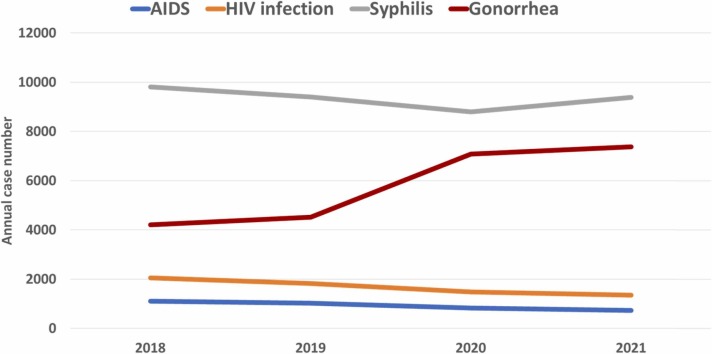
The annual case number of four sexually-transmitted NIDs was 17,176, 16,782, 18,191, and 18,843 in 2018, 2019, 2020 and 2021, respectively. The overall case number in the pandemic period was higher than those in pre-pandemic period (37,034 vs 33,958) and the monthly case number was significantly higher in pandemic period than pre-pandemic period (p < 0.05). In addition to gonorrhea, which has increased from the pre-pandemic period (n = 8732) to the pandemic period (n = 14,463), all the other sexually-transmitted diseases – AIDS, HIV infection and syphilis had reduced number during the pandemic period ( Fig. 2). The case number of domestic gonorrhea increased during pandemic (+5734) but the number of imported gonorrhea decreased (−3) ( Table 4). For syphilis, both domestic and imported cases had decreased during pandemic period (Table 4).
Fig. 2.
Annual case of overall sexually transmitted notifiable infectious disease from 2018 to 2022.
Table 4.
The case number of sexually-transmitted notifiable infectious diseases.
| Disease | All |
Domestic |
Imported |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No of cases |
Change (%) | No of cases |
Change (%) | No of cases |
Change (%) | ||||
| Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | Pre-pandemic period | Pandemic period | ||||
| AIDS | 2131 | 1552 | -27.2 | NA | NA | – | NA | NA | – |
| HIV infection | 3887 | 2828 | -27.2 | NA | NA | – | NA | NA | – |
| Syphilis | 19208 | 18191 | -5.3 | 19200 | 18188 | -5.3 | 8 | 3 | -62.5 |
| Gonorrhea | 8732 | 14463 | 65.6 | 8728 | 14462 | 65.7 | 4 | 1 | -75.0 |
NA, not available
4. Discussion
This study evaluated the effect of COVID-19 on the epidemiology of 24 non- airborne/droplet-transmitted NIDs in Taiwan. Although we found the overall case number of these 24 NIDs had an increase of 1143, which from 38,287 during pre-pandemic period to 39,430 during pandemic period, the increasing case number was largely driven by the change of gonorrhea with an increase of 5731 and further detailed analysis according to the transmission routes and specific NIDs showed the different findings. First, in addition to sexually-transmitted diseases, which had increased during pandemic, all the other types of infections, including fecal-oral-, vector-borne, and direct-contact transmitted NIDs had decreased during pandemic. Moreover, the decreasing trend remained unchanged in domestic NIDs for these three types of overall infections. Among 24 specific NIDs, 22 NIDs had decreased, one – toxoplasmosis remained the same and gonorrhea had large increase during pandemic. In addition, among domestic NIDs with available data for analysis, the decreased number was observed for 15 NIDs, but the increased number was noted in four fecal-oral transmitted NIDs, including typhoid fever (+1), acute hepatitis A infection (+4), toxoplasmosis (+5), and shigellosis (+32), and one sexually-transmitted NID - gonorrhea (+5734). In summary, this study demonstrated the most of fecal-oral-, vector-borne, and direct-contact transmitted NIDs in both overall and domestic cases.
The possible mechanisms of these reductions could be multifactorial. First, in the early 2020, the authority in Taiwan development NPIs, such as universal masking, social distancing, hand hygiene, and travel restriction. Until mid-2020, some restrictions such as travel restriction and social gathering have been gradually relaxed. However, the large outbreak in the mid-2021 brough another upgraded restrictions including school closure and ban indoor dining. Through the whole course, most of the NPIs, such as wearing mask, hand hygiene, and social distancing have been persistently conducted since their implementation in the early pandemic in Taiwan. Therefore, the impact of NPI could be sustained through the study period. However, the reduction of different NIDs would be result from the implementations of different NPIs. The reductions in vector-born and direct contact diseases would be likely largely driven by reductions in travel (both domestic and imported cases). The reductions in foodborne illnesses would be largely attributed to changes in food consumption patterns (more meals consumed inside the home and fewer social gatherings). Second, during COVID-19 outbreak, there could be a considerable avoidance of healthcare settings due to patients’ reluctance to visit hospitals. This issue could be associated with the under-diagnosis and under-reporting of NIDs, and would be a potential cause of the reductions of NIDs. However, this effect might be minimal due to the well-control of COVID-19 and the convenience of healthcare system in Taiwan.
The decreasing fecal-oral-, vector-borne, and direct-contact transmitted NIDs during pandemic were consistent with previous studies in other countries [12], [18], [19], [20]. In Netherland, gastrointestinal-related NIDs, such as norovirus disease, hepatitis A and shigellosis and travel-related NIDs, such as malaria, Zika, typhoid fever, cholera, yellow fever and paratyphus were reported 50% less often during the first wave of the COVID-19 epidemic than in the corresponding period in 2017–2019 [18]. In Japan, lower activity of several contact transmitted, and fecal-oral transmitted diseases was observed in 2020 than that in 2015–2019 [12]. In China, gastrointestinal NIDs, including bacterial dysentery, hepatitis A, hepatitis E, hand-food-mouth disease, typhoid and paratyphoid fever, and vector-borne NIDs, including malaria, dengue and hemorrhagic fever with renal syndrome had significant reduction during early pandemic [19]. In Germany, the decreased numbers of NIDs during COVID-19 pandemic were observed for gastrointestinal diseases and vector-borne diseases [20]. All these findings suggested that COVID-19-related NPIs through reducing human-to-human contact and environmental exposure might prevent the transmission of fecal-oral-, vector-borne, and direct-contact transmitted NIDs. In contrast to previous studies [12], [18], [19], [20], which investigated the short-term effect of NPI during early pandemic, our findings indicated that the effect of continuing NPIs can persistent for two years.
Second, we found the large increase of gonorrhea cases during pandemic and mild reduction of AIDS, HIV infection and syphilis. These findings were in line with the previous studies [21], [22] in 2020 in Taiwan. Although the exact cause remains unclear, the increasing risk of unsafe sexual behavior may be related to the several NPIs, such as the quarantine at home, and avoidance of outdoor activity, which may prolong the increasing duration of stay at home or indoors. Overall, our findings indicated the COVID-19-related NPIs could not effectively prevent sexually-transmitted NIDs.
Third, we observed the decreasing case number of all imported fecal-oral-, vector-borne, direct-contact and sexually-transmitted NIDs during pandemic. Moreover, several imported NIDs including enterohaemorrhagic E. coli infection, paratyphoid fever, acute flaccid paralysis and poliomyelitis, acute hepatitis E, listeriosis, toxoplasmosis, Japanese encephalitis, scrub typhus, enterovirus infection with severe complication, leptospirosis had reduced to zero during the pandemic. The main cause could be due to the strict border control in Taiwan. However, it is impossible to strictly conduct border control for a long time for the sake of the economy. Further surveillance investigation should be continued after gradually loosing border control and travel restriction.
This study had several limitations. The NPIs varied in different countries and the NPIs in Taiwan were strictly implemented, so the finding of this study in Taiwan may not be generalized to other countries. In addition, many NPI have been implemented during the study period, so we could not clarify the effect of specific NPI on the each NID. However, our findings indicated the importance of surveillance investigation during COVID-19 and suggested that every site should keep monitoring the epidemiology of NIDs to make further preventive policy.
In conclusion, most of the fecal-oral-, vector-borne, and direct-contact transmitted NIDs had declined during the pandemic in Taiwan, however, the large increase gonorrhea during pandemic became another serious concern.
Ethical approval and consent to participate
Not required.
Consent for publication
Not applicable.
Funding
None.
CRediT authorship contribution statement
Study concept and design: SHH, WTL and CCL; Collection and assembly of data: SHH, WTL and JHW; Data analysis and interpretation: All authors; Manuscript writing: CCL; Final approval of the manuscript: All authors.
Declaration of Competing Interest
No conflict of interest.
Acknowledgment
None.
References
- 1.Lai C.C., Wang C.Y., Wang Y.H., Hsueh S.C., Ko W.C., Hsueh P.R. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Heath Organization. 〈https://covid19.who.int/〉 Accessed on April 1, 2022.
- 3.Tully M.A., McMaw L., Adlakha D., Blair N., McAneney J., McAneney H., et al. The effect of different COVID-19 public health restrictions on mobility: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayouni I., Maatoug J., Dhouib W., Zammit N., Fredj S.B., Ghammam R., et al. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC Public Health. 2021;21:1015. doi: 10.1186/s12889-021-11111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C., Yen M.Y., Lee P.I., Hsueh P.R. How to keep COVID-19 at Bay: a Taiwanese perspective. J Epidemiol Glob Health. 2021;11:1–5. doi: 10.2991/jegh.k.201028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto H., Ishikane M., Ueda P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA. 2020;323:1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng J.L.L., Fok K.M.N., Lin K.P.K., Chan E., Ma Y., Lau S.K.P., et al. Substantial decline in invasive pneumococcal disease during coronavirus disease 2019 pandemic in Hong Kong. Clin Infect Dis. 2022;74:335–338. doi: 10.1093/cid/ciab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rana M.S., Usman M., Alam M.M., Mere M.O., Ikram A., Zaidi S.S.Z., et al. Impact of COVID-19 pandemic on measles surveillance in Pakistan. J Infect. 2021;82:414–451. doi: 10.1016/j.jinf.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong N.S., Leung C.C., Lee S.S. Abrupt subsidence of seasonal influenza after COVID-19 outbreak, Hong Kong, China. Emerg Infect Dis. 2020;26:2753–2755. doi: 10.3201/eid2611.200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh J.Y., Seong H., Yoon J.G., Song J.Y., Cheong H.J., Kim W.J. Social distancing against COVID-19: implication for the control of influenza. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Wang K., Zhong H., Zhao N., Xu W., Yang Y., et al. The effect of coronavirus 2019 disease control measures on the incidence of respiratory infectious disease and air pollutant concentrations in the Yangtze River Delta Region, China. Int J Environ Res Public Health. 2022;19(3):1286. doi: 10.3390/ijerph19031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibiya K., Iwata H., Kinjo T., Shinzato A., Tateyama M., Ueda S., et al. Incidence of common infectious diseases in Japan during the COVID-19 pandemic. PLoS One. 2022;17 doi: 10.1371/journal.pone.0261332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai C.C., Chen S.Y., Yen M.Y., Lee P.I., Ko W.C., Hsueh P.R. The impact of the coronavirus disease 2019 epidemic on notifiable infectious diseases in Taiwan: a database analysis. Travel Med Infect Dis. 2021;40 doi: 10.1016/j.tmaid.2021.101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latini A., Magri F., Donà M.G., Giuliani M., Cristaudo A., Zaccarelli M. Is COVID-19 affecting the epidemiology of STIs? The experience of syphilis in Rome. Sex Transm Infect. 2021;97:78. doi: 10.1136/sextrans-2020-054543. [DOI] [PubMed] [Google Scholar]
- 15.Jiang L., Liu Y., Su W., Liu W., Yang Z. Decreased dengue cases attributable to the effect of COVID-19 in Guangzhou in 2020. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen P.K., Lind A., Klundby I., Dudman S. The incidence of infectious diseases and viruses other than SARS-CoV-2 amongst hospitalised children in Oslo, Norway during the Covid-19 pandemic 2020-2021. J Clin Virol. 2022;2 doi: 10.1016/j.jcvp.2021.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin E.C., Tu H.P., Hong C.H. Halved incidence of scrub typhus after travel restrictions to confine a surge of COVID-19 in Taiwan. Pathogens. 2021;10(11):1386. doi: 10.3390/pathogens10111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Deursen B., Hagenaars M., Meima A., van Asten L., Richardus J.H., Fanoy E., et al. A sharp decrease in reported non-COVID-19 notifiable infectious diseases during the first wave of the COVID-19 epidemic in the Rotterdam region, the Netherlands: a descriptive study. BMC Infect Dis. 2022;22:208. doi: 10.1186/s12879-022-07209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng M.J., Zhang H.Y., Yu L.J., Lv C.L., Wang T., Che T.L., et al. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021;12:6923. doi: 10.1038/s41467-021-27292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullrich A., Schranz M., Rexroth U., Hamouda O., Schaade L., Diercke M., et al. Impact of the COVID-19 pandemic and associated non-pharmaceutical interventions on other notifiable infectious diseases in Germany: an analysis of national surveillance data during week 1-2016 - week 32-2020. Lancet Reg Health Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia C.C., Chao C.M., Lai C.C. Diagnoses of syphilis and HIV infection during the COVID-19 pandemic in Taiwan. Sex Transm Infect. 2021;97:319. doi: 10.1136/sextrans-2020-054802. [DOI] [PubMed] [Google Scholar]
- 22.Lee K.K., Lai C.C., Chao C.M., Tang H.J. Increase in sexually transmitted infection during the COVID-19 pandemic in Taiwan. J Eur Acad Dermatol Venereol. 2021;35:e171–e172. doi: 10.1111/jdv.17005. [DOI] [PubMed] [Google Scholar]