Abstract
Background
At present, no single efficacious therapeutic exists for acute COVID-19 management and a multimodal approach may be necessary. 2-deoxy-d-glucose (2-DG) is a metabolic inhibitor that has been shown to limit multiplication of SARS-CoV-2 in-vitro. We evaluated the efficacy and safety of 2-DG as adjunct to standard care in the treatment of moderate to severe COVID-19 patients.
Methods
We conducted a randomized, open-label, phase II, clinical study to evaluate the efficacy, safety, and tolerability of 2-DG administered as adjunct to standard of care (SOC). A total of 110 patients between the ages of 18 and 65 years with moderate to severe COVID-19 were included. Patients were randomized to receive 63, 90, or 126 mg/kg/day 2-DG in addition to SOC or SOC only. Times to maintaining SpO2 ≥ 94% on room air, discharge, clinical recovery, vital signs normalisation, improvement by 1 and 2 points on WHO clinical progression scale, negative conversion on RT-PCR, requirement for intensive care, and mortality were analyzed to assess the efficacy.
Results
Patients treated with 90 mg/kg/day 2-DG plus SOC showed better outcomes. Time to maintaining SpO2 ≥ 94% was significantly shorter in the 2-DG 90 mg compared to SOC (median 2.5 days vs. 5 days, Hazard ratio [95% confidence interval] = 2.3 [1.14, 4.64], p = 0.0201). Times to discharge from isolation ward, to clinical recovery, and to vital signs normalization were significantly shorter for the 2-DG 90 mg group. All three doses of 2-DG were well tolerated. Thirty-three (30.3%) patients reported 65 adverse events and were mostly (86%) mild.
Conclusions
2-DG 90 mg/kg/day as adjunct to SOC showed clinical benefit over SOC alone in the treatment of moderate to severe COVID-19. The promising trends observed in current phase II study is encouraging for confirmatory evaluation of the efficacy and safety of 2-DG in a larger phase III trial.
Trial registration: CTRI, CTRI/2020/06/025664. Registered 5th June 2020, http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=44369&EncHid=&modid=&compid=%27,%2744369det%27.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07642-6.
Keywords: COVID-19, SARS-CoV-2, 2-deoxy-d-glucose, Randomised clinical trial
Introduction
Coronavirus disease 2019 (COVID-19) is currently a major global public health crisis. While remarkable progress has been made in vaccine development, there are limited therapeutic interventions available. Although several treatment modalities have been tried, no curative treatment has been found to date for COVID-19, and it is increasingly apparent that a multimodal approach is necessary for acute COVID-19 management [1].
The synthetic glucose analogue 2-deoxy-d-glucose (2-DG, Additional file 1: Figure) has been identified as a potential treatment for COVID-19. It inhibits glycolysis in host cells infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2–4].
Upon infecting host cells, viruses reprogram host cell metabolism for their own rapid replication [5]. New virion assembly requires high levels and turnover of nucleotides and lipids, which are achieved by elevated levels of glucose transporters and enhanced aerobic glycolysis (i.e., Warburg effect) [6]. Administration of 2-DG leads to its preferential accumulation within virally infected host cells, on account of the higher number of glucose transporters on the membranes of these virally infected cells, as compared to uninfected cells. Subsequently, 2-DG blocks glycolysis, resulting in the depletion of adenosine triphosphate (ATP) and anabolic intermediates required for viral replication and packaging. In addition to this direct effect, 2-DG as a mannose analog interferes with N-linked glycosylation of nascent viral proteins to form defective progeny virions with low potential to secondarily infect neighbouring cells; altered/mis-glycosylation also leads to formation of mis-folded proteins causing endoplasmic reticulum stress, thereby activating the unfolded protein response which in turn inhibits viral synthesis and replication [2, 7, 8]. Moreover, 2-DG also exerts anti-inflammatory effects and was shown to inhibit viral infection and inflammation in lungs in a murine model [9].
The antiviral activity of 2-DG was demonstrated in a study in 36 women with herpes simplex infection, when applied topically [10]. Furthermore, in vitro studies have shown significant inhibition of SARS-CoV-2 replication by 2-DG [2–4].
Recent studies using Positron Emission Tomography (PET) with the radiotracer, 18FDG (fluorodeoxyglucose, an analogue of 2-DG) demonstrates substantial accumulation of the radiolabeled FDG in the inflamed lungs of COVID-19 patients [11, 12]. This indicates that 2-DG could also preferentially and disproportionately accumulate in the inflamed lungs of COVID-19 patients. Furthermore, 2-DG has been studied in several clinical trials for treatment of various cancers globally and has demonstrated acceptable tolerability in humans [13] .
This phase II clinical trial was conducted to evaluate the efficacy and safety of 2-DG as adjunctive therapy in the treatment of moderate to severe COVID-19. The starting daily dose of 2-DG, 63 mg/kg was chosen based on tolerability data from previous clinical studies in patients with solid tumours [14, 15]. Dose escalation to 90 mg/kg/day (nearly 1.5 ×) and 126 mg/kg/day (2 ×) were planned if no safety concerns were observed at the starting dose. It should be noted that tolerability up to 250 mg/kg was established in a previous clinical study in glioblastoma multiforme [16].
Methods
This was a phase II, multicentre, randomised, open label, parallel group clinical trial to evaluate the efficacy, safety, and tolerability of 2-DG administered adjunctly to standard of care (SOC), in comparison with SOC alone, in patients with moderate or severe COVID-19. SOC was based on the national guideline [17].
The trial was conducted under the supervision of Drugs Controller General of India (DCGI) and approved by appropriate ethics committees. The sample size (110 patients, 22 subjects in each arm) for this proof-of-concept study was mutually decided upon between the sponsors and DCGI based on the novelty of the drug and with limited efficacy and safety information available in non-cancer patients. The trial was prospectively registered on Clinical Trials Registry-India [CTRI/2020/06/025664 (Registered on: 05/06/2020)].
Participating patients
The trial enrolled male or female patients aged 18–65 years, who were admitted to isolation wards at 12 COVID-19 management hospitals in India. Critically ill patients as defined in the guideline were excluded from the study [17]. The diagnosis of COVID-19 was confirmed by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay of each patient’s nasopharyngeal/oropharyngeal swab specimen.
COVID-19 severity in each patient was assessed according to the guideline [17]. Moderate disease was defined as the presence of dyspnoea and/or hypoxia, fever, cough, including an oxygen saturation level (SpO2) of 90–94% on room air, and a respiratory rate of ≥ 24 per min. Severe disease was defined as the presence of clinical signs of pneumonia plus one of the following: respiratory rate > 30 per min, severe respiratory distress, SpO2 < 90% on room air, but not critically ill, i.e., no acute respiratory distress syndrome (ARDS), multiorgan failure, or septic shock. Since, 2-DG has previously been reported to cause QT prolongation, patients with cardiac conduction delay (QTc > 500 ms) or patients taking any medications known to prolong QT interval (including hydroxychloroquine and azithromycin), were not included in the study. Patients with malabsorption/gastrointestinal abnormalities that may affect drug absorption, and patients with body weight < 45 kg or > 130 kg were excluded from the study.
Trial design
The trial was conducted in two parts, Part A for proof-of-concept (clinical), and Part B for dose-ranging. Powder form of 2-DG was dissolved in 100 mL of potable water and an individualised volume of the solution was dosed orally to patients based on body weight. In Part A, 44 patients were randomised in a 1:1 ratio to receive treatment with either 2-DG 63 mg/kg/day plus SOC (the 2-DG 63 mg group) or SOC only (the SOC1 group). Centralized randomisation was carried out manually using Statistical Analysis Software (SAS, version 9.4), throughout the study. 2-DG was given in two split doses totaling 63 mg/kg/day, viz., 45 mg/kg in the morning and 18 mg/kg in the evening, until the patient was discharged or up to 28 days after the initiation of study treatment (i.e., Day 1), whichever occurred first. In the SOC1 group, SOC was provided as long as required. The dose-ranging Part B was conducted after the safety results from Part A had been reviewed and deemed acceptable. A total of 66 patients were randomised in a 1:1:1 ratio to receive 2-DG 90 mg/kg/day plus SOC (the 2-DG 90 mg group), 2-DG 126 mg/kg/day plus SOC (the 2-DG 126 mg group), and SOC only (SOC2 group). 2-DG was administered in two equally divided doses in the morning and evening, viz., 45 mg/kg for 90 mg group, and 63 mg/kg for 126 mg group. In the two active treatment groups, 2-DG was administered along with SOC for 10 days or until discharge, whichever occurred earlier. In the SOC2 group, SOC was provided as long as required.
For both parts of the study, data were collected through 28 days of study or until a patient was discharged from isolation ward of the hospital, whichever occurred earlier. The patient’s clinical status was evaluated every day by the treating physician using the World Health Organization (WHO) 10-point ordinal scale. The WHO clinical progression scale measures disease progression from 0 to 10, with increasing numbers reflecting the severity of symptoms and recovery in reverse order on the basis of supportive measures used within the health care system. It captures the complete spectrum of clinical illness of COVID-19 patients, ranging from 0 (virus-free) to 10 (death) with different score for patients treated in hospital, on supplemental oxygen, admitted to an intensive care unit or high-dependency unit etc. [18]. The following assessments were done daily; the severity of COVID-19-associated symptoms, vital signs, peripheral blood oxygen saturation (SpO2), partial physical examination, 12-lead electrocardiogram, random blood glucose, adverse events, and concomitant medication. Real-time RT-PCR assay was carried out on nasopharyngeal/oropharyngeal swab samples on Days 3, 7, 10, 14, and 28 (or day of discharge if earlier) during Part A of the study and on Days 1, 3, 5, 7, 9, 10, 14, and 28 (or day of discharge if earlier) during Part B. Clinical laboratory tests (haematology, serum biochemistry, and urinalysis) were performed on Days 7, 14, and 28 (or discharge if earlier). The severity of COVID-19-associated symptoms of cough, fever, nasal congestion, fatigue, body aches, sore throat, breathlessness, and diarrhea were self-scored by the patient every day using a 5-point Likert-type scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe/critical) for each symptom.
A Data Safety Monitoring Board (DSMB) was instituted to review the safety data throughout the trial, as per the DSMB charter.
Statistical methods
Continuous data were summarised using descriptive statistics. Categorical data were summarised using counts and percentage. ‘Time-to-event’ analyses compared between treatment and control (2-DG plus SOC versus SOC) groups using the Cox proportional hazard (CPH) model, with baseline clinical status scores as covariates. Age and sex were considered as additional covariates wherever relevant. Median estimates, Hazard ratio (HR) and its corresponding two-sided 95% confidence interval (CI), and two-sided p values at 5% level of significance are reported. Statistical comparisons were done using log-rank test and Kaplan-Meier plot wherever applicable. Proportions were compared using Chi-square or Fisher exact test.
There were no statistical power calculations for sample size. Efficacy data were collected based on several clinically meaningful measures, and no particular parameter was designated as the primary endpoint.
The 2-DG plus SOC treatment groups were compared with their contemporaneous SOC groups as well as with the pooled SOC (SOC1 + SOC2).
Results
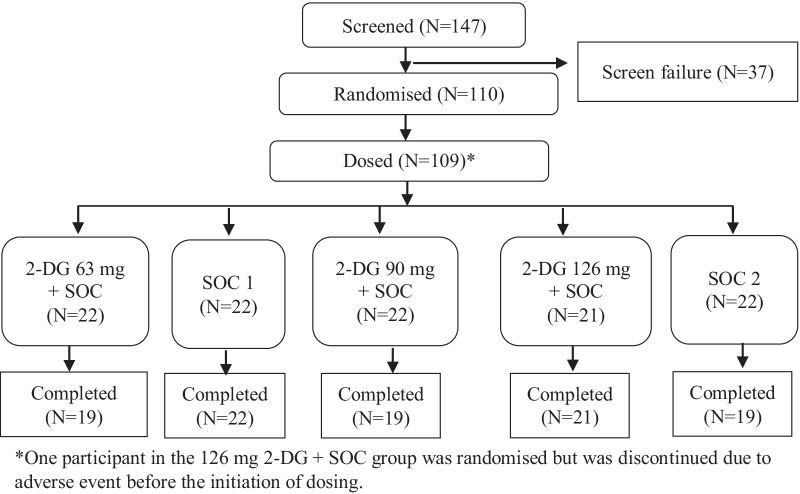
A total of 110 patients were randomised, between June 2019 and September 2020, with 22 patients in each of the five treatment groups: the 2-DG 63 mg and SOC1 groups in Part A and the 2-DG 90 mg, 2-DG 126 mg, and SOC2 group in Part B. 109 were dosed, and 1 patient in the 2-DG 126 mg group was discontinued before receiving any dose, due to an adverse event (Fig. 1).
Fig. 1.
Study flow chart displaying patient counts in each treatment group. 2-DG 2-deoxy-d-glucose, SOC standard of care, SOC1 SOC in Part A of the study, SOC2 SOC in Part B of the study
The demographic and baseline characteristics were similar across the treatment groups (Table 1). The mean (standard deviation [SD]) age was 44.9 (10.90) years, and the mean (SD) weight was 68.6 (11.39) kg for all randomised patients (Additional file 1: Tables). A majority of the patients (88 [80.7%]) were male. There were differences in certain baseline disease characteristics between patients enrolled in Parts A and B of the study (Additional file 1: Tables). The mean (SD) number of days since the onset of initial COVID-19 symptoms in patients enrolled in Part A were 7.2 (2.58) days for the SOC1 group and 6.6 (2.26) days for the 2-DG 63 mg group, and for those enrolled in Part B were 4.5 (1.41) days, 4.3 (1.46) days, and 4.4 (1.40) days for the 2-DG 90 mg, 2-DG 126 mg, and SOC2 groups, respectively (Table 1). All enrolled patients were assessed to have moderate COVID-19 as defined by the guideline [17], except 3 patients with missing severity data [one (4.5%) patient each from 2-DG 63 mg, SOC1, and SOC2 groups].
Table 1.
Patient demographic and baseline characteristics
| 2-DG 63 mg + SOC N = 22 n (%) |
SOC1 N = 22 n (%) |
2-DG 90 mg + SOC N = 22 n (%) |
2-DG 126 mg + SOC N = 21 n (%) |
SOC2 N = 22 n (%) |
||
|---|---|---|---|---|---|---|
| Age (years) | Mean (SD) | 44.2 (12.71) | 44.4 (9.59) | 46.3 (11.00) | 42.7 (9.33) | 46.6 (11.96) |
| Weight (kg) | Mean (SD) | 61.3 (9.35) | 67.9 (9.76) | 69.4 (10.52) | 74.2 (12.23) | 70.6 (11.77) |
| Gender [n (%)] | Female | 7 (31.8) | 6 (27.3) | 1 (4.5) | 5 (23.8) | 2 (9.1) |
| Male | 15 (68.2) | 16 (72.7) | 21 (95.5) | 16 (76.2) | 20 (90.9) | |
| Number of days since onset of first symptom of COVID-19 | Mean (SD) | 6.6 (2.26) | 7.2 (2.58) | 4.5 (1.41) | 4.3 (1.46) | 4.4 (1.40) |
| Clinical severity status as defined by MoH&FW [n (%)] | Group 1 (Mild) | 0 | 0 | 0 | 0 | 0 |
| Group 2 (Moderate) | 21 (95.5%)a | 21 (95.5%)a | 22 (100.0%) | 21 (100.0%) | 21 (95.5%)a | |
| Group 3 (Severe) | 0 | 0 | 0 | 0 | 0 | |
|
Oxygen saturation (SpO2%) |
N | 22 | 21 | 22 | 21 | 21 |
| Mean (SD) | 93.1 (2.39) | 93.0 (1.82) | 92.7 (1.55) | 92.5 (1.47) | 93.0(2.07) | |
| Median | 92.0 | 93.0 | 93.0 | 93.0 | 93.0 | |
| Heart rate (beats per minute) | N | 22 | 21 | 22 | 21 | 21 |
| Mean (SD) | 85.0 (11.32) | 86.3 (15.17) | 81.3 (10.39) | 84.9 (11.57) | 89.6 (8.48) | |
| Median | 80.0 | 84.0 | 80.0 | 88.0 | 89.0 | |
| Respiratory rate (per minute) | N | 22 | 21 | 22 | 21 | 21 |
| Mean (SD) | 22.1 (3.04) | 21.4 (3.20) | 23.9 (2.41) | 24.4 (2.09) | 24.7 ± 2.61 | |
| Median | 24.0 | 22.0 | 25.0 | 25.0 | 25.0 | |
| WHO clinical progression scale | N | 22 | 22 | 22 | 21 | 22 |
| Mean (SD) | 5.1 (0.35) | 5.0 (0.21) | 4.3 (0.48) | 4.3 (0.46) | 4.3 (0.48) | |
| Median | 5.0 | 5.0 | 4.0 | 4.0 | 4.0 |
2-DG 2-deoxy-d-glucose, SOC standard of care, SOC1 SOC in Part A of the study, SOC2 SOC in Part B of the study
N: Total number of patients in the specified treatment group
n: Total number of patients in a given category
Percentages are based on the total number of patients in the specified treatment group
MoH&FW: Ministry of Health & Family Welfare, Government of India
aSeverity assessment was missing for 1 patient each in these three groups
Efficacy
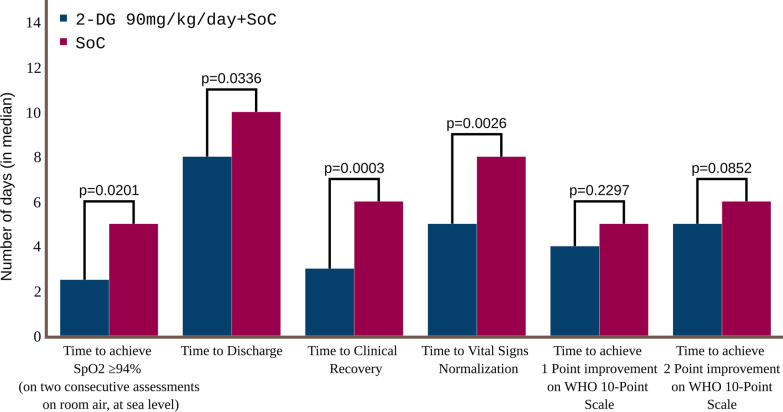
The median time to achieve and maintain SpO2 ≥ 94% on room air at sea level was the shortest in 2-DG 90 mg group (2.5 days, Fig. 2), followed closely by the 2-DG 126 mg group (3.0 days). The median time to achieving SpO2 ≥ 94% was 5.0 days across all the other three groups, viz., 63 mg, SOC1 and SOC2 (Table 2). The Hazard ratio (95% CI) for the 2-DG 90 mg group was 2.3 (1.14, 4.64) (p = 0.0201) compared with the SOC2 group (Table 2 and Kaplan meier plot is shown in Additional file 1: Fig. S2) and 2.6 (1.49, 4.70) (p = 0.0009) compared with the pooled SOC group (Additional file 1: Table S18). The comparisons between 2-DG 63 mg and SOC1 and between 2-DG 126 mg and SOC2 were not statistically significant.
Fig. 2.
Median time (days) to clinical endpoints compared between patients receiving 2-DG 90 mg/kg/day plus SOC and patients receiving standard of care only. 2-DG 2-deoxy-d-glucose, SOC standard of care, WHO World Health Organization
Table 2.
Efficacy endpoint comparisons between active (2-DG) and standard-of-care groups
| 2-DG 63 mg + SOC | SOC1 | 2-DG 90 mg + SOC | 2-DG 126 mg + SOC | SOC2 |
Pooled SOC (SOC1 + SOC2) |
||
|---|---|---|---|---|---|---|---|
| Time (days) to achieve SpO2 ≥ 94% (on two consecutive assessments on room air, at sea level) | N | 22 | 22 | 22 | 21 | 22 | 44 |
| Median (days) | 5 | 5 | 2.5 | 3 | 5 | 5 | |
| HR (95% CI)a | 1.277 (0.658, 2.477) | – | 2.3 (1.14, 4.642) | 0.975 (0.494, 1.925) | – | – | |
| P-value a | 0.4698 | – | 0.0201 | 0.9415 | – | – | |
| Time (days) to discharge | Median (days) | 12 | 11 | 8 | 11 | 10 | 10 |
| HR (95% CI)a | 0.791 (0.416, 1.504) | – | 2.238 (1.065, 4.703) | 0.679 (0.334, 1.38) | – | – | |
| P-value a | 0.4746 | – | 0.0336 | 0.2847 | – | – | |
| Time (days) to clinical recoveryb | Median (days) | 4.5 | 5 | 3 | 4 | 6 | 5 |
| HR (95% CI)a | 0.985 (0.526, 1.846) | – | 3.837 (1.853, 7.944) | 1.881 (0.922, 3.838) | – | – | |
| P-value a | 0.9629 | – | 0.0003 | 0.0824 | – | – | |
| Time (days) to vital signs normalisationc | Median (days) | 7 | 7 | 5 | 10 | 8 | 7 |
| HR (95% CI)a | 0.889 (0.472, 1.674) | – | 4.341 (1.669, 11.294) | 1.024 (0.418, 2.511) | – | – | |
| P-value | 0.7162 | – | 0.0026 | 0.958 | – | – | |
| Time (days) to achieve 2 points improvement on WHO clinical progression scale | Median (days) | 11 | 10.5 | 5 | 5 | 6 | 8 |
| HR (95% CI)a | 0.624 (0.318, 1.224) | – | 1.763 (0.924, 3.363) | 1.183 (0.63, 2.221) | – | – | |
| P-valuea | 0.1702 | – | 0.0852 | 0.6021 | – | – | |
| Time (days) to achieve 1 point improvement on WHO clinical progression scale | Median (days) | 5.5 | 6 | 4 | 3 | 5 | 5 |
| HR (95% CI) a | 1.414 (0.71, 2.818) | – | 1.483 (0.78, 2.822) | 1.301 (0.697, 2.427) | – | – | |
| P-valuea | 0.3247 | – | 0.2297 | 0.409 | – | – |
CI confidence interval, 2-DG 2-deoxy-d-glucose, HR hazard ratio, SOC standard of care, SOC1 SOC in Part A of the study, SOC2 SOC in Part B of the study, WHO World Health Organization
aEach 2-DG + SOC group was compared with its respective SOC group, in Parts A and B of the study. The 63 mg group was compared with SOC1, and the 90 mg and 126 mg groups were compared with SOC2
bTime to clinical recovery is a composite endpoint of number of days to achieving and maintaining blood oxygen saturation of ≥ 94% on room air and the number of days to achieving symptom severity score of ≤ 1 (on a 5-point Likert-type scale) for all COVID-19 associated symptoms, after start of study treatment
cTime to vital signs normalisation was defined as the earliest date when all the following vital signs parameters were satisfied: body temperature < 98.9 ℉, respiratory rate < 20 breaths per min, blood oxygen saturation (SpO2) > 95% on room air and heart rate < 90 bpm after start of study treatment
The median time to discharge from isolation ward was 8.0 days in the 2-DG 90 mg group (Fig. 2), which was the shortest among all groups (Table 2). The Hazard ratio (95% CI) for the 2-DG 90 mg group was 2.2 (1.07, 4.70) (p = 0.0336) compared with the SOC2 group (Table 2) and 2.2 (1.21, 4.04) (p = 0.01) compared with the pooled SOC group (Additional file 1: Table S18). Other two dose groups (2-DG 63 mg and 2-DG 126 mg groups) were not statistically significant, when compared with their respective SOC groups (Table 2).
Time to clinical recovery is a composite endpoint of number of days to achieving and maintaining SpO2 of ≥ 94% on room air and the number of days to achieve symptom severity score (self-assessed by patient) of ≤ 1 on a 5-point Likert-type scale, for all COVID-19 associated symptoms. The median time to clinical recovery was 4.5 days, 3 days, and 4 days in the 2-DG 63 mg, 2-DG 90 mg, and 2-DG 126 mg groups, respectively, and 5 days in SOC1 and 6 days in SOC2 groups (Table 2). The Hazard ratio (95% CI) for 2-DG 90 mg group was 3.8 (1.85, 7.94) (p = 0.0003) compared with the SOC2 group (Table 2) and 3.4 (1.90, 6.01) (p < 0.0001) compared with the pooled SOC group (Additional file 1: Table S18).
Similarly, time to vital signs normalisation was seen to be significantly better in the 2-DG 90 mg group as compared to SOC2 group. Median time to vital signs normalisation was 5 days in the 2-DG 90 mg group as compared to 8 days in the contemporaneous SOC2 group, Hazard ratio (95% CI) from the CPH model = 4.3 (1.67, 11.29) (p = 0.0026) (Table 2).
The median time to improvement in clinical status score by 2 points over baseline was 5 days in both 2-DG 90 mg group and 2-DG 126 mg group, even though the median time to improvement by 1 point was shortest in 2-DG 126 mg group (3 days) followed by 2-DG 90 mg group (4 days). The comparisons of these two groups with SOC2 group were not statistically significant (Table 2). Hazard ratio (95% CI) from CPH model was 1.8 (0.92, 3.36) (p = 0.0852) for 2-DG 90 mg vs. SOC2 group, and 1.18 (0.63, 2.22) (p = 0.6021) for 2-DG 126 mg vs. SOC2 group, for the time to achieve 2 points improvement over baseline in the clinical status score (Table 2). Only the comparison between the 2-DG 90 mg group and the pooled SOC group in terms of median time to 2 points improvement (HR = 2.364; 95% CI: 1.33, 4.18; p = 0.0032) was found significant (Additional file 1: Table S18).
Median time to first conversion to negative RT-PCR test for SARS-CoV-2 RNA was the shortest in the SOC1 group (3.5 days), followed by the 2-DG 90 mg group with 5.0 days as per CPH model (4.0 days from descriptive statistics) and the 2-DG 63 mg group with 7.0 days as per CPH model (6.0 days from descriptive statistics). The median time was 7.0 days for both the 2-DG 126 mg and SOC2 groups. None of the 2-DG groups was statistically significant compared with the corresponding SOC groups. However, 2-DG 90 mg group showed numerically superior trend with HR = 2.0 (0.94, 4.25; p = 0.0702) (Additional file 1: Table S7).
One patient each in 2-DG 90 mg group and the SOC2 group required Intensive Care Unit (ICU) admission during the study. One mortality (4.5%) was reported in 2-DG 90 mg group, which was reported unrelated to the drug by investigator. No meaningful comparison on ICU admission or mortality rates among treatment groups could be made due to the negligible number of events.
Safety
All three dose levels of 2-DG were well tolerated. A total of 65 treatment-emergent adverse events were reported in 33 (30.3%) patients. One serious adverse event was reported in a patient in the 2-DG 90 mg group who died of ARDS, which was considered not related to the study drug by the sponsor as well as the investigator. Fifty-six of the 65 adverse events (86%) were mild. Hyperglycaemia was the most commonly reported adverse event overall, with 14 events occurring in 10 patients (9.2%) across five groups (Table 3). This included 4 (18.2%) patients in the SOC 2 group, 2 patients each in SOC1 group (9.1%) and 2-DG 126 mg group (9.5%), and 1 (4.5%) patient each in 2-DG 63 mg group and 2-DG 90 mg group. Other common adverse events were palpitations in 4 (3.7%) patients, dizziness in 4 (3.7%) patients, and diarrhoea in 3 (2.8%) patients out of 109 patients, all of which were observed in the 2-DG 63 mg and 90 mg groups with incidence ranging 4.5–9.1% (Table 3).
Table 3.
Incidence of treatment-emergent adverse events occurring in > 2% of patients
| Preferred Term | 2-DG 63 mg + SOC N = 22 |
SOC1 N = 22 |
2-DG 90 mg + SOC N = 22 |
2-DG 126 mg + SOC N = 21 |
SOC2 N = 22 |
SOC (SOC1 + SOC2) N = 44 |
Total N = 109 |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Hyperglycaemia | 1 (4.5) | 2 (9.1) | 1 (4.5) | 2 (9.5) | 4 (18.2) | 6 (13.6) | 10 (9.2) |
| Palpitations | 2 (9.1) | 0 | 2 (9.1) | 0 | 0 | 0 | 4 (3.7) |
| Dizziness | 2 (9.1) | 0 | 2 (9.1) | 0 | 0 | 0 | 4 (3.7) |
| Diarrhea | 1 (4.5) | 0 | 2 (9.1) | 0 | 0 | 0 | 3 (2.8) |
| Hyperhidrosis | 2 (9.1) | 0 | 0 | 1 (4.8) | 0 | 0 | 3 (2.8) |
2-DG 2-deoxy-d-glucose, SOC standard of care, SOC1 SOC in Part A of the study, SOC2 SOC in Part B of the study
N: Total number of patients in the specified treatment group
n: Number of patients with the adverse events of the preferred term
No clinically significant prolongations of the cardiac QT interval were reported in 2-DG treatment arm. The greatest change in mean QTc intervals from baseline and the highest mean and median values were observed on Day 7 in the 2-DG 126 mg group, with a mean increase of 23.8 msec from baseline (data on file), mean value of 446.7 ms, and median value of 444.0 ms (Table 4).
Table 4.
Summary of QTcB (milliseconds) during the study period
| 2-DG 63 mg + SOC | SOC1 | 2-DG 90 mg + SOC | 2-DG 126 mg + SOC | SOC2 | ||
|---|---|---|---|---|---|---|
| Baseline | N | 22 | 21 | 22 | 21 | 18 |
| Mean (SD) | 407.0 (32.79) | 412.5 (29.45) | 429.0 (27.15) | 428.6 (21.21) | 418.9 (33.06) | |
| Median | 409.5 | 413.0 | 430.0 | 423.0 | 431.5 | |
| Day 3 | N | 22 | 22 | 20 | 21 | 21 |
| Mean (SD) | 419.1 (24.46) | 412.5 (24.19) | 425.7 (32.72) | 427.5 (29.36) | 427.0 (22.31) | |
| Median | 413.0 | 406.5 | 423.0 | 427.0 | 421.0 | |
| Day 7 | N | 19 | 19 | 10 | 15 | 12 |
| Mean (SD) | 415.7 (21.86) | 412.0 (31.39) | 433.5 (30.25) | 446.7 (61.33) | 411.8 (28.40) | |
| Median | 422.0 | 408.0 | 423.0 | 444.0 | 411.5 | |
| End of Treatment | N | 22 | 22 | 19 | 21 | 19 |
| Mean (SD) | 413.1 (24.69) | 410.4 (20.61) | 433.9 (37.82) | 435.2 (36.59) | 422.4 (23.20) | |
| Median | 417.0 | 411.0 | 423.0 | 440.0 | 422.0 | |
2-DG 2-deoxy-d-glucose, SOC standard of care, SOC1 SOC in Part A of the study, SOC2 SOC in Part B of the study
Discussion
The clinical experience of COVID-19 management suggests that no single agent is sufficient to treat all COVID-19 cases, and a multimodal approach is imperative [1]. In this context, we evaluated 2-DG as a potential therapeutic option for COVID-19 as an adjunct to standard of care. To the best of our knowledge, this is the first clinical study of 2-DG in COVID-19 patients. This study was based on extensive safety evidence for 2-DG from prior clinical studies, antiviral efficacy of topically applied 2-DG in a herpes simplex study, and in vitro inhibition of SARS-CoV-2 by 2-DG [2–4, 9, 13–15].
The starting dose used in this study, 63 mg/kg/day, was about 4 times lower than the maximum tolerated dose of 250 mg/kg reported in a previous trial of glioblastoma multiforme patients [16]. As COVID-19 management has evolved rapidly, some differences in SOC medications were seen between Parts A and B of the study; however, the SOC was comparable between each active treatment group and the corresponding SOC group during each part of the study.
On several key efficacy endpoints, the most favourable outcomes were observed in the 2-DG 90 mg group as compared with SOC. Statistically significant outcomes were seen with 2-DG 90 mg group in times to achieving and maintaining blood oxygen saturation ≥ 94%, clinical recovery, vital signs normalization and discharge as compared to respective SOC. Few favourable numerical trends were also seen in the 2-DG 126 mg group with regards to achieving blood oxygen saturation ≥ 94%, 1 point and 2 points improvement on the WHO clinical progression scale, and clinical recovery. However, these were not statistically significant when compared with either contemporaneous SOC or the pooled SOC. Further, median time to 2 points clinical improvement on WHO clinical progression scale in 90 mg group showed favourable numerical trend but was found insignificant in comparison with SOC2, possibly due to small sample size; because, when it is compared with pooled SOC group this outcome also showed significance. Although, the pharmacological plausibility of drugs being less effective at higher doses exists, pointing out the exact reason is difficult in this case given the small sample size of the study. However, it was demonstrated earlier that higher doses of 2-DG may induce insulin response [14], diverting 2-DG to muscle and fat tissues, thereby reducing the effective 2-DG concentration in virally infected host cells/ tissues. This could be a possible reason for 2-DG being less effective in 126 mg group as compared to 90 mg group.
The guidance document on trials for treatment and prevention of COVID-19, published by the United States Food and Drug Administration (USFDA), enunciates the return to room air or baseline oxygen requirement as one of the objective clinical outcome measures of sustained clinical improvement [18]. Several studies have considered oxygen saturation as one of the specific metrics for assessing lung injury and function [19]. Thereby, the time to achieving and maintaining SpO2 ≥ 94% is considered as a clinically meaningful endpoint for COVID-19 drug development [19]. Therefore, the results of this study have established proof-of-concept for 2-DG and justify the evaluation of the 90 mg/kg/day dose of 2-DG in a pivotal phase III study. These results also have important implications for the management of moderate to severe COVID-19 patients in the current pandemic context. In an earlier observational study of hospitalised COVID-19 patients in regular hospital ward (i.e., outside of ICU), the average duration on supplemental oxygen was 8 days [20]. In another observational study of severe COVID-19 patients, the median time to getting off supplemental oxygen was 6 days [21]. These data are comparable to what we observed in patients in the SOC groups in our current study, with median time to achieving and maintaining SpO2 ≥ 94% on room air of 5 days. Importantly, the time to SpO2 ≥ 94% on room air was significantly shorter in patients treated with 2-DG 90 mg/kg/day, with median time of 2.5 days (50% reduction). During the massive second wave of COVID-19 in India, shortages in hospital beds with medical-grade oxygen have been experienced at numerous locations. If any therapeutic can help in substantial reduction in supplemental oxygen requirement and hospital bed occupancy in COVID-19 patients, it can go a long way to ease the burden on a country’s healthcare resources.
The postulated biochemical mechanism of action of 2-DG can explain its efficacy in achieving and maintaining blood oxygen saturation. SARS-CoV-2 infects the respiratory tract epithelial cells, which leads to inflammation and impedes the transfer of oxygen in the lungs. As these infected cells have high metabolic demand, 2-DG may accumulate within the infected cells, leading to energy starvation and dearth of anabolic intermediates, which could potentially lead to inhibition of viral replication and host inflammatory response, ultimately translating to clinically meaningful benefits, such as improvement in oxygenation and early recovery. Also, this underlying biochemical mechanism of action would be agnostic to SARS-CoV-2 variants as 2-DG acts on host cell metabolism and does not target the fast-mutating viral proteins [2].
For the 90 mg/kg/day dose of 2-DG, benefits were observed for other efficacy endpoints, including time to discharge from isolation ward, time to clinical recovery, time to vital signs normalisation.
All three dose levels studied were well tolerated and were found to be reasonably safe. The overall incidence of adverse events was low, and the majority of adverse events were mild in intensity. One patient died of ARDS, which was considered not related to 2-DG treatment. Changes in blood glucose levels were evaluated in this study. As observed in previous oncology studies, glycolytic inhibition and competition between glucose and 2-DG for cellular uptake can result in transient hyperglycaemia at higher doses [14]. In this study, the incidence of hyperglycaemia was comparable between the active 2-DG and SOC groups. Moreover, these events of hyperglycaemia were mild and did not lead to study treatment discontinuation in any patients. There was no confirmed adverse event of hypoglycaemia in our study. Maximum plasma concentration achieved with a 45 mg/kg dose of 2-DG was found to be approximately 0.5 mM in an earlier study [15], which is only one-ninth of the plasma glucose concentration (80 mg/dL or 4.5 mM under fasting conditions). Therefore, theoretically the availability of glucose to normal cells, particularly glucose-hungry brain cells, is 9 times higher than 2-DG. It is noteworthy that due to limited mitochondrial function, SARS-CoV-2 infected cells use glycolysis to meet the high bioenergetic and anabolic demand, unlike the uninfected cells that use both glycolysis and mitochondrial respiration to fulfil normal cellular energy demands. 2-DG primarily inhibits glycolysis and its effect on ATP generation from mitochondrial oxidation in normal uninfected cells (without Warburg shift) would be negligible.
Another important safety consideration with 2-DG was its potential for cardiac QT prolongation. While QT prolongation have been reported in previous oncology studies, mostly at higher doses of 2-DG, these effects were transient and asymptomatic [14, 15]. In this study, the changes from baseline for mean and median QTc values for 2-DG arm were within acceptable ranges.
A limitation of this phase II study is that it was not adequately powered. A subsequent adequately powered (80%) phase III clinical study has been initiated with prespecified primary and secondary endpoints. The results from the phase III study are expected to be published in the near future.
For several patients in the current phase II study, a clinical status score of 4 (‘hospitalised, no oxygen therapy’) was recorded on the WHO clinical progression scale at baseline, despite their requiring oxygen treatment. 21 out of 22 patients had SpO2 < 95% at baseline. Due to shortage of oxygen supply, many eligible patients did not receive oxygen supplementation in their respective hospitals. It is possible that these patients were assigned a score of 4.
Another limitation was that meaningful comparison between the 2-DG groups and control groups on ICU admission and death was not possible due to small number of such events. In addition, the study was not placebo controlled or blinded.
Conclusions
Results of the current phase II study suggest 2-DG holds promise as adjunctive treatment to standard of care, in the management of moderate to severe COVID-19 and have encouraged confirmatory evaluation of its efficacy and safety in a larger phase III clinical trial. If confirmed, 2-DG may provide healthcare practitioners with another option to treat moderate to severe COVID-19 patients.
Supplementary information
Additional file 1: Fig. S1 Chemical structures of glucose (left) and 2-deoxy-D-glucose (2-DG) (right). Fig. S2 Probability of achieving and maintaining blood oxygen saturation of ≥94% in patients treated with 2-DG 90 mg/kg/day + SOC compared with the contemporaneous SoC group (SOC2). Table S1 Patient Demographics. Table S2 Medical History. Table S3 Summary of Time to Clinical Recovery. Table S4 Analysis of Time to Clinical Recovery. Table S5 Proportion of Patients Showing Negative Conversion of Detectable SARS-CoV-2 RNA on RT-PCR by Study Days 3, 7, 10, and EOT. Table S6 Summary of Time to First Negative Conversion of Detectable SARS-CoV-2 By RT-PCR Assay of a Nasopharyngeal/Oropharyngeal Swab Specimen. Table S7 Analysis of Time to First Negative Conversion of Detectable SARS-CoV-2 by RT-PCR Assay of a Nasopharyngeal/Oropharyngeal Swab Specimen. Table S8 Summary of Time to Discharge from Isolation Ward. Table S9 Analysis of Time to Discharge from Isolation Ward. Table S10 Summary of Time to Achieve Improvement by At Least 1 and At Least 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment. Table S11 Analysis of Median Time (no. of days) to Achieve Improvement by 1 Point and by 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment - Study part A. Table S12 Analysis of Median Time (no. of days) to Achieve Improvement by 1 Point and by 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment - Study part B. Table S13 Summary of Patients Requiring Management in Intensive Care Unit (ICU), Oxygen Supplementation, Non-Invasive and Invasive Mechanical Ventilation until Day 28. Table S14 Summary of Time to Achieve and Maintain Blood Oxygen Saturation of ≥94%. Table S15 Analysis of Time to Achieve and Maintain Blood Oxygen Saturation of ≥94%. Table S16 Summary of Time to Vital Signs Normalization. Table S17 Analysis of Time to Vital Signs Normalization. Table S18 Efficacy endpoint comparisons between active (2-DG) and Pooled SOC groups. Table S19 Number and Percentage of patients with TEAEs classified by SOC and PT.
Acknowledgements
The authors wish to thank Dr DM Rao and his team from Dr Reddy’s Laboratories Ltd. for his contributions to all regulatory activities related to 2 DG. The study was conducted by Navitas Life Sciences (Contract Research Organization, CRO). The authors would also like to thank all the members of the project team without whom the successful completion of the project would not have been possible.
Abbreviations
- 2-DG
2-deoxy-d-glucose
- SOC
Standard of care
- WHO
World Health Organization
Author contributions
ANB, SS and SM conceived the project and designed the study with inputs from SC and AS. AA and AVK were Investigators in the study and collected the clinical data. ANB, SS, SM, VC analyzed the data and interpreted the outcomes with the help of SC. All authors participated in reviewing and interpretation of the data and writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by Defence Research and Development Organization, Ministry of Defence, Govt. Of India, India and Dr Reddy’s Laboratories Limited, Hyderabad, India. The funding sources had no role in the design of this study, its execution, analyses, interpretation of the data, or reporting of the results in the publication. The drug, 2-DG is manufactured and provided by Dr Reddy’s Laboratories for packaging and supply to clinical sites by CRO.
Availability of data and materials
All the important data generated and analyzed during this clinical study are included in this published article (and its Additional file) and remaining data will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by Drug Controller General of India (CTRI/2020/06/025664). The study was conducted at 12 hospitals located in various parts of India. Ethics Approval was obtained from respective Hospital’s Ethics committees (details can be obtained on this link: http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=44369&EncHid=&modid=&compid=%27,%2744369det%27). Written informed consent was obtained from all study participants and the trial was conducted in accordance with the principles outlined in Declaration of Helsinki and Good Clinical Practice.
Consent for publication
Not applicable.
Competing interests
Drs Anant Narayan Bhatt, Vijayakumar Chinnadurai, Ratnesh Kanwar and Sudhir Chandna: The authors (from INMAS-DRDO) have no conflict of interest or financial relationships relevant to the submitted work to disclose. None of the INMAS-DRDO authors accepted any honoraria or consultancy fees directly or indirectly from industry. Drs Srinivas Shenoy, Sagar Munjal and A Shanavas: The authors are paid employees of Dr Reddy’s Laboratories Limited. None of the authors received any separate compensation for the 2 DG project directly or indirectly. Dr Apurva Agarwal and Dr A Vinoth Kumar hereby certify that there is no actual or potential conflict of interest in relation to this article/publication of 2 DG study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anant Narayan Bhatt, Email: anant.inmas@gov.in, Email: anbhatt@yahoo.com.
Srinivas Shenoy, Email: srinivasshenoyb@drreddys.com, Email: srinivass@rediffmail.com.
References
- 1.Cao B, Hayden FG. Antiviral monotherapy for hospitalised patients with COVID-19 is not enough. Lancet. 2020;396:1310–1. doi: 10.1016/S0140-6736(20)32078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt AN, Kumar A, Rai Y, Kumari N, Vedagiri D, Harshan KH, Chandna S. Glycolytic inhibitor 2-Deoxy-D-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions. BioRxiv. 2021 doi: 10.1101/2021.06.12.448175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Codo AC, Davanzo GG, de Brito Monteiro L, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–46. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bojkova D, Klann K, Koch B, Widera M, Krause D, Ciesek S, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–72. doi: 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardestani A, Azizi Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct Target Ther. 2021;6:112. doi: 10.1038/s41392-021-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang HT, Hwang ES. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not anymore, a low glucose mimetic. Life Sci. 2006;78:1392–9. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Mesri EA, Lampidis TJ. 2-Deoxy-d-glucose exploits increased glucose metabolism in cancer and viral-infected cells: relevance to its use in India against SARS-CoV-2. IUBMB Life. 2021;73(10):1198–204. doi: 10.1002/iub.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu K, Yang Y, Lin L, et al. Caloric restriction mimetic 2-deoxyglucose alleviated inflammatory lung injury via suppressing nuclear pyruvate kinase M2-signal transducer and activator of transcription 3 pathway. Front Immunol. 2018;9:426. doi: 10.3389/fimmu.2018.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blough HA, Giuntoli RL. Successful treatment of human genital herpes infections with 2-deoxy-D-glucose. JAMA. 1979;241:2798–2801. doi: 10.1001/jama.1979.03290520022018. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Zhou J, Xia L, Cheng X, Lu D. 18F-FDG PET/CT and serial chest CT findings in a COVID-19 patient with dynamic clinical characteristics in different period. Clin Nucl Med. 2020;45:495–6. doi: 10.1097/RLU.0000000000003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–6. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajak B, Siwiak E, Sołtyka M, et al. 2-Deoxy-d-glucose and its analogs: from diagnostic to therapeutic agents. Int J Mol Sci. 2019;21:234. doi: 10.3390/ijms21010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raez LE, Papadopoulos K, Ricart AD, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523–30. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 15.Stein M, Lin H, Jeyamohan C, et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010;70:1388–94. doi: 10.1002/pros.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh D, Banerji AK, Dwarakanath BS, et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181:507–14. doi: 10.1007/s00066-005-1320-z. [DOI] [PubMed] [Google Scholar]
- 17.Government of India. Clinical Management Protocol for COVID-19. Ministry of Health and Family Welfare Directorate General of Health Services 2020. Available at https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf. Accessed 17 Jun 2021.
- 18.Marshall JC, Murthy S, Diaz J, Adhikari N, Angus DC, Arabi YM, Blackwood BWHO. Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–7. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.USFDA. COVID-19. Developing Drugs and Biological Products for Treatment or Prevention, Guidance for Industry. US Department of Health and Human Services Food and Drug Administration 2021. https://www.fda.gov/media/137926/download. Accessed 6 Jun 2021.
- 20.Daher A, Balfanz P, Aetou M, et al. Clinical course of COVID-19 patients needing supplemental oxygen outside the intensive care unit. Sci Rep. 2021;11:2256. doi: 10.1038/s41598-021-81444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leulseged TW, Hassen IS, Edo MG, Abebe DS, Maru EH, Zewde WC, Jagema TB. Duration of oxygen requirement and predictors in severe COVID-19 patients in Ethiopia: a survival analysis. medRxiv 2020; 10. 10.1101/2020.10.08.20209122. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Chemical structures of glucose (left) and 2-deoxy-D-glucose (2-DG) (right). Fig. S2 Probability of achieving and maintaining blood oxygen saturation of ≥94% in patients treated with 2-DG 90 mg/kg/day + SOC compared with the contemporaneous SoC group (SOC2). Table S1 Patient Demographics. Table S2 Medical History. Table S3 Summary of Time to Clinical Recovery. Table S4 Analysis of Time to Clinical Recovery. Table S5 Proportion of Patients Showing Negative Conversion of Detectable SARS-CoV-2 RNA on RT-PCR by Study Days 3, 7, 10, and EOT. Table S6 Summary of Time to First Negative Conversion of Detectable SARS-CoV-2 By RT-PCR Assay of a Nasopharyngeal/Oropharyngeal Swab Specimen. Table S7 Analysis of Time to First Negative Conversion of Detectable SARS-CoV-2 by RT-PCR Assay of a Nasopharyngeal/Oropharyngeal Swab Specimen. Table S8 Summary of Time to Discharge from Isolation Ward. Table S9 Analysis of Time to Discharge from Isolation Ward. Table S10 Summary of Time to Achieve Improvement by At Least 1 and At Least 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment. Table S11 Analysis of Median Time (no. of days) to Achieve Improvement by 1 Point and by 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment - Study part A. Table S12 Analysis of Median Time (no. of days) to Achieve Improvement by 1 Point and by 2 Points Over Baseline in the Clinical Status Score on WHO 10-Point Ordinal Scale from Start of Study Treatment - Study part B. Table S13 Summary of Patients Requiring Management in Intensive Care Unit (ICU), Oxygen Supplementation, Non-Invasive and Invasive Mechanical Ventilation until Day 28. Table S14 Summary of Time to Achieve and Maintain Blood Oxygen Saturation of ≥94%. Table S15 Analysis of Time to Achieve and Maintain Blood Oxygen Saturation of ≥94%. Table S16 Summary of Time to Vital Signs Normalization. Table S17 Analysis of Time to Vital Signs Normalization. Table S18 Efficacy endpoint comparisons between active (2-DG) and Pooled SOC groups. Table S19 Number and Percentage of patients with TEAEs classified by SOC and PT.
Data Availability Statement
All the important data generated and analyzed during this clinical study are included in this published article (and its Additional file) and remaining data will be available from the corresponding author upon reasonable request.