Abstract
Substituted thiazoles are widely known as natural products, approved drugs, and a number of synthetic compounds as bioactive agents. Due to the worth of this heterocycle nucleus, a large number of synthetic methodologies have been reported over the years to synthesize its derivatives. In this perspective, recent advances in the synthesis of thiazole compounds by using domino/cascade and multicomponent approaches have been summarized.
Keywords: Domino/cascade reaction, multicomponent reaction, thiazole, thiazoline, fused-thiazole, anticancer, thiazolo-androstenone, isothiazole
1. INTRODUCTION
Thiazole (1,3-thiazole) is 5-membered nitrogen and sulfur-containing heterocycle, which is a sulfur analogue of oxazole and imidazole heterocycles. Thiazole shows the most aromatic character among these three five-membered heterocycles in the order of thiazole > imidazole > oxazole Fig. (1) [1]. This heterocycle is found in a number of drugs to treat different types of diseases and in natural products with a wide range of biological activities. Isothiazole or 1,2-thiazole is a lesser known isomer of thiazole [2]. Nevertheless, ziprasidone and perospirone drugs contain fused-isothiazole moiety [3, 4]. This perspective highlights the recent advances in the synthesis of thiazole derivatives by using domino and multicomponent methodologies.
Fig. (1).

Structure of thiazole and isothiazole.
A number of anticancer drugs such as alpelisib, bleomycin, dabrafenib, dasatinib, and ixabepilone contain thiazole nucleus. Dasatinib (1), a kinase inhibitor to treat leukemia, is among the top 100 prescribed drugs in the United States. A plethora of papers have been reported on thiazole derivatives as potent antiproliferative agents [5, 6]. Thiazole derivatives as potent antimicrobial agents have been reported in a number of papers [7]. More than 25 thiazole-containing small molecules have been approved as antibiotics (e.g., 3 and 4) to treat different types of bacterial infections. These antibiotics mainly include the cephalosporin class of antibacterial agents.
Several other classes of drugs contain the thiazole nucleus as a core structure. Ritonavir is a widely used anti-HIV drug, and a number of novel thiazole compounds have been reported as potent antiviral agents [8]. Several thiazole compounds have been reported as anti-TB, anti-inflammatory, anti-fungal agents, and compounds hypoglycemic properties [6, 9–11]. Last but not least, aminothiazole containing the drug, mirabegron (5) to treat overactive bladder is one of the top 100 drugs in the US in retail sales. This small molecule (5) is a β3-blocker Fig. (2). This molecule also shows several other useful clinical properties, including anti-obesity [12–14]. Due to the importance of thiazoles, a large number of synthetic methodologies have been reported over the years.
Fig. (2).
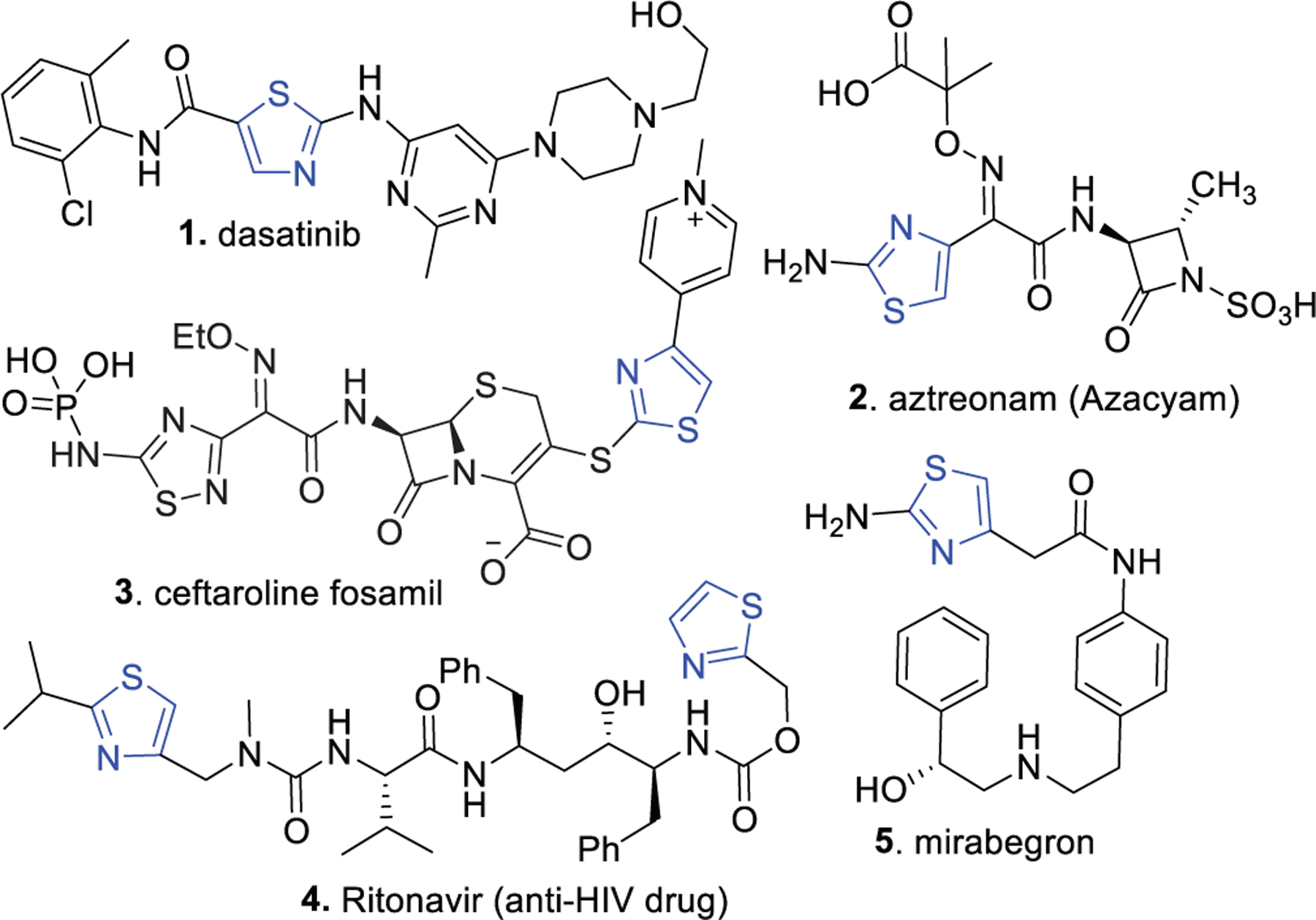
Representative examples of thiazole containing drugs.
Domino reactions involve the transformation of functionalities formed in the previous reaction steps under the same reaction condition to incorporate two or more chemical bonds. Domino approach offers multiple bond formation to synthesize complex natural products and heterocycles efficiently. Atom economy, lesser use of resources, materials, energy, time or money, and less generation of waste are the salient features of this benign protocol [15]. Therefore, using the domino concept to synthesize novel compounds and natural products is a part of green chemistry. Enzyme-mediated cascade/domino reactions are very common in biosynthesis to synthesize diverse natural products [16]. Domino reactions start with an intermolecular reaction followed by intramolecular reactions to form final products. Subsequent reactions can be intermolecular. In that case, domino reactions become multicomponent reactions.
2. DOMINO/CASCADE REACTIONS
In a continuation of our work to find therapeutic agents [17, 18], we have reported the synthesis and biological studies of a number of novel thiazole derivatives [19]. By using our previous reports on domino reactions [20, 21], we have synthesized a series of thiazoline and thiazole derivatives efficiently (Scheme 1) [22]. In this methodology, the reaction of thioamide with ethyl-4-bromocrotonate forms thiazoline derivatives (6) from 68% to 90% yields. A similar reaction with ethyl-4-bromo-3-ethoxycrotonate formed thiazole derivatives (7) in more than 90% yield. The mechanism of this reaction involves an SN2 reaction followed by the Michael addition to form thiazoline compounds (6). The thiazole derivatives (7) were formed by the domino mechanism involving SN2 reaction, Michael addition, E1cB reaction followed by [1,3]-H shift [22]. 1,1,1,3,3,3-Hexafluoroisopropanol (HFIP) is the solvent and a promotor for this methodology, which activates the substrate via strong hydrogen bonding.
Scheme 1.

Synthesis of thiazole and thiazoline derivatives.
A similar reaction of thiourea and thioamide derivatives with 6β-bromoandrostenedione (BS) formed fused thiazole derivatives (e.g., 8–19), entirely by a different mechanism (Scheme 2). The expected compound (TAD) did not form at all. In this novel methodology, the reaction of thiourea and its derivatives reacted with BS in ethanol under refluxing conditions. By using this methodology, 25 fused thiazolo-androstenone derivatives (e.g., 8–19) have been reported with yields as high as 92%. Several of these compounds are potent growth inhibitors of several cancer cell lines with 50% growth inhibition (GI50) values at submicromolar concentration [23]. A similar reaction of thioamide derivatives with 6β-bromoandrostenone in HFIP formed another series anticancer compounds. Some of these compounds are effective against NCI-60 cancer cell lines [24].
Scheme 2.

Synthesis of fused-thiazole derivatives, compounds 8–14 are aminothiazole and compounds 15–19 are thiazole derivatives.
We studied the potent compounds for their anti-melanoma properties [25]. These potent compounds are more potent than the positive control, dacarbazine, an approved drug to treat melanoma. These compounds caused caspase-dependent apoptosis and caspase-mediated cell death, as evident by the colocalization of TUNEL with endonuclease G (EndoG) and caspase-activated DNase (CAD) respectively. These compounds also induced oxidative injury to melanoma cell lines measured by colocalization of TUNEL with heme oxygenase-1 (HO1). The positive control (dacarbazine) caused caspase-independent apoptosis only [25].
To further expand the scope of the substrate, we used α,β-epoxyketone derivatives as electrophiles. The reaction of thiourea and thioamide derivatives with the epoxyketone electrophile formed several series of fused-thiazole derivatives (Scheme 3) [26]. Acetic acid is the key solvent for product formation in good yields. By using this methodology, we have synthesized 80+ thiazole-fused nootkatone (20 and 21), cholestenone (22), progesterone (23), and ethisterone (24 and 25) compounds in very good average yield. Thiazolo-ethisterone class of compounds are potent against several cancer cell lines with GI50 values at submicromolar concentration. Fused-thiazolo nootkatone compounds are effective antibacterial agents with minimum inhibitory concentration (MIC) values as low as 0.78 μg/mL. These compounds effectively inhibited the growth of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) bacteria. The most potent compound is bactericidal and active against S. aureus persisters [27]. This methodology (Scheme 3) is very attractive to synthesize thiazole-fused natural products. About 25% of natural products contain enone moiety (A), and several of these natural products can be converted to epoxyketones (B) by using established procedures [26, 27].
Scheme 3.

Synthesis of fused-thiazole derivatives from epoxy ketone.
Several other groups have utilized the domino protocol to synthesize novel thiazole derivatives. Lokman et al. have reported the synthesis of trisubstituted thiazole (26) by using a multicomponent domino approach (Scheme 4). This green methodology utilizes arylglyoxals, cyclic 1,3-dicarbonyls, and thioamides in an aqueous solvent without using any external reagents to get the products. Nevertheless, this methodology requires microwave irradiation for 15 min to synthesize phenyl, coumarin, pyrone, and dicarbonyl substituted thiazoles [28]. In another report, the same group has reported the synthesis of novel diphenyl-1,3-thiazole linked barbituric acid hybrids [29]. This catalyst-free methodology utilizes 3–4 drops of water in liquid assisted grinding (LAG) to react arylglyoxal, barbituric acid and thiobenzamide derivatives. Thiobenzamides were also prepared in situ by reacting aryl nitriles with ammonium sulfide in the aqueous medium. Some of these compounds are fluorescent with very good quantum yield in DMSO [29]. A related multi-component methodology has been reported to synthesize trisubstituted thiazole-derived hydrazones by reacting thiosemicarbazones, generated in situ, with arylglyoxals and active methylene compounds. This metal-free reaction utilizes ethanol as a solvent and acetic acid as a catalyst [30].
Scheme 4.

Cascade reactions to synthesize thiazole derivatives.
Castagnolo et al. have reported an efficient and simple protocol to synthesize thiazole derivatives under microwave irradiation condition. In this methodology, the reaction of propargyl amines with isothiocyanates in the presence of catalytic p-toluene sulfonic acid (PTSA) forms thiazole derivatives (27) at 160 °C (Scheme 4). Lower temperature leads to the formation of tautomeric products, methylenethiazoline derivatives This methodology afforded the products up to 85% yield [31]. Dai et al. have reported a facile and efficient approach to synthesis 2-hydroxybenzoyl-substituted thiazole derivatives (28) by using cascade reactions. This methodology exploits the reaction of thiobenzamides with 3-chlorochromone in ethanol and KOH as a base to get the products in good yields. The mechanism of this reaction involves the Michael reaction, elimination followed by intramolecular cyclization to get the substituted thiazole derivatives. Detailed mechanism and other possibilities of product formation have been explained by DFT calculations [32]. Cascade reactions to synthesize 2,4-disubstituted thiazoles (29) have been reported by Wang et al. This ligand-free palladium (II)-catalyzed C(sp)-C(sp2) bond formation is an efficient method to synthesize a series of thiazole derivatives by using various substrates and Pd(OAc)2 in toluene. a-Thiocyanomethyl ketones and readily available aryl boric acid are the substrates for this attractive methodology [33]. Tong et al. have reported an efficient synthesis of fused imidazolo-thiazoles (30) using a multi-step cascade protocol in ~90% yield. This methodology to synthesize a new class of fused-thiazoles utilizes Pd-mediated phosphorous-doped porous organic polymers (POPs) as a heterogeneous catalyst, which acts as both ligands and catalyst support. In this reaction, thiobenzamide reacts with isonitrile in the presence of Pd/porous organic ligand (POL) to form thiobenzamide-substituted thiazole, which is further converted to fused-imidazolo-thiazole by treatment with potassium carbonate [34]. Allenyl isothiocyanates have been used to synthesize substituted-thiazoles (31) in a multicomponent strategy. In this methodology, aromatic and sterically hindered aliphatic amines, vinyl nitro derivatives react with allenyl isothiocyanates under mild reaction conditions to form 1,3-substituted thiazole derivatives (31). This methodology generated novel thiazole compounds with bulky substituents (Scheme 4) [35].
3. MULTICOMPONENT REACTIONS
Deng et al. have reported a four-component reaction to synthesize polysubstituted thiazole derivatives in moderate to good yields (32). This metal-free condition utilizes readily available ketones, aldehydes, and ammonium salt and elemental sulfur as the sources of nitrogen and sulfur, respectively (Scheme 5) [36]. A multicomponent approach to synthesize pyrazolo-thiazole derivatives (33) has been reported by Dumas et al. This reaction utilizes bromoacetyl, acetoacetyl derivatives, and thiosemicarbazides as nitrogen and sulfur sources. This reaction happens with the combination of Hantzsch thiazole and Knorr pyrazole syntheses. The salient features of this methodology involve operational simplicity in refluxing ethanol and good to excellent yields of the products [37]. A cascade Ugi/Wittig cyclization for a four-component reaction has been utilized to synthesize polysubstituted thiazoles (34). The components of this reaction are aldehydes, odorless isocyano(triphenylphosphoranylidene)acetate, amines, and thiocarboxylic acids in the methanol solvent in the presence of triethylamine base [38].
Scheme 5.

Multicomponent approach to synthesize substituted thiazole.
CONCLUSION AND PERSPECTIVE
Domino and multicomponent reactions have been utilized over the years to synthesize different arrays of novel thiazole derivatives. Several of these compounds are potent/potential bioactive agents to treat different types of anomalies. These benign methods have a huge potential to generate a library of novel compounds for their potent biological applications.
FUNDING
This perspective was made possible by the Arkansas INBRE, a grant from the National Institute of General Medical Sciences (NIGMS), P20 GM103429 from the National Institutes of Health, the Winthrop P. Rockefeller Cancer Institute at the University of Arkansas for Medical Sciences (UAMS), Little Rock, USA, and the Arkansas Biosciences Institute (ABI) mini-grant.
Biography

Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- [1].Horner KE; Karadakov PB Shielding in and around oxazole, imidazole, and thiazole: How does the second heteroatom affect aromaticity and bonding? J. Org. Chem, 2015, 80(14), 7150–7157. 10.1021/acs.joc.5b01010 [DOI] [PubMed] [Google Scholar]
- [2].Favre HA; Powell WH Nomenclature of organic chemistry: IUPAC recommendations and preferred names; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- [3].Mandrioli R; Protti M; Mercolini L Evaluation of the pharmacokinetics, safety and clinical efficacy of ziprasidone for the treatment of schizophrenia and bipolar disorder. Expert Opin. Drug Metab. Toxicol, 2015, 11(1), 149–174. 10.1517/17425255.2015.991713 [DOI] [PubMed] [Google Scholar]
- [4].Ishibashi T; Ohno Y Perospirone hydrochloride: The novel atypical anti-psychotic agent with high affinities for 5-HT2, D2 and 5-HT1A receptors. In: Advances in neuroregulation and neuroprotection; Collin C; Minami M; Parvez H; Saito H; Parvez S; Qureshi, ; Reiss C, Eds.; CRC Press: FL, USA, 2005; pp. 347–357. [Google Scholar]
- [5].Pawar S; Kumar K; Gupta MK; Rawal RK Synthetic and medicinal perspective of fused-thiazoles as anticancer agents. Anticancer. Agents Med. Chem, 2021, 21(11), 1379–1402. 10.2174/1871520620666200728133017 [DOI] [PubMed] [Google Scholar]
- [6].Petrou A; Fesatidou M; Geronikaki A Thiazole ring-A biologically active scaffold. Molecules, 2021, 26(11), 3166. 10.3390/molecules26113166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ayati A; Emami S; Asadipour A; Shafiee A; Foroumadi A Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem, 2015, 97, 699–718. 10.1016/j.ejmech.2015.04.015 [DOI] [PubMed] [Google Scholar]
- [8].Sharma D; Bansal KK; Sharma A; Pathak M; Sharma PC A brief literature and review of patents on thiazole related derivatives. Curr. Bioact. Compd, 2019, 15(3), 304–315. 10.2174/1573407214666180827094725 [DOI] [Google Scholar]
- [9].Mishra R; Sharma PK; Verma PK; Tomer I; Mathur G; Dhakad PK Biological potential of thiazole derivatives of synthetic origin. J. Heterocycl. Chem, 2017, 54(4), 2103–2116. 10.1002/jhet.2827 [DOI] [Google Scholar]
- [10].Zhen Z; Bing S; Yaodong Z; Girdhar SD; Qing-Shan L 2,4,5-trisubstituted thiazole: A privileged scaffold in drug design and activity improvement. Curr. Top. Med. Chem, 2020, 20(28), 2535–2577. 10.2174/1568026620999200917153856 [DOI] [PubMed] [Google Scholar]
- [11].Gümüş M; Yakan M; Koca İ Recent advances of thiazole hybrids in biological applications. Future Med. Chem, 2019, 11(15), 1979–1998. 10.4155/fmc-2018-0196 [DOI] [PubMed] [Google Scholar]
- [12].Bel JS; Tai TC; Khaper N; Lees SJ Mirabegron: The most promising adipose tissue beiging agent. Physiol. Rep, 2021, 9(5), e14779. 10.14814/phy2.14779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leis K; Mazur E; Racinowski M; Świerczyński W; Baska A; Gałązka P Effect of mirabegron on the body’s exercise capacity: A review. Endocr. Metab. Immune Disord. Drug Targets, 2020, 20(9), 1448–1455. 10.2174/1871530320666200516164434 [DOI] [PubMed] [Google Scholar]
- [14].Allison SJ; Gibson W Mirabegron, alone and in combination, in the treatment of overactive bladder: Real-world evidence and experience. Ther. Adv. Urol, 2018, 10(12), 411–419. 10.1177/1756287218801282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borukhova S Domino reactions: Concepts for efficient organic synthesis. Green Proc. Synth, 2014, 3(6), 501–501. 10.1515/gps-2014-0078 [DOI] [Google Scholar]
- [16].Walsh CT; Moore BS Enzymatic cascade reactions in biosynthesis. Angew. Chem. Int. Ed. Engl, 2019, 58(21), 6846–6879. 10.1002/anie.201807844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nelson G; Alam MA; Atkinson T; Gurrapu S; Sravan Kumar J; Bicknese C; Johnson JL; Williams M Synthesis and evaluation of p-N,N-dialkyl substituted chalcones as anti-cancer agents. Med. Chem. Res, 2013, 22(10), 4610–4614. 10.1007/s00044-013-0469-8 [DOI] [Google Scholar]
- [18].Whitt J; Duke C; Ali MA; Chambers SA; Khan MMK; Gilmore D; Alam MA Synthesis and antimicrobial studies of 4-[3-(3-fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(4-fluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid as potent growth inhibitors of drug-resistant bacteria. ACS Omega, 2019, 4(10), 14284–14293. 10.1021/acsomega.9b01967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alam MA Synthesis of 3,4-thiazolo steroids for treating cancer. WO2020018997A1, 2020. [Google Scholar]
- [20].Alam MA; Alsharif Z; Alkhattabi H; Jones D; Delancey E; Gottsponer A; Yang T Hexafluoroisopropyl alcohol mediated synthesis of 2,3-dihydro-4H-pyrido[1,2-a]pyrimidin-4-ones. Sci. Rep, 2016, 6(1), 36316. 10.1038/srep36316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alsharif Z; Ali MA; Alkhattabi H; Jones D; Delancey E; Ravikumar PC; Alam MA Hexafluoroisopropanol mediated benign synthesis of 2H-pyrido[1,2-a]pyrimidin-2-ones by using a domino protocol. New J. Chem, 2017, 41(24), 14862–14870. 10.1039/C7NJ03376A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alsharif ZA; Alam MA Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Advances, 2017, 7(52), 32647–32651. 10.1039/C7RA05993K [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ali MA; Okolo C; Alsharif ZA; Whitt J; Chambers SA; Varma RS; Alam MA Benign synthesis of thiazolo-androstenone derivatives as potent anticancer agents. Org. Lett, 2018, 20(18), 5927–5932. 10.1021/acs.orglett.8b02587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Okolo C; Ali MA; Newman M; Chambers SA; Whitt J; Alsharif ZA; Day VW; Alam MA Hexafluoroisopropanol-mediated domino reaction for the synthesis of thiazolo-androstenones: Potent anticancer agents. ACS Omega, 2018, 3(12), 17991–18001. 10.1021/acsomega.8b02840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chambers SA; Newman M; Frangie MM; Savenka AV; Basnakian AG; Alam MA Antimelanoma activities of chimeric thiazole-androstenone derivatives. R. Soc. Open Sci, 2021, 8(8), 210395. 10.1098/rsos.210395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alnufaie R; Ali MA; Alkhaibari IS; Roy S; Day VW; Alam MA Benign synthesis of fused-thiazoles with enone-based natural products and drugs for lead discovery. New J. Chem, 2021, 45(13), 6001–6017. 10.1039/D1NJ00380A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alkhaibari IS; Raj K C H; Alnufaie R; Gilmore D; Alam MA Synthesis of chimeric thiazolo-nootkatone derivatives as potent antimicrobial agents. ChemMedChem, 2021, 16(17), 2628–2637. 10.1002/cmdc.202100230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karamthulla S; Pal S; Khan MN; Choudhury LH “On-water” synthesis of novel trisubstituted 1,3-thiazoles via microwave-assisted catalyst-free domino reactions. RSC Advances, 2014, 4(71), 37889–37899. 10.1039/C4RA06239F [DOI] [Google Scholar]
- [29].Mahata A; Bhaumick P; Panday AK; Yadav R; Parvin T; Choudhury LH Multicomponent synthesis of diphenyl-1,3-thiazole-barbituric acid hybrids and their fluorescence property studies. New J. Chem, 2020, 44(12), 4798–4811. 10.1039/D0NJ00406E [DOI] [Google Scholar]
- [30].Saroha M; Khurana JM Acetic acid mediated regioselective synthesis of 2,4,5-trisubstituted thiazoles by a domino multicomponent reaction. New J. Chem, 2019, 43(22), 8644–8650. 10.1039/C9NJ01717H [DOI] [Google Scholar]
- [31].Castagnolo D; Scalacci N; Pelloja C; Radi M Microwave-assisted domino reactions of propargylamines with isothiocyanates: Selective synthesis of 2-aminothiazoles and 2-amino-4-methylenethiazolines. Synlett, 2016, 27(12), 1883–1887. 10.1055/s-0035-1561985 [DOI] [Google Scholar]
- [32].Dai T; Cui C; Qi X; Cheng Y; He Q; Zhang X; Luo X; Yang C Regioselective synthesis of substituted thiazoles via cascade reactions from 3-chlorochromones and thioamides. Org. Biomol. Chem, 2020, 18(31), 6162–6170. 10.1039/D0OB01019G [DOI] [PubMed] [Google Scholar]
- [33].Wang Z-J; Chen W-T; He C; Luo H-F; Zhang G-L; Yu Y-P Cascade reactions to 2,4-disubstituted thiazoles via ligand-free palladium(II)-catalyzed C(sp)–C(sp2) coupling. Tetrahedron, 2020, 76(9), 130953. 10.1016/j.tet.2020.130953 [DOI] [Google Scholar]
- [34].Tong W; Li W-H; He Y; Mo Z-Y; Tang H-T; Wang H-S; Pan Y-M Palladium-metalated porous organic polymers as recyclable catalysts for the chemioselective synthesis of thiazoles from thiobenzamides and isonitriles. Org. Lett, 2018, 20(8), 2494–2498. 10.1021/acs.orglett.8b00886 [DOI] [PubMed] [Google Scholar]
- [35].Richter F; Seifert J; Korb M; Lang H; Banert K Real multicomponent reactions: Synthesis of highly substituted 2-aminothiazoles. Eur. J. Org. Chem, 2018, 2018(34), 4673–4682. 10.1002/ejoc.201800701 [DOI] [Google Scholar]
- [36].Jiang J; Huang H; Deng G -J. Four-component thiazole formation from simple chemicals under metal-free conditions. Green Chem, 2019, 21(5), 986–990. 10.1039/C8GC03895C [DOI] [Google Scholar]
- [37].Ben Mohamed S; Rachedi Y; Hamdi M; Le Bideau F; Dejean C; Dumas F An efficient synthetic access to substituted thiazolyl-pyrazolyl-chromene-2-ones from dehydroacetic acid and coumarin derivatives by a multicomponent approach. Eur. J. Org. Chem, 2016, 2016(15), 2628–2636. 10.1002/ejoc.201600173 [DOI] [Google Scholar]
- [38].Guan Z-R; Liu Z-M; Wan Q; Ding M-W One-pot four-component synthesis of polysubstituted thiazoles via cascade Ugi/Wittig cyclization starting from odorless Isocyano(triphenylphosphoranylidene)-acetates. Tetrahedron, 2020, 76(15), 131101. 10.1016/j.tet.2020.131101 [DOI] [Google Scholar]


