Abstract
In Methanobacterium thermoautotrophicum, sn-glycerol-1-phosphate (G-1-P) dehydrogenase is responsible for the formation of the Archaea-specific backbone of phospholipids, G-1-P, from dihydroxyacetonephosphate (DHAP). The possible G-1-P-forming activities were surveyed in cell-free extracts of six species of Archaea. All the archaeal cell-free homogenates tested revealed the ability to form G-1-P from DHAP. In addition, activities of G-3-P-forming glycerol kinase and G-3-P dehydrogenase were also detected in four heterotrophic archaea, while glycerol kinase activity was not detected in two autotrophic methanogens. These results show that G-1-P is produced from DHAP by G-1-P dehydrogenase in a wide variety of archaea while exogenous glycerol is catabolized via G-3-P.
The glycerophosphate (GP) backbone of glycerophospholipid in archaea is sn-glycerol-1-phosphate (G-1-P), which is the enantiomer of its bacterial and eucaryal counterpart (4). So far, no exception to this difference in the stereoconfiguration of GP has been found, and this is one of the most fundamental features of members of each domain. The mechanism of formation of the G-1-P structure of phospholipids in archaea was first studied by in vivo incorporation experiments by Kates et al. in 1970 (5) in Halobacterium cutirubrum. They reported that the tritium label at the 2 position of glycerol was not retained in the glycerol moiety of the lipids after the incorporation, while tritium at the 1 position of glycerol was retained in the lipids. Kakinuma et al. (3) showed that a stereochemical inversion of glycerol moiety took place during lipid biosynthesis from exogenously supplied glycerol. sn-Glycerol-3-phosphate (G-3-P)-specific dehydrogenase and glycerol kinase have been detected in H. cutirubrum cell-free homogenates (15).
On the other hand, Zhang et al. (17) have shown that G-1-P is the direct precursor of the ether lipid, and we have identified G-1-P dehydrogenase as the key enzyme of G-1-P formation in Methanobacterium thermoautotrophicum (9). These results suggest that different mechanisms of G-1-P formation might be operating in extreme halophiles and methanogens. In the present study, we sought to generalize direct G-1-P formation from dihydroxyacetonephosphate (DHAP) in archaea.
Growth of cells and preparation of cell-free homogenates.
Cells of Methanobacterium thermoautotrophicum ΔH (8), Methanosarcina barkeri DSM800 (7), Halobacterium salinarum JCM8981 (8), Pyrococcus furiosus JCM8422 (14), and Thermoplasma sp. strain HO-62 (16) were grown as described previously. Pyrococcus sp. strain KS8-1 was grown at 90°C in the medium for heterotrophic hyperthermophilic archaea (11) supplemented with 0.1% yeast extract and 20 g of sulfur/liter. The cells collected at the late logarithmic growth phase were disrupted in 50 mM K-phosphate buffer (pH 7.3) by passage through a French pressure cell at 12,500 lb/in2 (86,200 kPa). The supernatant fraction of cell homogenates obtained by centrifugation at 10,000 × g for 15 min was used for experiments. In the case of H. salinarum, 4 M NaCl was included in the buffer. Protein was determined by the bicinchoninic acid method (13).
GP-forming reactions.
The reaction mixture (5.0 ml) for DHAP reduction (GP dehydrogenase reaction) contained 10 mM DHAP, 8 mM NADH, 58 mM triethanolamine buffer (pH 7.3), 60 mM KCl, and cell-free homogenate containing 5 to 30 mg of protein. In the case of H. salinarum cell-free homogenates, NADH was replaced by 8 mM NADPH. The reaction mixture (4.0 ml) for glycerol kinase reaction contained 20 mM glycerol, 20 mM ATP, 20 mM MgCl2, 50 mM K-phosphate buffer (pH 7.3), and cell-free homogenate containing 0.5 to 10 mg of protein. NaCl was added to give a concentration of 4 M in the reaction mixtures for H. salinarum. The mixtures were incubated for 16 h at the temperatures indicated in Table 1.
TABLE 1.
Enantiomeric composition of the product (GP) of DHAP reduction and glycerol phosphorylation catalyzed by cell-free homogenates of various archaeaa
| Archaeon | Temp (°C) | GP formed from DHAP (nmol/16 h/mg of protein)b
|
GP formed from glycerol + ATP (nmol/16 h/mg of protein)b
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein (mg) | Total GP | G-3-P | G-1-P | Protein (mg) | Total GP | G-3-P | G-1-P | ||
| Methanobacterium thermoautotrophicum ΔH | 65 | 5 | 1,929 | 23 (1.2) | 1,833 (95) | 10 | n.d. | ||
| Methanosarcina barkeri DSM800 | 37 | 10 | 242 | n.d.c (0) | 222 (92) | 10 | n.d. | ||
| H. salinarum JCM8981 | 37 | 20 | 49 | 2 (3.9) | 44 (91) | 2 | 515 | 530 (103) | n.d. (0) |
| Pyrococcus sp. strain KS8-1 | 65 | 10 | 166 | 7 (4.6) | 161 (97) | 0.5 | 60,193 | 59,771 (99) | n.d. (0) |
| P. furiosus JCM8422 | 65 | 10 | 89 | n.d. (0) | 85 (95) | 1 | 7,032 | 7,384 (105) | n.d. (0) |
| Thermoplasma sp. strain HO-62 | 60 | 30 | 17 | 4 (24) | 12 (72) | 1 | 1,129 | 1,208 (107) | n.d. (0) |
Cell-free homogenates of the indicated archaea were incubated with DHAP plus NAD(P)H or glycerol plus ATP for 16 h as described in the text. After the reaction, total GP, G-3-P, and G-1-P were determined by gas-liquid chromatography, the G-3-P dehydrogenase-MTT method, and the G-1-P dehydrogenase-MTT method, respectively.
Values in parentheses are percentages of each enantiomer in total GP.
n.d., not detected (less than the lowest limit of determination).
Determination of total and individual GP.
After the GP-forming reaction was completed, total GP, G-1-P, and G-3-P were determined by gas-liquid chromatography and by two stereospecific enzymes, respectively. An aliquot of the reaction mixture received 30 μg of icosane as an internal standard, and total GP was trimethylsilylated with an excess of 1,1,1,3,3,3-hexamethyldisilazane and trimethylchlorosilane for 60 min at 110°C before gas chromatography. The lowest limit of determination of this method was 5 nmol of GP.
Another aliquot of the GP-forming reaction mixtures was deproteinized by the addition of perchloric acid and neutralized with KOH. In the case of GP dehydrogenase reaction, the nicotinamide adenine coenzyme in the deproteinized solution was removed by the addition of active carbon. The coenzyme-free solution was used for determination of each GP.
G-1-P was measured by using G-1-P dehydrogenase from Methanobacterium thermoautotrophicum and NAD. A NAD recycling system [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (MTT) (Dojindo Laboratories, Kumamoto, Japan) and thermophilic diaphorase from Bacillus stearothermophilus (Unitika Ltd., Osaka, Japan) were included. The mixture was incubated at 65°C for 90 min. After cooling, the absorbance at 578 nm was read. This method was specific to G-1-P, and G-3-P did not interfere with the G-1-P determination. The lowest limit of determination of this method was 15 nmol of G-1-P. Standard G-1-P was prepared from halobacterial archaeol (diphytanylglycerol) (8). G-3-P was assayed in essentially the same way as in the case of G-1-P measurement except that rabbit muscle G-3-P dehydrogenase and mesophilic diaphorase from Clostridium sp. (Toyobo Co., Ltd., Osaka, Japan) were used. The lowest limit of determination of this method was 2 nmol of G-3-P. Standard G-3-P and racemic α-GP were obtained from Nacalai Tesque (Kyoto, Japan). Details of the above methods will be published elsewhere.
G-1-P dehydrogenase activity.
The occurrence of G-1-P dehydrogenase and G-3-P dehydrogenase was examined in cell-free homogenates of six species of archaea, including methanogens, an extreme halophile, a thermoacidophile, and hyperthermophiles. The results are summarized in Table 1. Cell-free homogenates of all archaea tested contained G-1-P-forming activity from DHAP. Among those organisms, Methanobacterium thermoautotrophicum showed the highest specific activity. Cell-free homogenates of Methanobacterium thermoautotrophicum, Methanosarcina barkeri, two strains of Pyrococcus sp., and Thermoplasma sp. contained activities of NADH-dependent reduction of DHAP to G-1-P, while the similar activity of H. salinarum was NADPH dependent. The activities of Methanobacterium thermoautotrophicum and Thermoplasma sp. were also active to NADPH. These results show that all the archaeal species so far measured contained G-1-P dehydrogenase.
Activity of the reverse reaction, that is, G-1-P oxidation by NAD, was detected in cell-free homogenates of Pyrococcus sp. strain KS8-1 even though it was significantly lower than DHAP reduction (data not shown). Although G-1-P oxidation was not detected by the cell-free homogenate of H. salinarum, the activity was able to be observed when G-1-P was incubated with a protein fraction obtained by ammonium sulfate precipitation of the cell-free homogenate, in which G-1-P dehydrogenase was presumed to be concentrated. G-1-P oxidation activity has been reported to be 1/16th of DHAP reduction activity of the same enzyme in Methanobacterium thermoautotrophicum (8).
G-3-P dehydrogenase activity.
G-3-P was also detected in the reaction mixture after reaction completed with H. salinarum, Thermoplasma, and Pyrococcus sp. strain KS8-1, and Methanobacterium thermoautotrophicum (Table 1). While in the study reported here, the amount of G-3-P formed when cell-free homogenates of P. furiosus JCM8422 was incubated with DHAP and NADH was negligible, Noguchi et al. (10) observed accumulation of 2% G-3-P of total GP after DHAP and NADH were incubated with cell-free homogenates of the same organism at 51°C for 60 h. Furthermore, a fraction of P. furiosus JCM8422 cell-free homogenate eluted from a column of Cosmogel DEAE (Nacalai Tesque) with 0.2 M KCl showed an activity of G-3-P oxidation by NAD at 65°C. This activity was detected with a NAD recycling system using MTT and thermophilic diaphorase at pH 7.3. It is thus concluded that cell-free homogenates of these organisms also contained NAD-dependent G-3-P dehydrogenase. G-3-P-dependent 2,6-dichlorophenolindophenol reduction was also observed in the Thermoplasma HO-62 cell-free homogenate at 60°C. This activity suggests the presence of flavin-containing G-3-P dehydrogenase in this organism. G-3-P oxidation was confirmed in halobacterial homogenates.
Glycerol kinase activities.
Formation of GP was observed when glycerol and ATP were incubated with cell-free homogenates of H. salinarum, two Pyrococcus spp., and Thermoplasma sp. but not with cell-free homogenates of two methanogens (Table 1). All the products of these activities were G-3-P.
It is concluded from the results presented in previous work (10) and in this paper that (i) G-1-P dehydrogenase was present in all the archaea; (ii) G-1-P was not produced by glycerol kinase reaction in any archaea; (iii) heterotrophic archaea (H. salinarum, Pyrococcus spp., and Thermoplasma sp.) contained G-3-P-forming glycerol kinase, while autotrophic archaea (Methanobacterium thermoautotrophicum and Methanosarcina barkeri) did not; and (iv) organisms that contained G-3-P-forming glycerol kinase activity also contained G-3-P dehydrogenase. In Thermoplasma, flavin-linked G-3-P dehydrogenase may also be present.
The fact that only heterotrophic archaea contained a G-3-P-specific enzyme set (both G-3-P dehydrogenase and G-3-P-forming glycerol kinase) is possibly explained as follows. When these heterotrophic archaea utilize glycerol as a carbon or energy source, they would convert glycerol to G-3-P but not to G-1-P. The G-3-P produced would be further metabolized to DHAP by G-3-P dehydrogenase, and reactions connecting DHAP to central metabolic pathways have been already described (2). Therefore, G-3-P pathway would probably be a pathway for glycerol catabolism. In archaea, the catabolic and lipid biosynthetic pathways seem to be separated by enantiomers of GP (Fig. 1). The role of G-3-P dehydrogenase in an autotroph Methanobacterium thermoautotrophicum is not known.
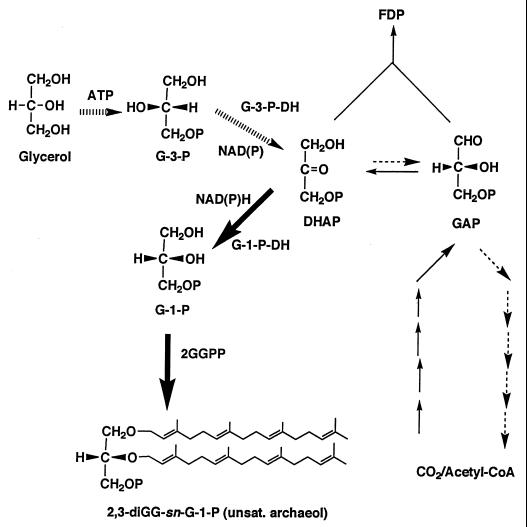
FIG. 1.
Possible metabolic pathway of GP in archaea. In autotrophic archaea (e.g., methanogens) triosephosphates (DHAP and glyceraldehyde-3-phosphate [GAP]) are synthesized from CO2 and DHAP is converted to G-1-P, which is the sole source of enantiomeric backbone of archaeal polar lipids. Although some autotrophic methanogens (e.g., Methanobacterium thermoautotrophicum) have G-3-P dehydrogenase, this enzyme is not necessary for lipid biosynthesis. In heterotrophic archaea, exogenously supplied glycerol is incorporated and phosphorylated by ATP to form G-3-P, which is catabolized via DHAP. Endogenous DHAP and glycerol-derived DHAP are mixed in the intracellular pool, and some fraction of it is incorporated into lipids. Thus sn-1 and sn-3 carbons of exogenous glycerol are inverted to sn-3 and sn-1 carbon of lipid glycerol backbone, and sn-2 hydrogen of exogenous glycerol is not retained in lipids. G-1-P-DH, G-1-P dehydrogenase; G-3-P-DH, G-3-P dehydrogenase; FDP, fructose diphosphate; GGPP, geranylgeranylpyrophosphate; 2,3-diGG-sn-G-1-P, 2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate; unsat. archaeol, unsaturated archaeol. The solid arrows indicate the anabolic route and the broken arrows indicate the catabolic route.
Earlier work (3, 5) based on the results of in vivo incorporation experiments for the formation of enantiomeric backbone of phospholipids in H. cutirubrum can be explained as follows. Isotopically labeled glycerol incorporated into halobacterial cells would be phosphorylated to G-3-P by glycerol kinase. The G-3-P should then be dehydrogenated to DHAP by G-3-P dehydrogenase. At this point, 3H at the sn-2 position must be lost. In the intracellular pool of DHAP, [3H]DHAP and unlabeled DHAP provided endogenously would be mixed and be rereduced to G-1-P, which is then incorporated into lipid. Apparent inversion of the glycerol stereostructure (3) should be a phenomenon that can be seen only in the case of the incorporation of exogenously supplied glycerol into lipids but that is not essential for lipid synthesis itself.
Complete genome sequences of three species of archaea recently published (Methanococcus jannaschii [1], Methanobacterium thermoautotrophicum [12], and Archaeoglobus fulgidus [6]) revealed the presence of G-1-P dehydrogenase genes (MJ0712, mt0610, and AF1674, respectively; Table 2). The biosynthetic G-3-P dehydrogenase gene (gpsA) of Bacteria has also been identified in genomes of Methanobacterium thermoautotrophicum and A. fulgidus (mt0368 and AF0871). A glycerol kinase gene (glpK; AF0866) and the catabolic G-3-P dehydrogenase gene (glpA; AF1328) have been found in the genome of a heterotroph A. fulgidus. G-3-P dehydrogenase gene (MJ1411) has been also found in Methanococcus jannaschii, in which glycerol kinase gene is not present. The distribution of the relevant genes is consistent with the conclusion of the present study.
TABLE 2.
Distribution of genes for GP metabolism in archaea whose complete genome sequences have been reported
| Archaeon | Nutrition | egsA | gpsA | glpK | glpA |
|---|---|---|---|---|---|
| Methanococcus jannaschii | Autotroph | + | (+)a | − | − |
| Methanobacterium thermoautotrophicum | Autotroph | + | + | − | − |
| Archaeoglobus fulgidus | Heterotroph | + | + | + | + |
A gene for G-3-P dehydrogenase has been identified but it has not been shown to be gpsA or glpA (1).
Acknowledgments
We are grateful to Mami Ohga for the growth of cells of Methanobacterium thermoautotrophicum ΔH, Methanosarcina barkeri DSM800, Halobacterium salinarum JCM8981, and Pyrococcus furiosus JCM8422.
REFERENCES
- 1.Bult C J, White O, Olsen G J, Zhou L X, Fleischmann R D, Sutton G G, Blake J A, Fitzgerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 2.Danson M J. Central metabolism of the archaea. In: Kates M, Kushner D J, Matheson A T, editors. The biochemistry of archaea (Archaebacteria). Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1993. pp. 1–24. [Google Scholar]
- 3.Kakinuma K, Yamagishi M, Fujimoto Y, Ikekawa N, Oshima T. Stereochemistry of the biosynthesis of sn-2,3-O-diphytanyl glycerol, membrane lipid of archaebacteria Halobacterium halobium. J Am Chem Soc. 1988;110:4861–4863. [Google Scholar]
- 4.Kates M. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog Chem Fats Other Lipids. 1978;15:301–342. doi: 10.1016/0079-6832(77)90011-8. [DOI] [PubMed] [Google Scholar]
- 5.Kates M, Wassef M K, Pugh E L. Origin of the glycerol moieties in the glycerol diether lipids of Halobacterium cutirubrum. Biochim Biophys Acta. 1970;202:206–208. doi: 10.1016/0005-2760(70)90238-9. [DOI] [PubMed] [Google Scholar]
- 6.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Peterson S, Reich C I, McNeil L K, Badger J H, Glodek A, Zhou L X, Overbeek R, Gocayne J D, Weidman J F, McDonald L, Utterback T, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Sadow P W, D’Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olsen G J, Fraser C M, Smith H O, Woese C R, Venter J C. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 7.Koga Y, Akagawa-Matsushita M, Ohga M, Nishihara M. Taxonomic significance of the distribution of the component parts of polar ether lipids in methanogens. Syst Appl Microbiol. 1993;16:342–351. [Google Scholar]
- 8.Nishihara M, Koga Y. Purification and properties of sn-glycerol-1-phosphate dehydrogenase from Methanobacterium thermoautotrophicum: characterization of the biosynthetic enzyme for the enantiomeric glycerophosphate backbone of ether polar lipids of archaea. J Biochem. 1997;122:572–576. doi: 10.1093/oxfordjournals.jbchem.a021791. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara M, Koga Y. sn-Glycerol-1-phosphate dehydrogenase in Methanobacterium thermoautotrophicum: key enzyme in biosynthesis of the enantiomeric glycerophosphate backbone of ether phospholipids of archaebacteria. J Biochem. 1995;117:933–935. doi: 10.1093/oxfordjournals.jbchem.a124822. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi S, Maeda M, Nishihara M, Koga Y, Sone N. Expression and use of Methanobacterium thermoautotrophicum sn-glycerol-1-phosphate dehydrogenase for the assay of sn-glycerol-1-phosphate in archaea. J Ferment Bioeng. 1998;86:266–270. [Google Scholar]
- 11.Robb F T, Place A R. Media for thermophiles. In: Robb F T, Place A R, editors. Archaea: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 167–168. [Google Scholar]
- 12.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H M, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D Y, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Mao J I, Rice P, Nolling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzaro M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Uemori T, Ishino Y, Toh H, Asada K, Kato I. Organization and nucleotide sequence of the DNA polymerase gene from the archaeon Pyrococcus furiosus. Nucleic Acids Res. 1993;21:259–265. doi: 10.1093/nar/21.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassef M K, Sarner J, Kates M. Stereospecificity of the glycerol kinase and the glycerophosphate dehydrogenase in Halobacterium cutirubrum. Can J Biochem. 1970;48:69–73. doi: 10.1139/o70-012. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda M, Oyaizu H, Yamagishi A, Oshima T. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl Environ Microbiol. 1995;61:3482–3485. doi: 10.1128/aem.61.9.3482-3485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D-L, Daniels L, Poulter C D. Biosynthesis of archaebacterial membranes. Formation of isoprene ethers by a prenyl transfer reaction. J Am Chem Soc. 1990;112:1264–1265. [Google Scholar]