Abstract
Objectives
Gonorrhoea caused by Neisseria gonorrhoeae is the second most notified sexually transmitted infection (STI) in Australia and the case numbers for this STI have been increasing globally. Progressive gonococcal infection may lead to disseminated gonococcal infection (DGI), which causes significant morbidity among patients. This study aims to examine the genetic diversity of N. gonorrhoeae isolates collected in Queensland from January 2010 to August 2015 and to determine factors associated with DGI in Queensland.
Design
Retrospective surveillance study for epidemiological purposes.
Setting
All gonorrhoeae isolates referred by private and public pathology laboratories to the state of Queensland, Australia Neisseria reference laboratory.
Methods
Between January 2010 and August 2015, 3953 N. gonorrhoeae isolates from both metropolitan and regional Queensland infections were typed with NG-MAST (N. gonorrhoeae multiantigen sequence typing) to assess the genetic diversity between strains. Whole-genome sequencing (WGS) was used to investigate strain-related factors associated with DGI.
Results
ST6876 was the most common NG-MAST type, detected in 7.6% of the isolates. DGI was significantly more likely in females <30 years (OR 13.02, p<0.0001) and in older males >30 years (OR 6.04, p<0.0001), with most cases originating from North Queensland (OR 8.5, p<0.0001). Strains harbouring PIA class of porB type were associated with DGI (OR 33.23, p<0.0001).
Conclusion
Genotyping techniques, such as NG-MAST and WGS, are proving instrumental in providing an insight into the population structure of N. gonorrhoeae, and genetic mechanisms of pathogenesis, such as for DGI.
Keywords: Epidemiology, INFECTIOUS DISEASES, MOLECULAR BIOLOGY
Strengths and limitations of this study.
Genetic diversity of over 3500 Neisseria gonorrhoeae isolates was assessed by N. gonorrhoeae multiantigen sequence typing genotyping.
Demographic factors associated with N. gonorrhoeae infection and disseminated gonococcal infection (DGI) cases were examined across all isolates.
Genotyping assisted in identification of populations associated with higher incidence of DGI.
Only a small number of strains were further characterised by whole-genome sequencing, meaning significance of associations with genetic markers such as pglA, PIA porB type and gonococcal genetic island could not be drawn and further studies with larger rates of whole-genome sequencing are needed to address this.
Introduction
Disseminated gonococcal infection (DGI) is a complication of gonorrhoea from bacteraemic spread of Neisseria gonorrhoeae. It typically presents as an arthritis-dermatitis syndrome but in rare cases can lead to death via septic shock.1 DGI primarily occurs in individuals with an asymptomatic untreated primary infection, most often female, although some studies have shown an association with males.2–4 Early diagnosis and treatment are required to avert significant morbidity.
Both host and N. gonorrhoeae strain-related factors can predispose to DGI. Host-associated risk factors include recent menstruation, pregnancy and complement deficiencies.5–7 Several strain-related factors have been proposed including exhibiting an arginine–hypoxanthine–uracil (AHU) auxotype, expressing particular phase-variable variants of the pilus glycosyl transferase A (pgtA) gene, opacity genes and harbouring a Protein IA (PIA) class of porB gene (as opposed to PIB).8–14 Gonococcal genetic island (GGI) is another speculated virulence factor encoding a type IV secretion system, which plays a role in horizontal gene transfer.15 However, except for the PIA gene, evidence to support the strain-related factors is limited. For example, an early study of DGI-causing gonococci in Australia found that none were of the AHU auxotype, while a later Australian study found no strong association with the phase-variable allele of the pgtA gene, subsequently referred to as the pglA gene.16 17 Previous studies assessing stain-related factors associated with DGI are limited by sample size and/or lack of comparison to non-DGI isolates.
We sought to assess the genetic diversity of N. gonorrhoeae isolates collected in Queensland from January 2010 to August 2015 and to determine factors associated with DGI in Queensland. In addition, we used whole-genome sequencing (WGS) to gain insight to any existing strain-related factors which may have contributed to the occurrence of DGI.
Methods
N. gonorrhoeae isolates from Queensland
From 2010 to 2015, the Australian state of Queensland reported 16 506 gonococcal infections over the 6 years, equivalent to an average notification rate of 60 cases per 100 000 population per year.18 Approximately 75% of these notifications were diagnosed by Nucleic Acid Amplification Test (NAAT) only, with no isolate available for further testing. Diagnostic methods used varied across the state, with 80% of cases in the northern Queensland (NQ) regions diagnosed by NAAT only, as opposed to 70% of cases from the rest of the state; with larger variations of between 58% and to 84% for individual health service districts. A total of 3953 N. gonorrhoeae isolates were included in this study, isolated from specimens collected between January 2010 and August 2015 in pathology laboratories servicing Queensland and surrounding areas and subsequently referred to the state reference laboratory at Queensland Health Forensic and Scientific Services. One isolate per patient episode, defined as not collected within 1 month of a previously included strain, was included. Data collected for each isolate included date of specimen collection, age, sex, postcode of residence and specimen type. To identify disease prevalence in specific age groups isolates from males and females were grouped into two groups, that is, <30 years and ≥30 years. Postcode of residence was used to assign broad geographical region categories of NQ and south-east Queensland (SEQ) and other (sparsely populated central Queensland, interstate or overseas). DGI was defined where the organism was isolated from blood culture, joint fluid and/or tissue.
N. gonorrhoeae multiantigen sequence typing typing and phylogenetic analysis
Crude DNA extracts of all the N. gonorrhoeae isolates received in the study period were routinely subjected to N. gonorrhoeae multiantigen sequence typing (NG-MAST) as previously described.19 These extracts were prepared by boiling 400 µL of TE buffer containing a loopful (1 µL) of pure N. gonorrhoeae colonies at 100°C for 10 min. NG-MAST comprises DNA sequencing of partial tbpB and porB genes and subsequent analysis via an online database to assign allele and sequence types (www.ng-mast.net). The porB sequence data from the NG-MAST was analysed to assign either PIA or PIB class.20 The analysis was based on a phylogenetic assessment, and comparison with previously recognised PIA and PIB sequences from GenBank.
WGS and bioinformatic analysis
To investigate strain-related factors associated with DGI, we selected 16 isolates from 8 different NG-MAST types, which were prevalent in DGI, in this study. Two strains from each NG-MAST were selected, comprising both DGI and non-DGI strains. DNA was extracted from isolates using the QIAsymphony SP, using the DSP DNA minikit (Qiagen, Germany), as per manufacturer’s guidelines. WGS was performed on the Illumina NextSeq 500 platform (Illumina, California, USA) using NextSeq 500 Mid Output V2 kit (Illumina) with Nextera XT library preparation. Reads were trimmed with Trimmomatic (V.0.36),21 corrected and assembled with Spades (V.3.10.1) (https://github.com/ablab/spades),22 and assemblies uploaded to pubMLST to determine presence/absence of the gonococcal genetic island,23 and analysed with Ridom SeqSphere +4.10.0 (Ridom, Germany) using alleles from 1649 N. gonorrhoeae cgMLST v1.0 loci24 and Neisseria spp. MLST.25 WGS assemblies are available on pubMLST with ID numbers 52753-52768. WGS raw sequence files and associated metadata have been submitted to the European Nucleotide Archive with Project Accession number PRJEB52601.
Statistical analysis
Descriptive analysis was performed using Microsoft Excel. Annual rates of reported cases were computed by using the number of cases reported as numerators, and statistics Queensland yearly population as denominators. Categorical variables were examined using the Fischer’s exact test performed in GraphPad Prism V.7 (GraphPad Software, California, USA). ORs with 95% CIs were obtained from logistic regression models in Microsoft Excel to quantify associations between independent variables and outcome. P values of <0.05 were considered statistically significant.
Patient and public involvement
There was no patient or public involvement in the study.
Results
Demographics and N. gonorrhoeae isolates
A total of 3953 N. gonorrhoeae isolates in this study from January 2010 to August 2015 consisted of genital (n=3099; 78.3%), invasive (n=64; 1.6%), anorectal (n=456; 11.5%), oropharyngeal (n=233; 5.8%), ocular (n=31; 0.7%) and other/not specified (n=70; 1.7%) specimen types, as listed in online supplemental data table 1. Overall, majority of these isolates were reported in SEQ (n=2403; 60.7%), followed by NQ (n=1193; 30.1%) and other regions (sparsely populated central Queensland, interstate or overseas), which constituted of 9% (n=357) of the total cases. The isolates comprised 73% (n=2898) from males (62.9 cases per 100 000 population) and 27% (n=1055) from females (22.9 cases per 100 000 population). Further breakdown into age groups showed that 20.4% (n=808) gonococcal infections were represented by females <30 years of age and 5.9% of infections were reported in females ≥30 years of age. Similar trend was observed in males where 44.3% (n=1754) of N. gonorrhoeae infections were noted in males <30 years of age and 28.3% (n=1119) of infections were present in males ≥30 years of age. PIB class of porB was assigned to 72.7% (n=2875) of isolates whereas 27.3% (n=1078) of the isolates belonged to porB class PIA. Table 1 shows a breakdown for all strains by PIA/PIB, NQ/SEQ and age group.
Table 1.
Demographics of Neisseria gonorrhoeae isolates, Queensland January 2010 to August 2015
| Demographics | N | % of total | |
| All cases | 3953 | 100 | |
| porB Class | PIA* | 1078 | 27.3 |
| PIB* | 2875 | 72.7 | |
| Geographical location | NQ | 1193 | 30.1 |
| SEQ | 2403 | 60.7 | |
| Others | 357 | 9.0 | |
| Sex | Female | 1055 | 26.6 |
| Male | 2898 | 73.3 | |
| Age groups | <30 Female | 808 | 20.4 |
| ≥30 Female | 234 | 5.9 | |
| <30 Male | 1754 | 44.3 | |
| ≥30 Male | 1119 | 28.3 | |
| Age not specified | 38 | 0.9 | |
*PorB class IA or IB
NQ, northern Queensland; SEQ, south-east Queensland.
bmjopen-2022-061040supp001.xls (805KB, xls)
NG-MAST typing and phylogenetic analysis
Among the 3953 isolates tested, 574 alleles of porB gene were identified, the most prevalent of which was the 4101 allele, present in 441 isolates (11.1%). The tbpB gene was represented by 250 alleles with the most frequent allele was 29, detected in 653 isolates (16.5%). Combinations of porB and tbpB alleles resulted in 823 NG-MAST types. Overall ST6876 was the most common NG-MAST type, detected in 302 isolates (7.6%). It was also the most prevalent in years 2010 and 2011, represented by 126 (16.1%) and 109 (14.4%) isolates, respectively. In 2012 and 2013, ST21 became more prevalent, represented by 8.6% and 6.0% of the isolates collected in those years. In 2014 and 2015 (up to August), ST4186 was found with high frequency with 7.1% and 10.1% of the isolates, respectively. 771 STs were represented by only one isolate, and these Sequence Types (STs) accounted for between 16.8% and 23.3% of total isolates each year. Table 2 shows a summary of the most frequent alleles and types over the 5 years.
Table 2.
NG-MAST typing of isolates collected in Queensland from January 2010 to August 2015
| Year | No of Neisseria gonorrhoeae isolates | No of porB alleles | Most frequent porB alleles | No of tbpB alleles | Most frequent tbpB alleles | Number of NG-MAST types | Most frequent NG-MAST types | porB class | Invasive (DGI) isolates* |
| 2010 | 782 | 165 | porB 4101 (18.7%) | 87 | tbpB 29 (17.6%) | 208 | 6876 (16.1%) | PIAˆ—255 (32.6%) PIBˆ—527 (67.3%) |
12 (1.5%) |
| porB 2280 (5.3%) | tbpB 1330 (16.3%) | 6863 (5.4%) | |||||||
| porB 908 (4.7%) | tbpB 1329 (8.4%) | 1407 (4.7%) | |||||||
| porB 1808 (4.4%) | tbpB 110 (6.6%) | 2992 (4.4%) | |||||||
| porB 4143 (3.7%) | tbpB 349 (6.1%) | 6940 (3.7%) | |||||||
| 2011 | 754 | 157 | porB 4101 (17.9%) | 85 | tbpB 29 (24.0%) | 206 | 6876 (14.4%) | PIA—240 (31.8%) PIB—514 (68.1%) |
8 (1.0%) |
| porB 1808 (8.8%) | tbpB 1330 (15.5%) | 2992 (8.8%) | |||||||
| porB 4099 (5.3%) | tbpB 349 (7.0%) | 6879 (4.7%) | |||||||
| porB 14 (4.7%) | tbpB 33 (6.7%) | 21 (4.4%) | |||||||
| porB 4104 (3.7%) | tbpB 1329 (6.1%) | 6937 (3.2%) | |||||||
| 2012 | 680 | 156 | porB 4101 (12.2%) | 91 | tbpB 29 (19.7%) | 203 | 21 (8.6%) | PIA—195 (28.6%) PIB—485 (71.3%) |
10 (1.4%) |
| porB 14 (8.6%) | tbpB 349 (12.3%) | 2992 (7.6%) | |||||||
| porB 4099 (7.7%) | tbpB 33 (10.8%) | 6879 (7.5%) | |||||||
| porB 1808 (7.6%) | tbpB 1330 (9.1%) | 6876 (7.0%) | |||||||
| porB 4104 (5.5%) | tbpB 1329 (7.0%) | 6937 (4.1%) | |||||||
| 2013 | 650 | 167 | porB 1808 (7.2%) | 93 | tbpB 29 (18.9%) | 217 | 21 (6.0%) | PIA—160 (24.6%) PIB—490 (75.3%) |
10 (1.5%) |
| porB 4101 (6.7%) | tbpB 349 (8.3%) | 4822 (6.0%) | |||||||
| porB 1903 (6.1%) | tbpB 33 (7.5%) | 6879 (5.3%) | |||||||
| porB 14 (6.0%) | tbpB 110 (6.9%) | 4186 (5.2%) | |||||||
| porB 4099 (5.3%) | tbpB 241 (6.9%) | 5533 (4.0%) | |||||||
| 2014 | 633 | 147 | porB 1808 (14.6%) | 90 | tbpB 241 (8.5%) | 201 | 4186 (7.1%) | PIA—140 (22.1%) PIB—493 (77.8%) |
18 (2.8%) |
| porB 2569 (7.2%) | tbpB 29 (7.8%) | 9654 (5.6%) | |||||||
| porB 147 (6.4%) | tbpB 4 (7.1%) | 4244 (4.8%) | |||||||
| porB 4101 (4.5%) | tbpB 110 (6.1%) | 10 039 (3.7%) | |||||||
| porB 5912 (3.7%) | tbpB 1744 (6.0%) | 5004 (2.3%), 6879 (2.3%) | |||||||
| 2015† | 454 | 115 | porB 1808 (12.1%) | 74 | tbpB 241 (16.5%) | 154 | 4186 (10.1%) | PIA—87 (19.1%) PIB—367 (80.8%) |
6 (1.3%) |
| porB 2569 (10.1%) | tbpB 4 (6.4%) | 4244 (5.2%) | |||||||
| porB 147 (8.14%) | tbpB 29 (5.9%) | 9654 (4.4%) | |||||||
| porB 2656 (5.0%) | tbpB 893 (5.7%) | 9909 (3.9%) | |||||||
| porB 543 (4.1%) | tbpB 1744 (5.3%) | 11 821 (3.7%) |
PorB class IA or IB
*Percentage of total isolates.
†Data collected up until August 2015.
DGI, disseminated gonococcal infection; NG-MAST, Neisseria gonorrhoeae multiantigen sequence typing.
Disseminated gonococcal infection
From January 2010 to August 2015, 64 DGI-related isolates were received by the reference laboratory, comprising 1.6% of total isolates; 49 cases (76.6%) were diagnosed from joint samples and 15 (23.4%) from blood samples. This study only had access to cultured gonococcal isolates, so any DGI cases diagnosed by NAAT only are not considered here. A summary of the demographics, porB class types and strain types (n=8) associated with DGI cases is provided in table 3. Even though the majority of total gonococcal isolates were from males, DGI was significantly more likely in females (OR 4.72, p<0.0001), particularly in those aged <30 years (OR 13.02, p<0.0001) and in older males aged >30 years (OR 6.04, p<0.0001) when compared with their younger counterparts. The majority of DGI cases (n=50; 78%) originated from the north Queensland, and cases from this region had higher odds of being associated with DGI (OR 8.5, p<0.0001). A total of 31 STs of the total 823 were observed among the 64 DGI cases. PIA porB type was significantly associated with DGI (OR 33.23, p<0.0001), and accounted for 59 (92.2%) of the 64 DGI cases. Seven of the prevalent NG-MASTs in DGI (ST758, ST6886, ST6937, ST6939, ST7126, ST8712 and ST10711), all with PIA class of porB, were found to be individually associated with DGI (table 3). However, the most prevalent NG-MAST in this study ST6876 (n=302), which is also PIA class of porB, was not found to be associated with DGI (n=6) (OR 1.2, p=0.6).
Table 3.
Demographic factors, porB type and NG-MAST types associated with disseminated gonococcal infection (DGI) in Queensland (January 2010 to August 2015)
| Risk factor | Non-DGI | DGI | Total | DGI as % | Univariate OR | P value | |
| porB class | PIA* | 1019 | 59 | 1078 | 5.5 | 33.23 | <0.0001 |
| PIB* | 2870 | 5 | 2875 | 0.2 | 0.03 | ||
| Geographical location | NQ | 1143 | 50 | 1193 | 4.2 | 8.5 | <0.0001 |
| SEQ | 2394 | 9 | 2403 | 0.4 | 0.1 | ||
| Other | 352 | 5 | 357 | 1.4 | 0.85 | ||
| Sex | Female | 1015 | 40 | 1055 | 3.8 | 4.72 | <0.0001 |
| Male | 2874 | 24 | 2898 | 0.8 | 0.2 | ||
| Age group (years) | <30 female | 779 | 29 | 808 | 3.58 | 13.02 | <0.0001 |
| <30 male | 1749 | 5 | 1754 | 0.2 | 0.07 | ||
| ≥30 female | 223 | 11 | 234 | 4.7 | 2.85 | 0.01 | |
| ≥30 male | 1100 | 19 | 1119 | 1.69 | 0.35 | ||
| <30 female | 779 | 29 | 808 | 3.58 | 0.75 | 0.44 | |
| ≥30 female | 223 | 11 | 234 | 4.7 | 1.32 | ||
| <30 male | 1749 | 5 | 1754 | 0.2 | 0.16 | <0.0001 | |
| ≥30 male | 1100 | 19 | 1119 | 1.69 | 6.04 | ||
| Age not specified | 38 | 0 | 38 | 0.0 | |||
| Prevalent NG-MAST types | ST758 | 17 | 2 | 19 | 10.5 | 3.67 | 0.03 |
| ST6876 | 296 | 6 | 302 | 2.0 | 1.2 | 0.6 | |
| ST6886 | 30 | 3 | 33 | 9.1 | 6.3 | 0.001 | |
| ST6937 | 80 | 14 | 94 | 14.9 | 13.3 | <0.0001 | |
| ST6939 | 34 | 3 | 37 | 8.1 | 5.57 | 0.02 | |
| ST7126 | 22 | 3 | 25 | 12.0 | 8.6 | 0.007 | |
| ST8712 | 17 | 5 | 22 | 22.7 | 19.3 | <0.0001 | |
| ST10711 | 15 | 3 | 18 | 16.7 | 12.7 | 0.002 | |
| Other STs | 3378 | 25 | 3403 | 0.7 | 0.09 | ||
| Total | 3889 | 64 | 3953 | 1.6 | |||
*PorB class IA or IB
NG-MAST, Neisseria gonorrhoeae multiantigen sequence typing; NQ, northern Queensland; SEQ, south-east Queensland.
Whole-genome sequencing
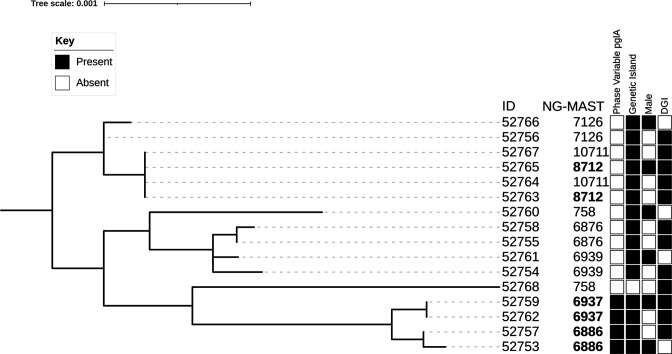
Figure 1 shows a core genome phylogeny of the 16 strains from 8 different NG-MAST, all of which were PIA types, selected for WGS. The four ST6886 and ST6937 strains contained phase variable pglA (NEIS0213) alleles with long homopolymeric tracts of Gs, while the others did not. These four strains grouped together by cgMLST, despite one of them sharing an MLST profile with other strains that did not form part of this group. Only 1 of the 16 strains sequenced did not possess the gonococcal genetic island.
Figure 1.
Core genome maximum likelihood phylogeny of 16 PIA strains of Neisseria gonorrhoeae from Queensland based on cgMLST. The tree is rooted at centre point and annotated with strain ID, NG-MAST associated with DGI, and sequence types derived from MLST and NG-MAST, with presence/absence of phase variable pglA and gonococcal genetic island. The phylogenetic distance is indicated by the length of the horizontal lines. Visualised with iTOL.27 DGI, disseminated gonococcal infection; NG-MAST, Neisseria gonorrhoeae multiantigen sequence typing.
Discussion
This study investigated the burden of gonococcal infections in Queensland and identified those most at risk of developing DGI. The increasing gonorrhoea rates among males could be a result of rapidly increasing rates of gonorrhoea in the MSM population. Another explanation would be that gonorrhoea is more symptomatic in men and as a result they are more likely to seek healthcare. In this study, we applied N. gonorrhoeae NG-MAST genotyping to a large, diverse, consecutively collected N. gonorrhoeae isolate collection in a setting where DGI is not uncommon and used WGS to investigate other strain-related factors. We have used a dataset of strains that encompassed both metropolitan and regional populations and has subsequently highlighted different populations vulnerable to DGI. We observed a higher likelihood of DGI in females, in cases reported in NQ when compared with SEQ and with strains harbouring the PIA gene. This finding agrees with the previous studies suggesting female predominance2 but contradicts a more recent study from the Northern Territory of Australia, which did not show any significant association between DGI and sex,26 but did not examine strain-related factors such as PIA/PIB. Moreover, our data may indicate that DGI is associated with certain PIA gene positive NG-MAST types, suggesting that additional mechanisms possessed by particular PIA bearing genotypes may be at play. PIA has previously been reported to be associated with DGI due to a diminished inflammatory response, which increases the chances that a mucosal infection may go untreated and, therefore, progress to DGI.12
Our data identified pglA phase variation present within certain NG-MAST types that were associated with DGI, however, further studies with larger diversity of strain collections are required, as this finding is inconsistent with the work of Power et al.17 Our sequencing work did not show any evidence that DGI is associated with the gonococcal genetic island as seen by Dillard et al,15 however, with the limited number of strains we sequenced, no statistical significance can be drawn and further studies are required to confirm or refute this. Data on the coexistence of genital infections for DGI cases were not consistently available, however, there were DGI cases that did not have a genital infection recorded in the dataset. Absence of a genital infection suggests cases may have cleared a mucosal infection before progressing to DGI, which would be more likely for asymptomatic infections in females rather than via anorectal injury infection in males.
The majority of gonorrhoea cases yielding an isolate are represented by males who continue to have higher notification rates than females in Queensland. DGI is not a rare occurrence, being noted in 1.6% of culture-positive cases and younger females showing higher rates than males. High DGI rates among younger women are concerning as infertility is one of the potential outcomes of untreated gonorrhoea infection, which has downstream social and economic impacts. Genotyping techniques, such as NG-MAST and WGS, are proving instrumental in providing an insight into the population structure of N. gonorrhoeae, and genetic mechanisms of pathogenesis, such as for DGI.
Supplementary Material
Acknowledgments
This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria) developed by Keith Jolley and sited at the University of Oxford.23 The development of this site has been funded by the Wellcome Trust and European Union. We thank all laboratories for referring isolates included in this study, and Public Health Microbiology Staff for technical work.
Footnotes
Contributors: AJ and DMW conceptualised the study. CJDG conducted the laboratory investigation. CJDG, SS, CLL, CB and ET performed formal data analysis. All authors contributed to the writing and review of the manuscript. AJ accepts full responsibility for the work and conduct of the study, had access to all data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. WGS raw sequence files and associated metadata have been submitted to the European Nucleotide Archive with Project Accession number PRJEB52601.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by Forensic and Scientific Services Human Ethics Committee (FSS-HEC, EC00305). HEC Ref Number HEC18_01.Waiver for consent was delivered by Forensic and Scientific Services Human Ethics Committee (FSS-HEC, EC00305). HEC Ref Number HEC18_01.
References
- 1. Thiéry G, Tankovic J, Brun-Buisson C, et al. Gonococcemia associated with fatal septic shock. Clin Infect Dis 2001;32:e92–3. 10.1086/319204 [DOI] [PubMed] [Google Scholar]
- 2. Tuttle CSL, Van Dantzig T, Brady S, et al. The epidemiology of gonococcal arthritis in an Indigenous Australian population. Sex Transm Infect 2015;91:497–501. 10.1136/sextrans-2014-051893 [DOI] [PubMed] [Google Scholar]
- 3. Suzaki A, Hayashi K, Kosuge K, et al. Disseminated gonococcal infection in Japan: a case report and literature review. Intern Med 2011;50:2039–43. 10.2169/internalmedicine.50.5586 [DOI] [PubMed] [Google Scholar]
- 4. Belkacem A, Caumes E, Ouanich J. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009–2011. Sex Transm Infect 2013;9:613–5. [DOI] [PubMed] [Google Scholar]
- 5. Bleich AT, Sheffield JS, Wendel GD, et al. Disseminated gonococcal infection in women. Obstet Gynecol 2012;119:597–602. 10.1097/AOG.0b013e318244eda9 [DOI] [PubMed] [Google Scholar]
- 6. Phupong V, Sittisomwong T, Wisawasukmongchol W. Disseminated gonococcal infection during pregnancy. Arch Gynecol Obstet 2005;273:185–6. 10.1007/s00404-005-0057-3 [DOI] [PubMed] [Google Scholar]
- 7. Petersen BH, Lee TJ, Snyderman R, et al. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann Intern Med 1979;90:917–20. 10.7326/0003-4819-90-6-917 [DOI] [PubMed] [Google Scholar]
- 8. Banerjee A, Wang R, Supernavage SL, et al. Implications of phase variation of a gene (pgtA) encoding a pilin galactosyl transferase in gonococcal pathogenesis. J Exp Med 2002;196:147–62. 10.1084/jem.20012022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roth A, Mattheis C, Muenzner P, et al. Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections. Infect Immun 2013;81:2358–70. 10.1128/IAI.00128-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knapp JS, Holmes KK. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis 1975;132:204–8. 10.1093/infdis/132.2.204 [DOI] [PubMed] [Google Scholar]
- 11. Brunham RC, Plummer F, Slaney L, et al. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae. J Infect Dis 1985;152:339–43. 10.1093/infdis/152.2.339 [DOI] [PubMed] [Google Scholar]
- 12. van Putten JP, Duensing TD, Carlson J. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J Exp Med 1998;188:941–52. 10.1084/jem.188.5.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plummer FA, Chubb H, Simonsen JN, et al. Antibodies to opacity proteins (opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest 1994;93:1748–55. 10.1172/JCI117159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ram S, Cullinane M, Blom AM, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med 2001;193:281–96. 10.1084/jem.193.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol 2001;41:263–77. 10.1046/j.1365-2958.2001.02520.x [DOI] [PubMed] [Google Scholar]
- 16. Tapsall JW, Phillips EA, Shultz TR, et al. Strain characteristics and antibiotic susceptibility of isolates of Neisseria gonorrhoeae causing disseminated gonococcal infection in Australia. members of the Australian gonococcal surveillance programme. Int J STD AIDS 1992;3:273–7. 10.1177/095646249200300408 [DOI] [PubMed] [Google Scholar]
- 17. Power PM, Ku SC, Rutter K, et al. The phase-variable allele of the pilus glycosylation gene pglA is not strongly associated with strains of Neisseria gonorrhoeae isolated from patients with disseminated gonococcal infection. Infect Immun 2007;75:3202–4. 10.1128/IAI.01501-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Notifiable Diseases Surveillance System . Australian government department of health. Available: http://www9.health.gov.au/cda/source/cda-index.cfm [Accessed 16 July 2019].
- 19. Martin IMC, Ison CA, Aanensen DM, et al. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis 2004;189:1497–505. 10.1086/383047 [DOI] [PubMed] [Google Scholar]
- 20. van der Ley P, Heckels JE, Virji M, et al. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun 1991;59:2963–71. 10.1128/iai.59.9.2963-2971.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30:2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012;19:455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolley KA, Maiden MCJ. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010;11:595. 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison OB, Cehovin A, Skett J, et al. Neisseria gonorrhoeae population genomics: Use of the gonococcal core genome to improve surveillance of antimicrobial resistance. J Infect Dis 2020;222:1816–25. 10.1093/infdis/jiaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maiden MC, Bygraves JA, Feil E, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 1998;95:3140–5. 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birrell JM, Gunathilake M, Singleton S, et al. Characteristics and Impact of Disseminated Gonococcal Infection in the "Top End" of Australia. Am J Trop Med Hyg 2019;101:753–60. 10.4269/ajtmh.19-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letunic I, Bork P. Interactive tree of life (iTOL) V3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 2016;44:W242–5. 10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061040supp001.xls (805KB, xls)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. WGS raw sequence files and associated metadata have been submitted to the European Nucleotide Archive with Project Accession number PRJEB52601.