Abstract
Background:
For relapsing-remitting multiple sclerosis (RRMS), there is a need for biomarker development beyond clinical manifestations and MRI. Soluble neurofilament light chain (sNfL) has emerged as a biomarker for inflammatory activity in RRMS. However, there are limitations to the accuracy of sNfL in identifying relapses. Here, we sought to identify a panel of biomarkers that would increase the precision of distinguishing patients in relapse compared to sNfL alone.
Methods:
We used a multiplex approach to measure levels of 724 blood proteins in two distinct RRMS cohorts. Multiple t-tests with covariate correction determined biomarkers that were differentially regulated in relapse and remission. Logistic regression models determined the accuracy of biomarkers to distinguish relapses from remission.
Results:
The discovery cohort identified 37 proteins differentially abundant in active RRMS relapse compared to remission. The verification cohort confirmed four proteins, including sNfL, were altered in active RRMS relapse compared to remission. Logistic regression showed that the 4-protein panel identified active relapse with higher accuracy (AUC = 0.87) than sNfL alone (AUC = 0.69).
Conclusion:
Our studies confirmed that sNfL is elevated during relapses in RRMS patients. Furthermore, we identified three other blood proteins, uPA, hK8 and DSG3 that were altered during relapse. Together, these four biomarkers could be used to monitor disease activity in RRMS patients.
Keywords: Multiple Sclerosis, Biomarkers, Relapses, Proteomics
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) that afflicts over 2.5 million people around the world. It is thought to be initiated by an autoimmune response to CNS antigens which leads to the destruction of myelin and neurons and renders patients disabled(Hafler, 2004). Approximately 85% of MS patients are diagnosed with a relapsing and remitting disease course. Relapses (MS flare or exacerbations) are defined as new or worsening clinical symptoms which are then followed by periods of remission where patients have partial or full recovery. It is often difficult to determine whether a patient is experiencing a true relapse or a pseudo-relapse confounded by infection and comorbidities(Mills et al., 2017). Gadolinium MRI contrast scans are often used to validate a clinically defined relapse(Avasarala, 2017). However, MRIs are expensive and may miss lesion location. In many instances, MRIs are used when clinical signs are already evident and inflammatory damage has occurred. Therefore, an inexpensive, non-invasive method for detecting relapses is much in need and will benefit both clinical trials and clinical practice.
Neurofilaments are released from damaged axons into the cerebrospinal fluid (CSF) and the blood. Soluble neurofilament light chain (sNfL) can be detected in the blood of MS patients having clinical relapse(Disanto et al., 2017; Kuhle et al., 2016; Thebault et al., 2021; Varhaug et al., 2018). However, elevated sNfL are also associated with other neurodegenerative diseases and increased age in a healthy population(Ashton et al., 2021; Delaby et al., 2020; Khalil et al., 2020). Even though sNfl levels show statistical differences in large clinical and interventional cohorts, there is minimal utility of sNfl in individual patients. These studies raise questions about the relevance of sNfL alone indicating MS disease activity or relapse. Additionally, reliable detectability of sNfL levels in blood is a concern. Only a small fraction of sNfL that leaks from the damaged CNS is detected in the blood. Therefore, minor disease-relevant fluctuations in sNfL levels may not be detected even with the most sensitive single molecular array (SiMoA) technology, questioning the sensitivity of using only sNfL as a blood-based biomarker for MS(Myhr and Torkildsen, 2020).
In this study, we used a large-scale, non-targeted multiplexed assay to discover relapse-associated protein markers in MS patients. Our goal is to identify blood proteins that are associated with relapse in MS patients and to determine if a panel of protein markers would increase accuracy of identifying relapse compared to using sNfL as the sole marker.
Materials and Methods
Patient Cohorts
Cross-sectional cohort:
Serum samples were collected from 62 RRMS patients and 13 healthy controls at the Oklahoma Medical Research Foundation (OMRF) Multiple Sclerosis Center of Excellence between December 2012 to April 2016. Demographic information for OMRF MS patients and healthy controls is shown in Supplemental Table 1. Twenty-three patients were having an active MS relapse, which was defined as new or worsening clinical symptoms lasting more than 48 hours in absence of fever, infection, or other confounding medical conditions. It was further determined using both clinical assessment as well as presence of increased gadolinium-enhanced lesions using magnetic resonance imaging (MRI) (Thompson et al., 2018). Thirty-nine patients were in remission, which was defined as the absence of gadolinium-enhancing lesions and no clinical worsening of neurological signs for at least six weeks. All serum samples were obtained before the initiation of disease-modifying therapies (DMTs). Healthy controls were defined as not being diagnosed with inflammatory neurological or autoimmune diseases (including meningitis, multiple sclerosis, neuromyelitis optica spectrum disorder, rheumatoid arthritis, systemic lupus erythematosus (SLE), and myasthenia gravis). Blood samples were collected in BD Vacutainer Serum tubes, allowed to clot for 60 minutes, centrifuged at 1300g for 10 minutes then frozen at −80°C.
Longitudinal cohort:
Paired plasma samples were collected from 12 RRMS patients from the Stanford MS Clinic between March 2018 to September 2019. Blood samples were collected in BD Vacutainer Tubes containing heparin and then centrifuged at 1300g for 10 minutes and plasma aliquots were frozen at −80°C. Demographics and information on disease-modifying treatments (DMTs) given to patients is shown in Supplemental Table 2. Longitudinally-paired samples were collected from individual patients during active relapse and during remission (between 0 to 19 months) from these patients.
Prior to study entry, all subjects were evaluated and screened for study eligibility at Stanford and OMRF MS clinics respectively. For this retrospective biomarker study, both cohorts of patients were diagnosed with RRMS using the McDonald criteria(Thompson et al., 2018) by a board-certified neurologist. Written informed consent was obtained from individuals prior to participation in the study, which was approved by the Oklahoma Medical Research Foundation’s and Stanford University’s Institutional Review Board. This was a convenience series of subjects who were available and willing to donate blood for these studies. Assays on the samples were performed blinded from the clinical data.
Protein Quantification
Proteins were measured with the Olink Cardiometabolic, Cardiovascular III, Development, Inflammation, Neuro-exploratory, Neurology, Oncology II and Oncology III panels using proximity extension assay (PEA) technology. The PEA assay has similar or better sensitivity to ELISA (Assarsson et al., 2014). In total, we measured expression levels of 724 proteins. Data are presented as log base-2 normalized protein expression (NPX) values.
Statistical Analysis
Pearson’s correlation coefficient (r) analysis was performed using cor function from stats v4.0.5 in R(Team, 2021) to test the correlation between NPX values for 724 proteins across two independent runs. Significantly different proteins were determined using multiple t-tests corrected by the Bonferroni and Hochberg method with a False Discovery Rate (FDR) of 5% using limma v3.46(Ritchie et al., 2015) in the Bioconductor suite in R. Principal Components Analysis (PCA) was performed using prcomp function in R(Team, 2021) and principal components with the highest variation – PC1 and PC2 – were plotted against each other. Gene Ontology analysis was performed using the STRING database(Szklarczyk et al., 2021) for functional annotation of the significantly different proteins.
Logistic regression analysis was performed using caret v6.0–88 in R(Kuhn, 2008) to generate univariate or multivariate predictive models that estimate the probability of identifying active relapse in RRMS patients. Repeated 10-fold cross-validation(Kuhn, 2008) was used to correct for the bias arising from validating models on the same dataset used to generate the models. Receiver Operating Characteristics (ROC) curves were generated using the probabilities from each model and the efficacy of each model for classifying active relapse in MS patients was evaluated using MLeval v0.3 in R(John, 2020). Area under the curve for the ROC Curve (AUC) was used to determine the classification accuracy for each model. All these statistical analyses were performed in R (3.6.2, 4.0.5)(Team, 2021). We compared AUCs with a panel of serum markers to a sNfl only as reference.
Individual serum protein levels were compared using unpaired t-tests with a significance of p < 0.05. Serum proteins that were significantly different between paired relapse and remission in the longitudinal cohort were determined using multiple t-tests with a significance of p < 0.05. sNfL and FKBP5 NPX values were correlated to weeks after cessation of steroid treatment using linear regression.
Results
Quality Control of Proximity Extension Assay (PEA), a multiplexed protein biomarker assay
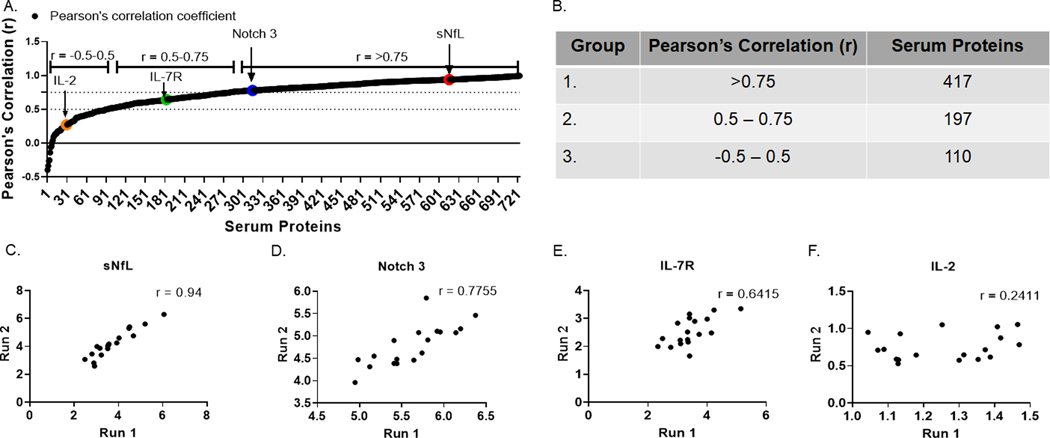
Measuring serum proteins using a multiplex approach has been widely used in many diseases including MS (Hartung et al., 2013; Hegen et al., 2016). Accuracy and reproducibility of the serum proteins measurement is paramount for the discovery of robust biomarkers. In this study, we used proximity extension assay (PEA) to measure the levels of 724 serum proteins in RRMS patients and healthy controls, with the aim to identify potential biomarkers associated with disease activity in patients. PEA is a relatively new antibody-based analyte detection technology. For the quality-control of PEA (Assarsson et al., 2014), expression levels of all 724 serum proteins were measured from two independent runs of 18 individuals from the OMRF cohort and were further correlated across the two runs (Fig 1A). Based on correlation values, we found that 417 proteins were highly reproducible with r > 0.75 (Group 1), 197 proteins were moderately reproducible with r values between 0.5 – 0.75 (Group 2) and 110 proteins were poorly reproducible with r values between −0.5 – 0.5 (group 3) (Fig 1B). Examples of highly reproducible proteins were sNfL (Fig 1C) and Notch 3 (Fig 1D). An example of a moderately reproducible protein was interleukin-7 receptor (IL-7R) (Fig 1E). Interleukin-2 (IL-2) was an example of a poorly reproducible protein (Fig 1F). For this study, we considered the 417 proteins with r > 0.75 to have passed quality control (QC) and these were used for the subsequent analysis of both cohorts.
Figure 1.
Quality Control (QC) of Proximity Extension Assay (PEA). Expression levels of 724 serum proteins were assessed across two independent runs (Run 1 and Run 2). (A) Pearson’s correlation coefficient (r) was determined for all 724 proteins. (B) Table showing serum proteins belonging to each group defined by the range of r. Correlation plots for Group 1 serum proteins (C) sNfL, (D) Notch 3. Group 2 serum protein (E) IL-7R. Group 3 serum protein (F) IL-2 are shown.
Serum protein signatures distinguish MS patients from healthy controls
We first compared levels of serum proteins in 62 RRMS patients and 13 healthy controls. We adjusted for age and sex covariates and performed multiple t-tests with two thresholds of significance, a stringent threshold with FDR q-values of less than 0.05 and a less stringent threshold with p-values less than 0.05. We identified 38 proteins (q-value < 0.05) and 69 proteins (p-value < 0.05) were elevated in RRMS patients compared to controls. We also identified 21 proteins (q-value < 0.05) and 35 proteins (p-value < 0.05) were reduced in RRMS patients compared to controls (Fig 2A,Supplemental Data 2).
Figure 2.
Serum protein profiles distinguish RRMS patients from healthy controls. (A) Serum protein profiles of RRMS (n = 62) and healthy controls (n = 13) were compared. Significant proteins were determined using unpaired t-tests with multiple comparisons corrected by Bonferroni and Hochberg method setting a False Discovery Rate (FDR) using q-value < 0.05 and p-value of 0.05. (B) PCA analysis of significantly different proteins between RRMS and controls was performed. Gene Ontology (GO) analysis of serum proteins (C) elevated in RRMS and (D) controls was also performed.
To determine if RRMS patients and controls can be distinguished based on their serum protein signatures, we performed principal components (PC) analysis using expression levels of the proteins that had q-values less than 0.05. Based on PC1 and PC2, which explained 25.5% and 16.4% of the variance respectively, we could cluster RRMS patients and healthy controls into two distinct groups (Fig 2B).
To identify the biological pathways to which these significantly different proteins belong to, we performed a Gene Ontology (GO) database analysis using the serum proteins with p-values less than 0.05. Of the 104 proteins, 102 mapped to five GO pathways which belonged to immune (GO: 0002376, GO:0002682, GO:0006952), nervous (GO:0007399) and other (GO:0009987) systems. Of the serum proteins elevated in RRMS patients, 26.47% mapped to immune system pathways, 32.35% mapped to nervous system pathways and 41.18% mapped to pathways belonging to other systems (Fig 2C). However, for the proteins elevated in healthy controls, 70.58% mapped to immune system pathways and 29.42% mapped to pathways belonging to other systems. None of the proteins elevated in controls mapped to nervous system pathways (Fig 2D). These data highlight the critical involvement of the nervous system in MS pathogenesis and provide evidence of neurological damage in MS.
Serum protein signatures can differentiate relapse from remission in MS patients
In our cross-sectional cohort, 23 patients were having inflammatory disease activity (relapse) and 39 patients were not experiencing inflammatory disease activity (remission) at the time of blood draw. We determined if serum protein levels could distinguish relapsing patients from patients in remission. We compared levels of serum proteins in relapse patients to remission patients using multiple unpaired t-tests adjusted for age and sex as co-variates and used two thresholds of significance, a more stringent threshold with FDR q-values of less than 0.2 and a less stringent threshold with p-values less than 0.05. We identified sNfL (q-value < 0.2) and 5 proteins (p-value < 0.05) were elevated in relapsing patients compared to remission patients. We also identified 6 proteins (q-value < 0.2) and 25 proteins (p-value < 0.05) were reduced in relapsing patients compared to remission patients (Fig 3A, Supplemental Data 3). Unsupervised hierarchical cluster analysis using serum proteins with p-values less than 0.01 divided patients into two groups – Group 1 which was largely composed of relapsing MS patients and Group 2 was remission patients (Fig 3B). Therefore, these data show that MS patients experiencing clinical relapse can be distinguished based on their serum protein signatures.
Figure 3:
Serum protein profiles distinguish relapse and remission in MS patients. (A) Serum protein profiles of Relapse (n = 23) and Remission (n = 39) MS patients were compared. Significant proteins were determined using unpaired t-tests with multiple comparisons corrected by Bonferroni and Hochberg method setting a False Discovery Rate (FDR) using q-value < 0.2 and p-value of 0.05 (B) Unsupervised hierarchical cluster analysis of significantly different proteins (p-value < 0.01) between patients in relapse and remission classified patients into two groups, Group 1 and Group 2.
Effects of steroid treatment on serum protein profiles of relapsing MS patients
High dose corticosteroids are short-term medications used to treat clinical relapses in MS patients(Beck et al., 1992; Morrow et al., 2004). In our cross-sectional cohort, 13 out of 23 relapse patients received IV and/or oral methylprednisolone during or before blood draw while the remaining ten did not receive steroids (Fig 4A). No patients in remission were given steroids. To determine whether steroid treatment influenced the serum protein profiles of relapsing patients, we performed three comparative analyses: (1) Relapsing patients on steroids vs remission patients, (2) relapsing MS patients without steroids vs remission patients, and (3) relapsing patients on steroids vs relapsing patients without steroids. We used multiple unpaired t-tests with a p-value threshold of < 0.05 and adjusted for age and sex as co-variates for these analyses. We found that 15 proteins were elevated, and 23 proteins were reduced in relapsing patients on steroids compared to remission patients (Fig 4B, Supplemental Data 4). We also identified that three proteins were elevated, and 32 proteins were reduced in relapsing patients without steroids compared to remission patients (Fig 4C, Supplemental Data 5). Additionally, we determined that 19 proteins were elevated, and three proteins were reduced in relapsing patients on steroids compared to relapsing patients without steroids (Fig 4D, Supplemental Data 6).
Figure 4:
Steroids alter serum protein profiles of MS Relapse patients. (A) Classification of RRMS patients based on disease status and steroids. Serum protein profiles of (B) MS Relapse patients on steroid treatment and MS Remission patients, (C) MS Relapse patients without steroid treatment and MS Remission patients and (D) MS Relapse patients on steroid treatment and MS Relapse patients without steroid treatment were compared. Significant proteins were determined using unpaired t-tests with multiple comparisons setting a p-value threshold of ≤ 0.05. (E) Venn diagram of differentially-abundant serum proteins of relapse steroids vs remission and relapse steroids vs relapse no steroids comparisons. (F) Serum FKBP5 levels from MS relapse patients on steroids, MS relapse patients without steroids, MS remission patients and healthy controls were compared by one-way ANOVA. (G) Serum FKBP5 levels in MS relapse patients were correlated with weeks after steroids cessation by Pearson’s correlation. (H) Serum sNfl levels from MS relapse patients on steroids, MS relapse patients without steroids, MS remission patients and healthy controls were compared by one-way ANOVA. (I) Serum sNfl levels in MS relapse patients were correlated with weeks after steroids cessation by Pearson’s correlation. p-values of <0.05 were considered significant.
To identify proteins with a strong association with steroid treatment, we examined differentially abundant proteins that were common between the following comparisons: (1) relapsing patients on steroids vs remission patients and (2) relapsing patients on steroids vs relapsing patients without steroids. We observed six common proteins in this analysis (Fig 4E). Of the serum proteins that were significantly altered in steroid patients, FK506-binding protein 5 (FKBP5) and sNfL were of interest. Expression of FKBP5 is directly increased in response to steroids engaging glucocorticoid receptor on cells(Binder, 2009; Vermeer et al., 2003). In this cohort of patients, levels of FKBP5 were significantly increased in relapsing MS patients on steroids compared to relapsing MS patients without steroids, remission MS patients and healthy controls (Fig 4F). Interestingly, we also found that FKBP5 levels were elevated in both relapsing MS patients without steroids and remission MS patients compared to healthy controls, suggesting that endogenous glucocorticoid levels are higher in MS compared to healthy controls. We also found that levels of FKBP5 significantly diminished with time after the cessation of steroid treatment in the relapsing MS patients (Fig 4G). These data show that the biological effects of steroid therapy can be detected with serum levels of FKBP5. It was also intriguing that sNfL levels were significantly increased in relapsing MS patients on steroids compared to relapsing MS patients without steroids, remission MS patients and healthy controls (Fig 4H). However, sNfL serum levels did not significantly decrease with time after stoppage of steroid treatment (Fig 4I). This suggests that the elevated levels of sNfL were not a direct effect of steroid treatment in these patients. These data demonstrate the need to consider steroid treatment for biomarker discovery.
Longitudinal changes in blood proteins from relapse to remission
We performed proteomic analysis of a second longitudinal blood sample set (Supplemental Table 2), where 12 patients had plasma collected during a relapse and during remission. We compared levels of proteins in relapse and remission using multiple paired t-tests with a p-value threshold of < 0.05. In this cohort, we identified that ten proteins were elevated, and 26 proteins were reduced in relapsing MS patients compared to remission MS patients (Fig 5A, Supplemental Data 7). Notably, sNfL was the most elevated blood protein in relapse and levels of sNfL were negatively correlated with the length of time taken from relapse to blood draw (Fig 5B). In this cohort, only two patients in relapse were on acute steroid therapy at blood draw. sNfL levels were not highly abundant in the blood of these relapsing patients on steroids (Fig 5C). This further demonstrates that steroid treatments do not directly affect blood sNfL levels during an active relapse.
Figure 5:
Monitoring longitudinal changes in plasma proteins from relapse to remission. (A) Fold Change of plasma proteins that were significantly different between MS relapse and remission. Significant proteins were determined using paired t-tests with multiple comparisons setting a p-value threshold of ≤ 0.05. (B) Protein levels of sNfL were compared between MS relapse patients with steroid treatment, MS relapse patients with no steroid treatment and MS remission patients using ordinary one-way ANOVA. P-values < 0.05 were considered significant. (C) Regression of sNfL levels with weeks after relapse in MS Relapse. P-values < 0.05 were considered significant.
A panel of blood proteins identifies MS relapse with higher accuracy than sNfL alone
We next identified the common proteins that were significantly different in relapse and remission in the cross-sectional and the longitudinal cohorts (Fig 6A). We found five proteins that were common between the two cohorts. sNfL was the only protein that was elevated in relapse in both studies. However, urokinase plasminogen activator (uPA), kallikrein-8 (hK8) and desmoglein-3 (DSG3) were all reduced in relapse in both cohorts. Although, mannose binding lectin 2 (MBL2) was significantly different in both cohorts, we found that it was reduced in relapse in the cross-sectional cohort but increased in relapse in the longitudinal cohort (Fig 6B).
Figure 6:
A panel of serum proteins classifies MS relapse with higher accuracy than sNfL alone. (A) Venn diagram comparing significantly-abundant proteins between MS Relapse and MS Remission from OMRF and Stanford cohort. (B) Table shows the five serum analytes that are significantly different between Relapse and Remission in OMRF and Stanford cohorts. (C) Receiver Operating Characteristics (ROC) curves examining the predictive performance of serum protein biomarkers for identifying MS relapse in the longitudinal cohort. (D) Table showing AUC scores, 95% CI, specificity, and sensitivity for all the regression models.
Even though blood sNfL is considered a marker of relapse in MS patients, there are questions about its accuracy to determine relapses. Therefore, we compared the effectiveness of sNfL alone with that of the panel of four serum proteins, verified in both cohorts, to distinguish patients in relapse or remission. Due to sample size restrictions, we performed this analysis on the cross-sectional cohort. We generated logistic regression models using one or more serum proteins as predictor variables using the cross-sectional cohort. We tested the logistic regression models using repeated 10-fold cross-validation on the cross-sectional cohort and plotted receiver operating characteristic (ROC) curves for each model (Novakova et al., 2017). We found that a regression model with a combination of sNfL, uPA, hK8 and DSG3 was able to classify relapse with an area under the curve (AUC) of 0.87 (95% CI 0.77 – 0.97) compared to an AUC of 0.69 (95% CI 0.55 – 0.83) using sNfL alone (Fig 6C). Models using individual proteins other than sNfL were also tested to classify relapse (Fig 6D). The models were generated and tested on the same dataset; therefore, they are inherently optimistic. However, our goal was to display that a combination of protein markers outperforms sNfL alone in classifying MS relapse. Our data suggest that a regression model using a combination of sNfL, uPA, hK8 and DSG3 can classify relapse in MS patients with higher AUC than sNfL alone.
Discussion
There is emerging interest in measuring sNfL levels in the blood to monitor neurological damage and identify active relapses in MS patients. However, using only sNfL to monitor disease activity in MS has limitations. First, confounders like age and other neurological comorbidities could affect the utility of sNfL alone in identifying MS relapses. Second, fluctuations in sNfL levels may not be sensitive when detecting relapses in all patients. Our study, using high throughput proteomics of two distinct MS cohorts, identified that a panel of serum markers has higher accuracy in identifying relapses compared to using sNfL only.
For the discovery of relapse-associated blood biomarkers, we used a cross-sectional cohort of MS patients who were either in relapse or remission. We minimized confounding factors by recruiting RRMS patients early after their diagnosis and before they were put on disease modifying therapies (DMTs) and also by correcting for age and sex during our analysis. In this cohort, we also considered the effects that glucocorticoid treatment had on blood proteins during relapse. Here, we identified that levels of FKBP5 are sensitive to glucocorticoids and may be used to monitor the short-lived biological activity of this therapy.
To verify the relapse-associated blood biomarkers, we used a longitudinal cohort of 12 RRMS patients where blood specimens were first taken at the time of an acute relapse and a second specimen was taken during remission and within 12 months of the initial relapse. This confirmatory cohort differed from the discovery cohort in two ways. First, the discovery cohort was serum, and the confirmatory cohort was plasma. Although there are differences in protein concentrations between serum and plasma, previous literature has shown that relative amounts of most proteins correlated across the two body fluids in matched specimens (Dossus et al., 2009; Huebschmann et al., 2020; O’Neal et al., 2014; Sejbaek et al., 2019). Second, the discovery cohort patients were treatment naïve, and patients from the second cohort were on various treatments. The rationale to use these cohorts for discovery and confirmation was to determine if there are robust blood markers that could distinguish relapse and remission in such disparate patient samples. Despite the differences in the two cohorts, we identified four common proteins, sNfL, uPA, hK8 and DSG3, that were significantly different in relapse and remission in RRMS patients(Adamczyk-Sowa et al., 2017).
A goal of this study was to evaluate biomarkers for their accuracy in classifying relapse from remission. We measured the performance of classification models using area under the ROC curve (AUC)(Lasko et al., 2005). We found that a ROC curve using sNfL alone had an AUC of 0.69 (95% CI 0.55 – 0.83) to classify relapse, which is similar to an AUC of 0.663 (95% CI 0.591–0.735) that was reported previously for sNfL(Novakova et al., 2017). When we used the other three proteins together with sNfL, the regression model was more accurate at classifying a relapse (with an AUC of 0.87) than using sNfL alone. A limitation of our study is that the classification models were generated and tested on the same dataset due to the lack of a powered validation cohort. Therefore, future studies validating the utility of these specific biomarkers are needed. Nevertheless, our data provides evidence that a panel of biomarkers improves the accuracy of identifying relapses than sNfl alone.
The biological significance of the proteins altered during relapse and remission in RRMS patients has not been fully elucidated. However, the established function of these molecules may provide clues to their effects in MS. sNfL has been shown to be elevated in the blood of relapsing MS patients and is considered a marker of neuronal damage in MS. Plasminogen activators, such as uPA, play an important role in the clearance of fibrin/fibrinogen deposits from sites of inflammation, thus preventing further inflammatory activity(Zhukovsky et al., 2021). Mice lacking uPA and its receptor uPAR have more severe experimental autoimmune encephalomyelitis (EAE) compared to wild-type mice(Gur-Wahnon et al., 2013). Here, we found lower levels of uPA during a relapse compared to remission highlighting a protective role of uPA in RRMS. We also found lower levels of hK8, a member of the kallikrein family of proteases, during relapse in patients. Kallikreins, specifically KLK1 and KLK6, are associated with secondary progressive MS(Scarisbrick et al., 2008). However, the role of kallikreins like hK8 during relapses in MS is unknown. Finally, we found lower levels of desmoglein-3 (DSG3) during relapse. DSG3, which is a component of intercellular desmosome junctions, is important for maintaining tight junctions in mucosal epithelial barriers in the intestines(Ungewiss et al., 2017). Therefore, dysregulation of DSG3 could be an indicator of intestinal permeability, which is a biological process that has been linked to MS pathology(Buscarinu et al., 2017; Buscarinu et al., 2018; Nouri et al., 2014).
Our current study shows that a panel of biomarkers can distinguish clinically confirmed relapses to remission. There is emerging interest in identifying pseudo-relapses in RRMS, which are identified as worsening of clinical signs and symptoms without the presence of gadolinium enhanced lesions. Future biomarkers studies comparing pseudo-relapses and true relapses may provide biological distinction between these clinically defined episodes in RRMS.
Our study provides important information on biomarker discovery for MS relapse. We confirm that sNfL is statistically elevated in relapse compared to remission. We present new data on the discovery of blood-based biomarkers for identifying and monitoring relapses in MS. Our data highlight the importance in identifying confounding factors that may influence biomarker discovery, including age, sex, treatment, and blood sample type. Finally, we have identified a panel of biomarkers that increases the accuracy of identifying relapse compared to using sNfL alone.
Data Availability
Anonymized data will be made available by request from any qualified investigator.
Supplementary Material
Highlights:
Large-scale proteomics reveal serum markers associated with ongoing relapse in MS.
Steroids alter serum proteins and must be considered for biomarker discovery.
A 4-biomarker panel is more accurate at identifying relapse compared to sNfL only.
Study Funding
This study was supported by grants from the NIH (R01AI137047 and R01EY027346) awarded to RCA and by grants from NIH NIAID Autoimmune Center of Excellence (UM1-AI110557-05, UM1 AI144298-01) awarded to YM-D.
Footnotes
Disclosure
RCA has consulted for Roche, EMD Serono, and Biogen Idec and is on the advisory board for Progentec Diagnostics Inc. GP has served on advisory boards and/or speakers’ bureau for Biogen Idec, Celgene/Bristol Myers Squibb, EMD Serono, Greenwich Biosciences, Janssen Pharmaceuticals, Novartis Pharmaceuticals, Roche/Genentech, Sanofi-Genzyme, TG Therapeutics, VielaBio/Horizon Therapeutics, and is a member of the Scientific Advisory Board of Progentec Diagnostics Inc. YM-D has served as a consultant and/or received grant support from: Acorda, Bayer Pharmaceutical, Biogen Idec, Celgene/Bristol Myers Squibb, EMD Serono, Sanofi-Genzyme, Genentech-Roche, Novartis, Questor, Horizon, Janssen, and Teva Neuroscience.
References
- Adamczyk-Sowa M, Galiniak S, Zyracka E, Grzesik M, Naparlo K, Sowa P, Bartosz G, Sadowska-Bartosz I, 2017. Oxidative Modification of Blood Serum Proteins in Multiple Sclerosis after Interferon Beta and Melatonin Treatment. Oxid Med Cell Longev 2017, 7905148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NJ, Janelidze S, Al Khleifat A, Leuzy A, van der Ende EL, Karikari TK, Benedet AL, Pascoal TA, Lleo A, Parnetti L, Galimberti D, Bonanni L, Pilotto A, Padovani A, Lycke J, Novakova L, Axelsson M, Velayudhan L, Rabinovici GD, Miller B, Pariante C, Nikkheslat N, Resnick SM, Thambisetty M, Scholl M, Fernandez-Eulate G, Gil-Bea FJ, Lopez de Munain A, Al-Chalabi A, Rosa-Neto P, Strydom A, Svenningsson P, Stomrud E, Santillo A, Aarsland D, van Swieten JC, Palmqvist S, Zetterberg H, Blennow K, Hye A, Hansson O, 2021. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun 12(1), 3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S, 2014. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 9(4), e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasarala J, 2017. Redefining Acute Relapses in Multiple Sclerosis: Implications for Phase 3 Clinical Trials and Treatment Algorithms. Innovations in clinical neuroscience 14(3–4), 38–40. [PMC free article] [PubMed] [Google Scholar]
- Beck RW, Cleary PA, Anderson MM Jr., Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, et al. , 1992. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 326(9), 581–588. [DOI] [PubMed] [Google Scholar]
- Binder EB, 2009. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34 Suppl 1, S186–195. [DOI] [PubMed] [Google Scholar]
- Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, Mechelli R, Romano S, Fornasiero A, Mattei G, Piras E, Angelini DF, Battistini L, Simmaco M, Umeton R, Salvetti M, Ristori G, 2017. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler 23(3), 442–446. [DOI] [PubMed] [Google Scholar]
- Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, Renie R, Cerasoli B, Morena E, Romano C, Loizzo ND, Umeton R, Salvetti M, Ristori G, 2018. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics 15(1), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaby C, Alcolea D, Carmona-Iragui M, Illan-Gala I, Morenas-Rodriguez E, Barroeta I, Altuna M, Estelles T, Santos-Santos M, Turon-Sans J, Munoz L, Ribosa-Nogue R, Sala-Matavera I, Sanchez-Saudinos B, Subirana A, Videla L, Benejam B, Sirisi S, Lehmann S, Belbin O, Clarimon J, Blesa R, Pagonabarraga J, Rojas-Garcia R, Fortea J, Lleo A, 2020. Differential levels of Neurofilament Light protein in cerebrospinal fluid in patients with a wide range of neurodegenerative disorders. Sci Rep 10(1), 9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J, Swiss Multiple Sclerosis Cohort Study, G., 2017. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81(6), 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S, 2009. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods 350(1–2), 125–132. [DOI] [PubMed] [Google Scholar]
- Gur-Wahnon D, Mizrachi T, Maaravi-Pinto FY, Lourbopoulos A, Grigoriadis N, Higazi AA, Brenner T, 2013. The plasminogen activator system: involvement in central nervous system inflammation and a potential site for therapeutic intervention. J Neuroinflammation 10, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA, 2004. Multiple sclerosis. J Clin Invest 113(6), 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Steinman L, Goodin DS, Comi G, Cook S, Filippi M, O’Connor P, Jeffery DR, Kappos L, Axtell R, Knappertz V, Bogumil T, Schwenke S, Croze E, Sandbrink R, Pohl C, 2013. Interleukin 17F level and interferon beta response in patients with multiple sclerosis. JAMA Neurol 70(8), 1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegen H, Adrianto I, Lessard CJ, Millonig A, Bertolotto A, Comabella M, Giovannoni G, Guger M, Hoelzl M, Khalil M, Fazekas F, Killestein J, Lindberg RL, Malucchi S, Mehling M, Montalban X, Rudzki D, Schautzer F, Sellebjerg F, Sorensen PS, Deisenhammer F, Steinman L, Axtell RC, 2016. Cytokine profiles show heterogeneity of interferon-beta response in multiple sclerosis patients. Neurol Neuroimmunol Neuroinflamm 3(2), e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebschmann NA, Luoto TM, Karr JE, Berghem K, Blennow K, Zetterberg H, Ashton NJ, Simren J, Posti JP, Gill JM, Iverson GL, 2020. Comparing Glial Fibrillary Acidic Protein (GFAP) in Serum and Plasma Following Mild Traumatic Brain Injury in Older Adults. Front Neurol 11, 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CR, 2020. MLeval: Machine Learning Model Evaluation. R package version 0.3. [Google Scholar]
- Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, Benkert P, Ropele S, Enzinger C, Fazekas F, Schmidt R, Kuhle J, 2020. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nature Communications 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, Yaldizli O, Regeniter A, Derfuss T, Canales M, Schluep M, Du Pasquier R, Krueger G, Granziera C, 2016. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler J 22(12), 1550–1559. [DOI] [PubMed] [Google Scholar]
- Kuhn M, 2008. Building Predictive Models in R Using the caret Package. Journal of Statistical Software 28(5), 1–26.27774042 [Google Scholar]
- Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L, 2005. The use of receiver operating characteristic curves in biomedical informatics. J Biomed Inform 38(5), 404–415. [DOI] [PubMed] [Google Scholar]
- Mills EA, Mirza A, Mao-Draayer Y, 2017. Emerging Approaches for Validating and Managing Multiple Sclerosis Relapse. Front Neurol 8, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM, 2004. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology 63(6), 1079–1080. [DOI] [PubMed] [Google Scholar]
- Myhr KM, Torkildsen O, 2020. Serum NFL levels should be used to monitor multiple sclerosis evolution - No. Mult Scler J 26(1), 19–21. [DOI] [PubMed] [Google Scholar]
- Nouri M, Bredberg A, Westrom B, Lavasani S, 2014. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One 9(9), e106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova L, Zetterberg H, Sundstrom P, Axelsson M, Khademi M, Gunnarsson M, Malmestrom C, Svenningsson A, Olsson T, Piehl F, Blennow K, Lycke J, 2017. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89(22), 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal WK, Anderson W, Basta PV, Carretta EE, Doerschuk CM, Barr RG, Bleecker ER, Christenson SA, Curtis JL, Han MK, Hansel NN, Kanner RE, Kleerup EC, Martinez FJ, Miller BE, Peters SP, Rennard SI, Scholand MB, Tal-Singer R, Woodruff PG, Couper DJ, Davis SM, Investigators S, 2014. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med 12, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK, 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7), e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Linbo R, Vandell AG, Keegan M, Blaber SI, Blaber M, Sneve D, Lucchinetti CF, Rodriguez M, Diamandis EP, 2008. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol Chem 389(6), 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejbaek T, Nielsen HH, Penner N, Plavina T, Mendoza JP, Martin NA, Elkjaer ML, Ravnborg MH, Illes Z, 2019. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J Neurol Neurosurg Psychiatry 90(12), 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, Jensen LJ, von Mering C, 2021. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49(D1), D605–D612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Thebault S, Bose G, Booth R, Freedman MS, 2021. Serum neurofilament light in MS: The first true blood-based biomarker? Mult Scler, 1352458521993066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA, 2018. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2), 162–173. [DOI] [PubMed] [Google Scholar]
- Ungewiss H, Vielmuth F, Suzuki ST, Maiser A, Harz H, Leonhardt H, Kugelmann D, Schlegel N, Waschke J, 2017. Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci Rep 7(1), 6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varhaug KN, Barro C, Bjornevik K, Myhr KM, Torkildsen O, Wergeland S, Bindoff LA, Kuhle J, Vedeler C, 2018. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 5(1), e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M, 2003. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab 88(1), 277–284. [DOI] [PubMed] [Google Scholar]
- Zhukovsky C, Herman S, Wiberg A, Cunningham JL, Kultima K, Burman J, 2021. Urokinase, CX3CL1, CCL2, TRAIL and IL-18 induced by interferon-beta treatment. Acta Neurol Scand 143(6), 602–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be made available by request from any qualified investigator.