Significance
Nematode worms rely primarily on olfaction for information about the external world and encode over 1,000 olfactory receptors in their genomes. All olfactory receptors in the worm appear to signal through the same few downstream molecules, and many receptors are expressed in each sensory neuron, raising the question of how worms can distinguish one smell from another. We show that olfactory discrimination occurs through desensitization of stimulated olfactory receptors by the protein arrestin, that the specific receptors to be desensitized are tagged by the protein GRK-1, and that these processes are not required for discrimination between smells sensed by different neurons. This resolves a long-standing question in nematode olfactory biology and reveals a fundamental mechanism of intraneuronal olfactory discrimination.
Keywords: olfaction, signaling, discrimination, arrestin, GRK-1
Abstract
In the mammalian olfactory system, cross-talk between olfactory signals is minimized through physical isolation: individual neurons express one or few olfactory receptors among those encoded in the genome. Physical isolation allows for segregation of stimuli during signal transduction; however, in the nematode worm Caenorhabditis elegans, ∼1,300 olfactory receptors are primarily expressed in only 32 neurons, precluding this strategy. Here, we report genetic and behavioral evidence that β-arrestin–mediated desensitization of olfactory receptors, working downstream of the kinase GRK-1, enables discrimination between intraneuronal olfactory stimuli. Our findings suggest that C. elegans exploits β-arrestin desensitization to maximize responsiveness to novel odors, allowing for behaviorally appropriate responses to olfactory stimuli despite the large number of olfactory receptors signaling in single cells. This represents a fundamentally different solution to the problem of olfactory discrimination than that which evolved in mammals, allowing for economical use of a limited number of sensory neurons.
Olfaction is an enormously multidimensional sense (1) through which signals generated by many odors are transduced through the nervous system to provide information about the external world. In mammals, cross-talk among these signals is minimized by physically isolating the neurons transducing them. Individual olfactory sensory neurons (OSNs) lining the olfactory mucosa stochastically express one or few olfactory receptors from the diverse repertoire encoded in the genome. Each OSN projects a single, unbranched axon, which passes through the cribriform plate to converge with the axons of other OSNs expressing the same receptor, forming one or few glomeruli specific to the receptor (2). Within these glomeruli, OSN axons synapse onto the dendrites of mitral and tufted cells, which in turn project to the primary olfactory cortex. Thus, by ensuring physical isolation of nerves transducing signals from individual receptors, mammalian olfaction allows for clear separation of olfactory stimuli during early signal transduction.
The nematode worm Caenorhabditis elegans has a highly developed olfactory sense, with ∼1,300 putative G protein-coupled receptor (GPCR) olfactory receptors (3) encoded in its genome, granting the ability to sense diverse chemical ligands (4). Primary sensation of olfactory stimuli appears to be localized to ∼32 sensory neurons (5), with individual putative chemoreceptors usually being strongly expressed only in one bilateral pair of neurons (6, 7). Sensation of many odorants seems to depend primarily on the G protein α-subunit ODR-3 (4, 8), with the other G protein α-subunits present in chemosensory neurons, GPA-2, GPA-3, GPA-5, GPA-6, and GPA-13, being able to partially compensate for its loss in experiments testing chemotaxis to some odorants (9). The rich resource of olfactory receptors encoded in the worm’s genome presents a signaling conundrum: How can 1,300 receptors, all working through a limited set of partially redundant G proteins, maintain signaling identity (avoid cross-talk) when expressed in comparatively few chemosensory neurons (7, 10)?
Evidence that cross-talk is avoided comes primarily from experiments in which an attractive odorant sensed exclusively by one neuron pair is placed on an agar plate containing a uniform concentration of a second attractive odorant, sensed exclusively by the same neuron pair. C. elegans is generally able to find the point source of the first odorant, despite the presence of the second, suggesting that olfactory discrimination between the two odorants is occurring. Importantly, when the uniform odor and the point odor are the same, animals are unable to locate the point, suggesting that attraction to the point does not result merely from a greater intensity of stimulus; instead, the response to the uniform odor has been “saturated” (4).
We wondered if the apparent intraneuronal olfactory discrimination exhibited by C. elegans in this paradigm might result from desensitization of the receptor or receptors required to sense the saturating odorant. Since the saturating odor is in the agar, and is in direct contact with the animal while the point odor can only reach it by diffusion, we reasoned that an activity-dependent process might affect receptors for the saturating odorant to a far greater extent than those for the point odorant, resulting in enhanced signaling through the latter relative to the former. This process would allow for chemotaxis toward the point by desensitizing the receptor or receptors responding to the saturating odorant, resulting in the receptors responsive to the point odorant being responsible for the majority of G protein signaling occurring in the neuron.
Results
Arrestin Is Required in Sensory Neurons for Intraneuronal Olfactory Discrimination.
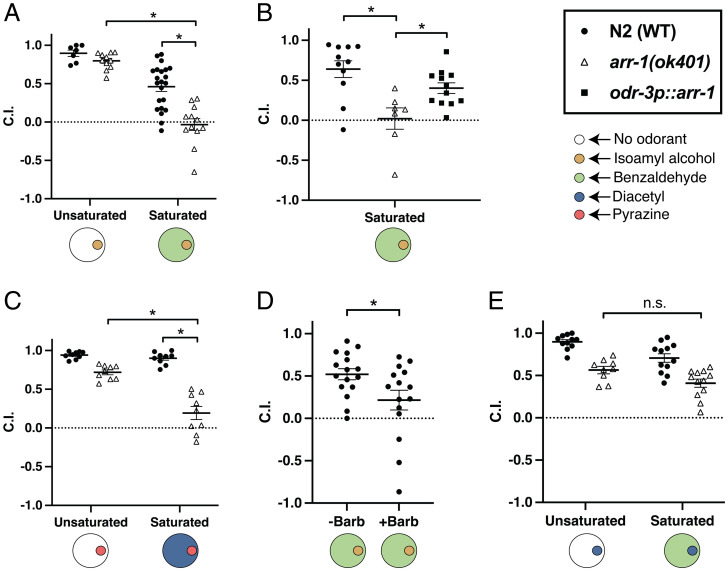
β-Arrestins are the canonical desensitizers of activated GPCRs, and the β-arrestin family is represented in the C. elegans genome solely by the gene arr-1 (11). To determine whether arrestin-mediated desensitization was responsible for the apparent ability of worms to perform intraneuronal olfactory discrimination, we first tested animals carrying arr-1–null alleles in a saturation assay. The arr-1(ok401) homozygous worms showed a severely abrogated ability to locate a point of isoamyl alcohol, sensed by the paired AWC neurons, within a saturating field of the AWC-sensed (4) odorant benzaldehyde. Conversely, in the absence of a saturating concentration of benzaldehyde, loss of arrestin resulted in only a very modest decrease in chemotaxis toward the point (Fig. 1A): This modest decrease may result from perturbation of chemosensory signaling, or may involve a role for ARR-1 in regulating nonchemosensory GPCRs required for optimal movement, and was observed in chemotaxis by arr-1 mutants to all odorants tested. These results were recapitulated in a second, probable null allele of arr-1 (SI Appendix, Fig. S1). Rescue of wild-type arr-1 under the odr-3 promoter, driving expression primarily in AWC, mostly restored chemotaxis to a point of isoamyl alcohol within a saturating field of benzaldehyde (Fig. 1B), suggesting that expression of arrestin within AWC alone was sufficient to restore most discrimination between odors sensed by these neurons. We next sought to determine whether chemotaxis toward AWA-sensed odorants was similarly affected, and found that worms homozygous for arr-1(ok401) exhibited a significant, but not complete, block of chemotaxis to pyrazine within a saturating field of diacetyl, again with only a mild chemotactic deficit under unsaturated conditions (Fig. 1C).
Fig. 1.
Olfactory discrimination depends on ARR-1. (A) Chemotaxis of wild-type N2 animals and arr-1(ok401) animals to a point of the AWC-sensed odorant isoamyl alcohol on unsaturated plates and plates containing a saturating concentration of the AWC-sensed odorant benzaldehyde. A two-way ANOVA revealed a significant effect of the interaction of strain and saturation condition (F = 7.606, P < 0.01), and a t test revealed a significant difference between N2 and arr-1(ok401) in the benzaldehyde saturated conditions (t = 4.997, P < 0.01) and a significant difference between arr-1(ok401) in the unsaturated and saturated conditions (t = 9.900, P < 0.01). (B) Chemotaxis of wild-type N2 animals, arr-1(ok401) animals and animals in which arr-1 has been selectively rescued primarily in AWC to a point of isoamyl alcohol on plates containing a saturating concentration of benzaldehyde. A t test revealed a significant difference between arr-1(ok401) and odr-3p::arr-1 (t = 2.56, P < 0.05). (C) Chemotaxis of wild-type N2 animals and arr-1(ok401) animals to a point of the AWA-sensed odorant pyrazine on unsaturated plates and plates containing a saturating concentration of diacetyl. A two-way ANOVA revealed a significant effect of the interaction of strain and saturation condition (F = 25.89, P < 0.01), while a t test revealed a significant difference between N2 and arr-1(ok401) in the diacetyl saturated conditions (t = 7.96, P < 0.01) and a significant difference between arr-1(ok401) in the unsaturated and saturated conditions (t = 5.824, P < 0.01). (D) Chemotaxis of wild-type N2 animals to a point of the AWC-sensed odorant isoamyl alcohol on plates saturated with benzaldehyde, in the absence (−Barb) and presence (+Barb) of the β-arrestin inhibitor Barbadin. A t test revealed a significant difference between the –Barb and +Barb groups (t = −2.27, P < 0.05). (E) Chemotaxis of wild-type N2 animals and arr-1(ok401) animals to a point of the AWA-sensed odorant diacetyl on unsaturated plates and plates containing a saturating concentration of the AWC-sensed odorant benzaldehyde. A two-way ANOVA revealed no significant interaction between strain and saturation condition (F = 0.171, P > 0.05), *P < 0.05.
When tested in the presence of Barbadin, a well-characterized small-molecule inhibitor of the mammalian β-arrestin/AP2 complex, wild-type worms displayed a similar, albeit less complete, inhibition of discrimination between AWC-sensed odors, and were unable to locate isoamyl alcohol in a saturating field of benzaldehyde (Fig. 1D), despite lacking any impairment of chemotaxis under unsaturated conditions (SI Appendix, Fig. S2). These data show that Barbadin is capable of partially phenocopying arr-1–null mutations in C. elegans, suggesting that it is an efficacious inhibitor of ARR-1, albeit (at least at the concentrations we employed) not a complete one. Since in mammalian cells Barbadin’s mechanism of action is specific to inhibition of the β-arrestin/AP2 complex, this partial recapitulation of a discrimination deficit suggests that arrestin is at least partially acting to enable olfactory discrimination by mediating endocytosis of the receptors for the saturating odor in clathrin-coated pits (12).
Arrestin Is Dispensable for Interneuronal Olfactory Discrimination.
Our hypothesized mechanism of intraneuronal olfactory discrimination should, if correct, play a minimal role in interneuronal olfactory discrimination, where, as in the mammalian olfactory system, physical separation of signaling allows for the avoidance of cross-talk. To test this prediction, we evaluated arr-1(ok401) animals for chemotaxis to a point of an AWA-sensed odorant, diacetyl, in a saturating concentration of the AWC-sensed odorant benzaldehyde. As expected, arr-1(ok401) animals were able to locate the point of diacetyl both in the absence and presence of a saturating concentration of benzaldehyde (Fig. 1E), suggesting that arrestin-mediated desensitization does not play a role in discrimination in contexts in which differential activation of sensory neurons is sufficient to distinguish between odorants.
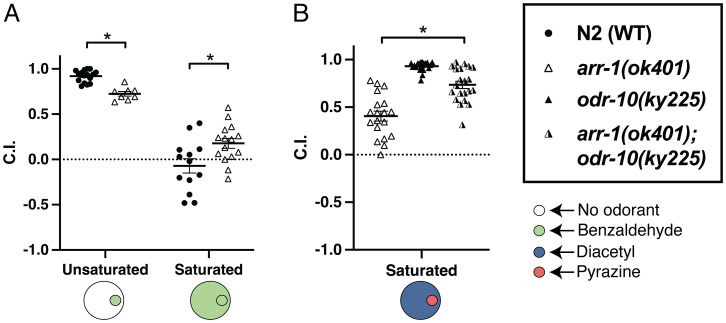
Previous reports have determined saturating concentrations in this paradigm by finding the minimum concentration of the odorant that prevented C. elegans from locating a point of the same odorant (4). We wondered if arrestin might, by desensitizing activated receptors, create a ceiling on receptor signaling potential, resulting in saturation at lower levels. To test this, we evaluated arr-1(ok401) animals for chemotaxis to a point of benzaldehyde in a saturating concentration of benzaldehyde. Consistent with previous reports, we found that wild-type N2 animals were unable to find the point of benzaldehyde; however, loss of arrestin resulted in a modest but significant increase in the ability to find the point (Fig. 2A), and this result was recapitulated when evaluating animals for chemotaxis to a point of diacetyl in a saturating concentration of diacetyl (SI Appendix, Fig. S3). This result, demonstrating an increase in the dynamic range of odorant-induced signaling, is a novel prediction of our hypothesis, further supporting a role for arrestin in constraining the dynamic range of response to ubiquitously present odors.
Fig. 2.
Experimental support for model predictions. (A) Chemotaxis of wild-type N2 animals and arr-1(ok401) animals to a point of benzaldehyde on both unsaturated agar plates, and plates containing a saturating concentration of benzaldehyde. A two-way ANOVA revealed a significant interaction between strain and saturation condition (F = 16.60, P < 0.01), and a t test indicated a significant difference between N2 and arr-1(ok401) in the benzaldehyde saturated condition (t = −2.58, P < 0.05). (B) Chemotaxis of arr-1(ok401), odr-10(ky225), and arr-1(ok401);odr-10(ky225) animals to a point of pyrazine on plates containing a saturating concentration of diacetyl. A t test revealed a significant difference between arr-1(ok401) and arr-1(ok401);odr-10(ky225) animals (t = −5.07, P < 0.01), *P < 0.05.
Mutations in Saturating Olfactory Receptors Genetically Suppress the Arrestin Mutant Discrimination Phenotype.
If discrimination of point odorants from saturating odorants occurs via arrestin-mediated desensitization of the receptors corresponding to the saturating odorants, then genetic elimination of these receptors should suppress the discrimination defects we observed in arr-1 mutant animals. We exploited a mutant in odr-10, the sole C. elegans receptor responsive to low concentrations of diacetyl (13, 14), to test our proposed mechanism of intraneuronal discrimination by examining chemotaxis to a point of pyrazine in a saturating concentration of diacetyl. odr-10(ky225) animals, which carry a deletion in the majority of the odr-10 gene, have wild-type responses to pyrazine but no chemotactic responses to low to moderate concentrations of diacetyl (14). As expected, odr-10(ky225) and arr-1(ok401);odr-10(ky225) animals showed minimal approach to diacetyl (SI Appendix, Fig. S4). In subsequent experiments testing the approach to pyrazine in a saturating concentration of diacetyl, odr-10(ky225) animals exhibited robust chemotaxis to the point, while arr-1(ok401) animals showed only a weak approach. In arr-1(ok401);odr-10(ky225) double mutants, however, approach to pyrazine was largely restored, indicating suppression of arr-1 by odr-10 (Fig. 2B). This finding—that elimination of the ability to sense one odorant can enhance the ability of the animal to sense a second—is predicted by our model, but would be surprising if signal transduction for different odorants within single neurons was qualitatively different downstream of the receptor, and thus further supports our hypothesis.
grk-1 Acts Upstream of Arrestin in Interneuronal Discrimination.
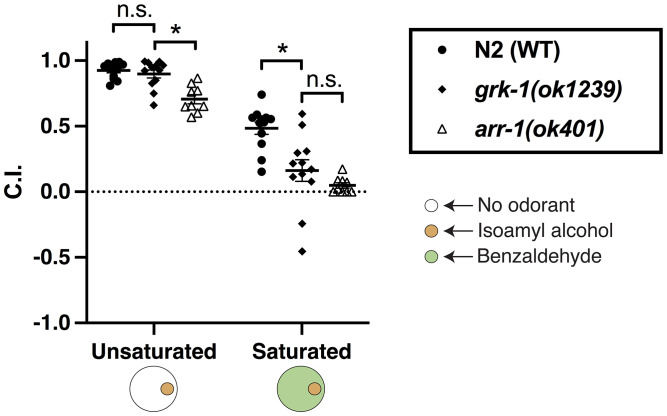
In mammals, arrestin-mediated desensitization occurs following phosphorylation of the activated receptor by GPCR kinases (GRKs) (15). Although the C. elegans genome contains two predicted GRKs, grk-1 and grk-2, loss-of-function mutations in these genes have been found to result in surprising phenotypes. While in mammals loss of GRK3 (expressed in the olfactory epithelium) results in olfactory desensitization deficits similar to those caused by loss-of-function mutations in arrestin (16, 17), loss of C. elegans grk-2 appears to completely abrogate chemosensation (18), while the only phenotype known to result from mutations in grk-1 is an alteration in dopamine-mediated swimming-induced paralysis (19, 20). We wondered whether arrestin-mediated olfactory discrimination might constitute a sensitized assay capable of revealing subtle chemosensory deficits in grk-1 mutant animals. When tested for chemotaxis to a point of isoamyl alcohol in a saturating field of benzaldehyde, grk-1 mutants showed severe chemotactic deficits. Unlike in arr-1 mutants, no chemotaxis deficit to isoamyl alcohol in unsaturated conditions was observed, suggesting that loss of grk-1 may result in either more specific (affecting fewer GPCRs) or less complete (affecting the same GPCRs to a lesser extent) inhibition of desensitization than loss of arr-1 (Fig. 3).
Fig. 3.
GRK-1 activity is required for olfactory discrimination. Chemotaxis of wild-type N2 animals, arr-1(ok401) and grk-1(ok1239) animals to a point of isoamyl alcohol on unsaturated agar plates and on plates containing a saturating concentration of benzaldehyde. A two-way ANOVA revealed a significant interaction between strain and saturation condition (F = 5.695, P < 0.01), and a t test indicated a significant difference between N2 and grk-1(ok1239) in the benzaldehyde saturated condition (t = 3.40, P < 0.01), and between grk-1(ok1230) and arr-1(ok401) in the unsaturated condition (t = 4.158, P < 0.01), but no significant difference between N2 and grk-1(ok1239) in the unsaturated condition (t = −0.76, P > 0.05) or between grk-1(ok1329) and arr-1(ok401) in the saturated condition (t = 1.333, P > 0.05), P < 0.05.
Discussion
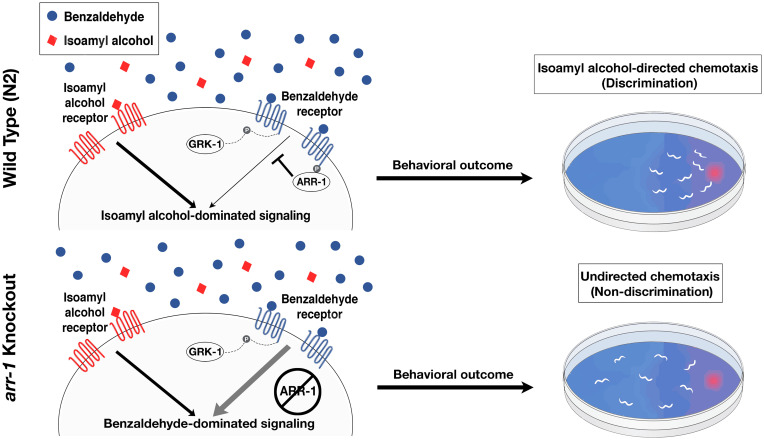
Nematodes and mammals face a similar problem in ensuring signal segregation between the many chemosensory receptors providing their senses of olfaction. While mammals have solved this problem by expressing olfactory receptor genes in a one or few genes per neuron fashion, our findings suggest that nematodes have enacted a radically different solution, in which many chemosensory receptors are expressed within a given neuron, but discrimination is enabled through desensitization of ubiquitously active receptors (Fig. 4). In mammals, physical isolation between signaling pathways preserves a qualitative difference between olfactory stimuli deep into the nervous system. In nematodes, however, our findings suggest that an initial qualitative difference at the receptors is converted by arrestin to a quantitative difference in signaling, which, by resulting in selective responses to odorants, nonetheless results in qualitatively different behavioral responses.
Fig. 4.
Diagram of discrimination model. In olfactory neurons of wild-type N2 animals (Upper), ARR-1 acts to desensitize the receptors for saturating odorants, here shown as the AWC-sensed odorant benzaldehyde, leaving only signaling from the point odorant, here shown as the AWC-sensed odorant isoamyl alcohol, to determine chemotactic behavior. In arr-1 mutant animals (Lower), ubiquitous signaling from the receptor for the saturating odorant overwhelms signaling from the point odorant, preventing chemotaxis toward it.
Despite remarkable morphological and functional diversity (21), crown clade nematodes, including C. elegans, have a reduced and largely conserved set of neurons, despite a massive expansion of (primarily chemosensory) GPCRs (22). The simultaneous expansion of the chemosensory receptor repertoire and reduction of neurons in which to express them may appear paradoxical; however, our findings suggest that the evolutionary adaptation of discrimination by arrestin-mediated desensitization of receptors may have enabled these processes to occur concurrently. It may be interesting to examine the chemosensory systems of more basal nematodes, in which development is indeterminate and the chemosensory receptor repertoire more limited, to better understand how this unusual mechanism for discrimination evolved.
The data we present for intraneuronal olfactory discrimination is specific to the particular circumstance in which a saturating concentration of an odorant sensed by one neuron is present along with a lower concentration of a second odorant sensed by the same neuron. While the use of arrestin to desensitize active receptors is presumably relatively generalized across diverse odorants under conditions broadly similar to those we’ve employed, other mechanisms have been recently proposed and likely also play a role (23).
Expression of multiple chemosensory receptors within single neurons is not unique to nematodes, and recent reports suggest it may be happening in both mosquitos (24) and fruit flies (25). Notably, neither group is known to employ GPCRs as chemosensory receptors, precluding the use of the discrimination approach employed in C. elegans, although analogous mechanisms based on receptor desensitization but employing different proteins may nonetheless play a role.
Cross-adaptation between odorants and between tastes has been shown in many organisms, including flies (26), mice (27), and humans (28, 29). Previous work from our group and others (30, 31) has analyzed cross-adaptation in C. elegans, whereby animals exposed to one odorant show altered subsequent responses to a second odorant sensed by the same neuron. We previously divided these responses into two categories: 1) nonassociative habituation, which occurs irrespective of the presence of food, and 2) associative memories, which form from the paired association of starvation and an odorant (30). Our finding that habituation depends on the activity of ARR-1 is consistent with arrestin-mediated desensitization of olfactory receptors being the molecular mechanism underlying behavioral habituation, and the cross-adaptation we report between benzaldehyde associative learning and isoamyl alcohol is most easily explained by signal transduction downstream of the receptors, including that required for associative learning, being shared between odorants sensed by a single neuron (as proposed by the model we outline in the present work). Some aspects of these results, however, including isoamyl alcohol adaptation being independent of food while benzaldehyde responses are dependent on the absence of food, suggest that these odorants are not completely interchangeable activators of signal transduction within the cell. We propose that the differences in efficacy among odorants, whereby some odorants activate additional pathways by inducing greater intracellular signaling, may explain these differences.
Several mechanistic questions remain about how arrestin is acting to mediate intraneuronal olfactory discrimination in C. elegans. If arrestin is acting through endocytosis and degradation of activated receptors, one might expect to see an arrestin-dependent decrease of fluorescent intensity in diacetyl stimulated ODR-10::GFP animals; however, we have not observed this difference in preliminary experiments. It may be that arrestin is acting through an endocytosis-independent mechanism, which would suggest that Barbadin acts in a different way in C. elegans than in mammalian cells: confirmation of a shared mechanism of Barbadin between mammalian cells and C. elegans would aid in clarifying this. We also have not been able to identify odorant concentrations, which would allow for behavioral discrimination given the reversal of saturating and point odors in the experiments described here, although our model suggests this should generally be possible: identifying these conditions would allow for further tests of our model.
Our model, in which discrimination occurs solely at the level of the receptor, raises questions about the limits of what can be called discrimination: that the behavioral responses to the two odorants differ is clear, and this would appear to necessarily constitute discrimination in some sense, but downstream components of the nervous system of the worm have no access to the qualitative distinction between these odorants. We propose that the model we present here represents a highly simplified type of sensory discrimination.
Evolution in nematodes, as in all organisms, operates within constraints. In C. elegans, 5% of genes and over 10% of neurons appear to be dedicated to chemosensation (32), suggesting the crucial importance of chemosensation in the animal’s ecology, a suggestion reinforced by evidence of strong selective pressure on at least some receptors (3). Despite the considerable portion of neurons dedicated to chemosensation, the large number of chemosensory genes encoded in the genome results in far more genes than can be expressed in a one-to-one fashion in neurons. Our findings suggest that nematodes have escaped this constraint by evolving a discrimination mechanism in which desensitization allows for the expression and discrimination of a plurality of receptors in any one cell. By allowing for expression of far more olfactory receptors than the worm has neurons to express them in, this strategy greatly enhances the olfactory repertoire of C. elegans.
Materials and Methods
C. elegans Strains and Maintenance.
Animals were maintained at 20 °C on NGM plates and fed Escherichia coli OP50. All experiments were performed at 20 °C.
The C. elegans strains N2, RB660 arr-1(ok401), RB1194 grk-1(ok1239), and CX3410 odr-10(ky225) were provided by the Caenorhabditis Genetics Center. UT1313 arr-1(ok401);odr-10(ky225) was produced by crossing RB660 and CX3410. arr-1(vs96) and the Ex[odr-3p::arr-1] arr-1(ok401);lin-15(n765ts) rescue line were kindly provided by Jeffrey Benovic of Thomas Jefferson University, Philadelphia, USA.
Preparation of Worms for Behavioral Assays.
Worms were age-synchronized using a sodium hypochlorite bleaching protocol. Briefly, eggs were isolated following treatment of worms with bleach solution and incubated on a rocker overnight at 20 °C in ∼2.5 mL M9 buffer to allow hatching, after which ∼1,000 L1 larvae were plated on an NGM plate seeded with E. coli OP50 and allowed to grow for 53.5 to 54 h at 20 °C (to the young adult stage).
Young adult worms were first washed from their cultivation plates with ∼1.5 mL M9 buffer and collected into 1.5-mL microcentrifuge tubes with a Pasteur pipette. Worms were washed twice with additional volumes of M9 to remove any remaining bacteria. During the wash steps, the largest worms were selected: worms were resuspended in buffer and only those worms that settled within ∼1 min were collected. Worms were starved in 1.5 mL of M9 buffer for ∼90 min before the assay.
Behavioral Assays.
Population chemotaxis assays were performed as previously described (4). Briefly, assay plates were 10-cm Petri plates containing 10 mL of assay agar (1.6% agar, 5 mM KH2PO4 pH 6.0, 1 mM CaCl2, 1 mM MgSO4). All attractant points were diluted in anhydrous ethanol to the following concentrations: benzaldehyde (Sigma-Aldrich) 1:200, isoamyl alcohol (Bioshop) 1:10, pyrazine (Sigma-Aldrich) 1:100, diacetyl (Fluka) 1:1,000.
Assays testing detection of one attractant in the saturating presence of another, or testing detection of one attractant in the saturating presence of the same attractant, were performed on plates to which undiluted attractant was added in a 1:10,000 ratio directly to the agar before pouring. Agar was first liquefied in a microwave and then cooled to ∼60 °C by incubating in a water bath for at least 1 h. Both odorant-saturated plates and unsaturated control plates were sealed with Parafilm after pouring.
Approximately 50 to 200 53.5- to 54-h-old (at 20 °C) worms were transferred to the center of an assay plate using a calibrated glass micropipette in ∼10 μL of M9 buffer. Next, 1 µL of 1 M sodium azide (Sigma-Aldrich) was spotted at opposite sides of the plate, 1 cm from the edge, to paralyze worms at the spots where attractant odorant would be spotted. Worms were allowed to settle in the small volume of M9 buffer at the center of the assay plate for 2 min before 1-μL attractant odorant diluted in ethanol and 1 µL of 100% ethanol were spotted on opposing sides of the plate, on top of the sodium azide points. A corner of a Kimwipe twisted into a point was then used to dry the worms. Worms were observed under the microscope during drying to ensure the surface of the agar was not broken with the Kimwipe; plates with any large breaks introduced at the origin during drying were not scored. Plates were again sealed with Parafilm and left undisturbed for 60 min, after which a chemotaxis index was calculated as (number of worms within 1 cm of the ethanol counter attractant subtracted from the number of worms within 1 cm of attractant)/(total number of worms on the plate). Any worms having not moved from a defined 1-cm by 0.5-cm rectangle, where they were initially placed were considered injured and omitted from this calculation. Any plates with fewer than 20 total worms were excluded from analysis.
Barbadin Experiments.
Worms were added to the center of the test plate in an ∼10-μL drop of 1 mM Barbadin (Toronto Research Chemicals) (or DMSO in control conditions) dissolved in 2% pluronic acid (F-127, Sigma Aldrich) in M9. Barbadin was prepared as a stock solution at 100 mM in DMSO. In addition to Barbadin in the drop at the center of the plate, the agar in these experiments contained 100 µM Barbadin (or DMSO in control conditions) and 2% pluronic acid.
Statistical Analysis.
No statistical test was performed to predetermine sample sizes. Mean chemotactic index and the SEM were calculated using R. In experiments with multiple independent variables, comparisons between chemotactic index were made by two-way ANOVA and follow-up t tests to determine between-strain differences within a testing condition. In experiments with a single independent variable, comparisons were made by t test alone. In experiments with more than one test, t test results were adjusted using Bonferroni correction. Differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank Srinidhi Krishnakumar for technical assistance. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Additional strains were provided by Jeffrey Benovic. Funding was provided by Natural Sciences and Engineering Research Council (NSERC) RGPIN Grant 8319 and NSERC CREATE in Manufacturing, Materials, Mimetics.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2116957119/-/DCSupplemental.
Data Availability
All data are available in the main text and SI Appendix.
References
- 1.Bushdid C., Magnasco M. O., Vosshall L. B., Keller A., Humans can discriminate more than 1 trillion olfactory stimuli. Science 343, 1370–1372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassar R., et al. , Topographic organization of sensory projections to the olfactory bulb. Cell 79, 981–991 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Thomas J. H., Kelley J. L., Robertson H. M., Ly K., Swanson W. J., Adaptive evolution in the SRZ chemoreceptor families of Caenorhabditis elegans and Caenorhabditis briggsae. Proc. Natl. Acad. Sci. U.S.A. 102, 4476–4481 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargmann C. I., Hartwieg E., Horvitz H. R., Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527 (1993). [DOI] [PubMed] [Google Scholar]
- 5.White J. G., Southgate E., Thomson J. N., Brenner S., The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986). [DOI] [PubMed] [Google Scholar]
- 6.Ward S., Chemotaxis by the nematode Caenorhabditis elegans: Identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. U.S.A. 70, 817–821 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., et al. , Identification of a nematode chemosensory gene family. Proc. Natl. Acad. Sci. U.S.A. 102, 146–151 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roayaie K., Crump J. G., Sagasti A., Bargmann C. I., The G α protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20, 55–67 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Lans H., Rademakers S., Jansen G., A network of stimulatory and inhibitory Galpha-subunits regulates olfaction in Caenorhabditis elegans. Genetics 167, 1677–1687 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troemel E. R., Chou J. H., Dwyer N. D., Colbert H. A., Bargmann C. I., Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207–218 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Palmitessa A., et al. , Caenorhabditus elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J. Biol. Chem. 280, 24649–24662 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Beautrait A., et al. , A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat. Commun. 8, 15054 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi G., Uozumi T., Kiriyama K., Kamizaki T., Hirotsu T., Screening of Odor-Receptor Pairs in Caenorhabditis elegans Reveals Different Receptors for High and Low Odor Concentrations Science signaling 7, ra39 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Sengupta P., Chou J. H., Bargmann C. I., odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84, 899–909 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Moore C. A. C., Milano S. K., Benovic J. L., Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 69, 451–482 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Peppel K., et al. , G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J. Biol. Chem. 272, 25425–25428 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Mashukova A., Spehr M., Hatt H., Neuhaus E. M., β-Arrestin2-mediated internalization of mammalian odorant receptors. J. Neurosci. 26, 9902–9912 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuto H. S., et al. , G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron 42, 581–593 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Wood J. F., Ferkey D. M., “GRK roles in C. elegans” in G Protein Coupled Receptor Kinases, 2016th Ed., Gurevich V. V., Gurevich E. V., Tesmer J. J. G., Eds. (Humana Press, 2016), pp. 283–299. [Google Scholar]
- 20.Wani K. A., et al. , D1 dopamine receptor signaling is modulated by the R7 RGS protein EAT-16 and the R7 binding protein RSBP-1 in Caenoerhabditis elegans motor neurons. PLoS One 7, e37831 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Ley P., A quick tour of nematode diversity and the backbone of nematode phylogeny. WormBook Jan 25, 1–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schafer W., Nematode nervous systems. Curr. Biol. 26, R955–R959 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Khan M., et al. , Context-dependent inversion of the response in a single sensory neuron type reverses olfactory preference behavior. bioRxiv [Preprint] (2021). https://www.biorxiv.org/content/10.1101/2021.11.08.467792v1. Accessed 25 May 2022.
- 24.Younger M. A., et al. , Non-canonical odor coding ensures unbreakable mosquito attraction to humans. bioRxiv [Preprint] (2022). https://www.biorxiv.org/content/10.1101/2020.11.07.368720v2. Accessed 25 May 2022.
- 25.Task D., et al. , Widespread polymodal chemosensory receptor expression in Drosophila olfactory neurons. bioRxiv [Preprint] (2020). https://www.biorxiv.org/content/10.1101/2020.11.07.355651v1. Accessed 25 May 2022.
- 26.Boyle J., Cobb M., Olfactory coding in Drosophila larvae investigated by cross-adaptation. J. Exp. Biol. 208, 3483–3491 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Kelliher K. R., Ziesmann J., Munger S. D., Reed R. R., Zufall F., Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc. Natl. Acad. Sci. 100, 4299–4304 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meiselman H. L., Adaptation and cross-adaptation of the four gustatory qualities. Percept. Psychophys. 4, 368–372 (1968). [Google Scholar]
- 29.Pierce J. D. Jr, Zeng X.-N., Aronov E. V., Preti G., Wysocki C. J., Cross-adaptation of sweaty-smelling 3-methyl-2-hexenoic acid by a structurally-similar, pleasant-smelling odorant. Chem. Senses 20, 401–411 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Pereira S., van der Kooy D., Two forms of learning following training to a single odorant in Caenorhabditis elegans AWC neurons. J. Neurosci. 32, 9035–9044 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colbert H. A., Bargmann C. I., Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14, 803–812 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Bargmann C. I., Chemosensation in C. elegans. WormBook. Oct. 25, 1–29 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text and SI Appendix.