Significance
The complex evolutionary history of bears has been considered to be among the most enigmatic aspects of mammalian evolution. The present results indicate that the Asiatic black bear was established through a hybrid speciation process. This type of speciation is thought to be rare in mammals, as opposed to plants, which can accommodate polyploidy. In addition, we identified key genes involved in the intermediate body size phenotype of the Asiatic black bear and propose its likely reproductive isolation from its parental lineages through alternate inheritance of diverged loci. This study sheds light on the enigmatic evolution of bears in greater depth and highlights the role of hybridization as a catalyst for new species formation and phenotypic evolution in mammals.
Keywords: Ursinae, hybridization, Asiatic black bear, population genomics
Abstract
Bears are fascinating mammals because of their complex pattern of speciation and rapid evolution of distinct phenotypes. Interspecific hybridization has been common and has shaped the complex evolutionary history of bears. In this study, based on the largest population-level genomic dataset to date involving all Ursinae species and recently developed methods for detecting hybrid speciation, we provide explicit evidence for the hybrid origin of Asiatic black bears, which arose through historical hybridization between the ancestor of polar bear/brown bear/American black bears and the ancestor of sun bear/sloth bears. This was inferred to have occurred soon after the divergence of the two parental lineages in Eurasia due to climate-driven population expansion and dispersal. In addition, we found that the intermediate body size of this hybrid species arose from its combination of relevant genes derived from two parental lineages of contrasting sizes. This and alternate fixation of numerous other loci that had diverged between parental lineages may have initiated the reproductive isolation of the Asiatic black bear from its two parents. Our study sheds further light on the evolutionary history of bears and documents the importance of hybridization in new species formation and phenotypic evolution in mammals.
Bears are fascinating mammals with a complex pattern of speciation and rapid evolution of distinct phenotypes (1). Natural hybrids and genetic studies have indicated that pervasive interspecific hybridization shaped their complex evolutionary history, particularly among the six bear species of the subfamily Ursinae (2–4). Ursinae comprises six genetically closely related and morphologically distinct extant species (polar bear, brown bear, American black bear, sun bear, sloth bear, and Asiatic black bear) whose origin can be traced back to the extremely recent early-Pliocene epoch (∼5 Ma) (4). The most striking aspect of bear evolution is the puzzle of the evolutionary relationship between polar bears and brown bears, which are known to mate and produce viable and fertile hybrids both in the wild and in captivity (5, 6). Although polar bears and brown bears have been considered to be two distinct lineages on the basis of nuclear genomic and Y chromosome data studies (1, 7, 8), analyses of mitochondrial (mt) DNA identified polar bear mt haplotypes as falling within the range of diversity of brown bear mt haplotypes, with the polar bear mt haplotypes being most closely related to those of brown bear on Alaska’s ABC islands (9). After more than two decades of extensive genetic research and debates, recent studies found that the brown bears of the ABC islands originated from sex-biased hybridization between brown bears and polar bears (10, 11).
Interspecific hybridization is not limited to the most recently diverged polar and brown bears; it has been reported to be universal among extant Ursinae bears (3, 4) and even between them and extinct bears (12), as inferred from extensive phylogenetic discordance observed across loci. In particular, ancient introgression between Asiatic black bears and the ancestor of polar, brown and American black bears has been used to explain the observed conflict between gene trees and the consensus species tree (4). The Asiatic black bear was placed as a sister group to the polar bear/brown bear/American black bear clade in most gene trees, while it grouped with the sun bear/sloth bear clade in the consensus species tree (3, 4), suggesting a complex evolutionary history for the Asiatic black bear. In addition, as seen from morphological features and geographic distributions, all species in the polar/brown/American black bear clade are large bears and inhabit high-latitude regions (hereafter referred to as the North Group) (13, 14). The sun/sloth bear clade consists of two species of small bears distributed in low-latitude regions (hereafter referred to as the South Group) (13, 14). Interestingly, the Asiatic black bear is an intermediate-sized bear, being smaller than North Group bears and larger than South Group bears, and it is widely distributed in regions of intermediate latitude, partially overlapping with the distributions of the other two clades of bears (13, 14) (Fig. 1A and SI Appendix, Table S1). The conflicting phylogenetic relationships, intermediate phenotype, and ecological divergence with partial spatial overlap have raised interest in the evolutionary origin of the Asiatic black bear. Whether this species arose through hybridization between the ancestor of the North Group and the ancestor of the South Group has hitherto remained unexplored.
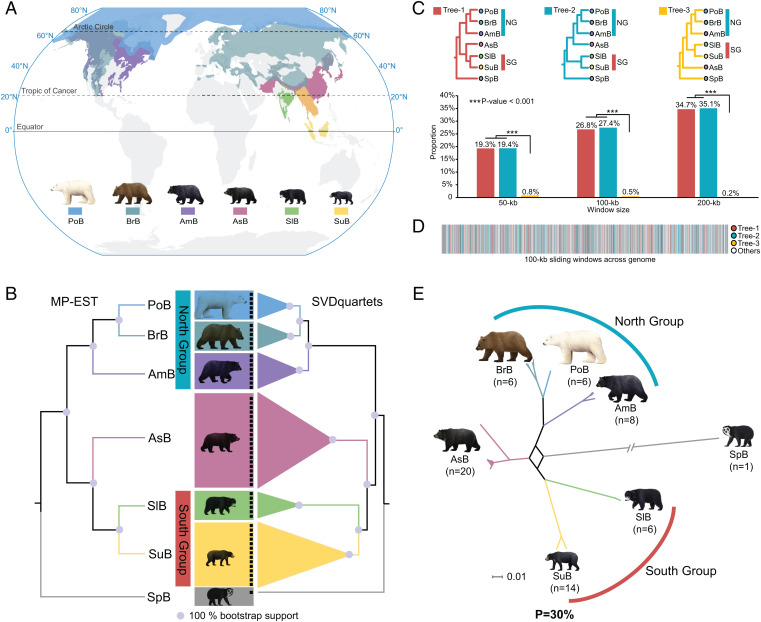
Fig. 1.
Geographic distribution and phylogenetic analyses of Ursinae. (A) Geographic distributions of six Ursinae species (IUCN, www.iucnredlist.org). PoB: polar bear; BrB: brown bear; AmB: American black bear; AsB: Asiatic black bear; SuB: sun bear; SlB: sloth bear. (B) Species trees of six Ursinae species constructed by MP-EST and SVDquartets. Both analyses produced the same topology, with strong support (100%) for all interspecific-level branches. Spectacled bear (SpB) was used as the outgroup. In the SVDquartets tree, the size of triangles for the species represents the number of individuals. (C) Distributions of phylogenetic trees based on three window sizes (50, 100, and 200 kb). Tree-1, Tree-2, and Tree-3 correspond to the three phylogenetic topologies described in the text. NG represents the North Group. SG represents the South Group. (D) Distributions of the phylogenetic tree topologies (Tree-1, Tree-2, Tree-3, and Others) across the Asiatic black bear genome based on 100-kb sliding window. (E) Phylogenetic network analysis with a threshold level of 30% showing the reticulate evolutionary history of the Asiatic black bear.
To address this issue, we newly generated 43 bear genomes and analyzed these data alongside 18 publicly available genomes. Based on the largest population-level genomic dataset to date involving all Ursinae bears and multiple methods for detection of hybrid speciation, which can be disentangled from the effects of incomplete lineage sorting (ILS) and introgression as well as ancestral subdivision, we provide explicit evidence for the hybrid origin of the Asiatic black bear, which emerged from historical hybridization between the ancestor of the polar bear/brown bear/American black bear and the ancestor of the sun bear/sloth bear. In addition, we identified key genes involved in the intermediate body size phenotype and propose the likely mechanism of reproductive isolation (RI) from the parental lineages. This study sheds further light on the evolutionary history of bears and documents the importance of hybridization in new species formation and phenotypic evolution in mammals.
Results
Genomic Sequencing and Single-Nucleotide Polymorphism (SNP) Calling.
Individual genome resequencing of 60 bears from Ursinae and one spectacled bear used as the outgroup, at an average of 31.59 ± 8.33-fold, was carried out for population genomic analyses (SI Appendix, Table S2). The average rate of alignment to the reference genome was 91.60% ± 7.19% (SI Appendix, Table S2). SNP calling and filtering produced a total of 100.96 million high-quality variants.
Phylogenetic Conflict across Nuclear Autosomal Genomes of Bears.
Two phylogenetic methods, MP-EST (based on all 100-kb window sequences and protein-coding gene sequences, respectively) and SVDquartets (based on all autosomal SNPs), produced the same topology as that proposed in previous studies (3, 4), with strong support (100%) for all interspecific-level branches, in which the six Ursinae bears are divided into two lineages, one consisting of American black, polar, and brown bears (i.e., the North Group) and the other of Asiatic black, sun, and sloth bears (i.e., Asiatic black bear and the South Group) (Fig. 1B).
Further phylogenetic trees reconstruction from sliding windows across the genome showed that, regardless of window size (50, 100, or 200 kb), two tree topologies (referred to as Tree-1 and Tree-2) accounted for the greatest proportion of trees, and the proportions of Tree-1 and Tree-2 were similar (Fig. 1C and SI Appendix, Fig. S1) (Tree-1 represented 19.3%, 26.8%, and 34.7% for window sizes of 50, 100 and 200 kb, respectively; Tree-2 made up 19.4%, 27.4%, and 35.1% for window sizes of 50, 100, and 200 kb, respectively). Tree-1 was identical to the species tree, while Tree-2 grouped Asiatic black bears with the North Group. In comparison, the other tree topologies, including one that clustered North and South group bear species together as sister taxa, with the exclusion of the Asiatic black bear (referred as Tree-3) (0.8%, 0.5%, and 0.2% for window sizes of 50, 100, and 200 kb, respectively), occurred at relatively much lower frequency (P < 0.05 for Tree-1 vs. Tree-3 and for Tree-2 vs. Tree-3) (Fig. 1C and SI Appendix, Fig. S1). Sliding window tree analyses along the Asiatic black bear genome also revealed mosaic clustering of Asiatic black bear with the North Group or the South Group (Fig.1D). In addition, network analyses identified conflicting phylogenetic signals, particularly among Asiatic black bear, the North Group, and the South Group, as seen from the complex network with square and cuboid-like structures that they formed (Fig. 1E and SI Appendix, Fig. S2).
It should be noted that most of the observed phylogenetic conflict could be attributed to Tree-2, which occupied a high proportion, almost equal to that of the species tree Tree-1 (Fig. 1C and SI Appendix, Fig. S1). This observation is unlikely to be explained by ILS, as one would expect that under this scenario Tree-2 and Tree-3 would occur in equal proportions due to the random sorting of ancestral polymorphisms. Additional D-statistics analyses, in which D-values were significantly different from zero (SI Appendix, Table S3), further supported the ancient hybridization event and the hybrid origin of Asiatic black bear.
Asiatic Black Bear Is of Hybrid Origin Based on Its Admixed Genome.
According to the results of phylogenetic analyses and D-statistics for genomic variations, the Asiatic black bear originated from ancient hybridization between the ancestor of the North Group and the ancestor of the South Group. To test this hypothesis of the hybrid origin of the Asiatic black bear, we used HyDe analyses in which the ancestral North Group and the ancestral South Group were set as the two putative parental lineages. A significant signal of hybridization was observed between the ancestral North Group and the ancestral South Group lineages to produce the hybrid species Asiatic black bear (SI Appendix, Table S4) (P < 0.05). The γ parameter estimated from HyDe analysis indicated the genomic hybrid origin of the Asiatic black bear with 57.5% contribution from the ancestral North Group and 42.5% from the ancestral South Group to the Asiatic black bear in this case. This almost equal genomic contribution from two parental lineages to the admixed genome of the Asiatic black bear is consistent with the observation that nearly equal proportions of the two tree topologies grouped this hybrid species with the two parental lineages respectively in the windowed tree analyses (Fig. 1C). Estimates of γ for each Asiatic black bear individual sampled across the whole species range (the south continental, the north continental, and the Japanese populations) (15) were remarkably similar (ranging from 56.1 to 58.3%; all P < 0.05) (SI Appendix, Table S5 and Fig. 2A). Additional HyDe windowed scans across the genome showed that the dual parental ancestries were distributed across the whole genome of all Asiatic black bears (Fig. 2B). These results are compatible with an ancient hybridization between the ancestor of the North Group and the ancestor of the South Group that produced the Asiatic black bear, rather than with recent introgression after it had originated. If the latter case had applied, only a few, rather than all, Asiatic black bear individuals from a wide geographic distribution would show genetic mixture.
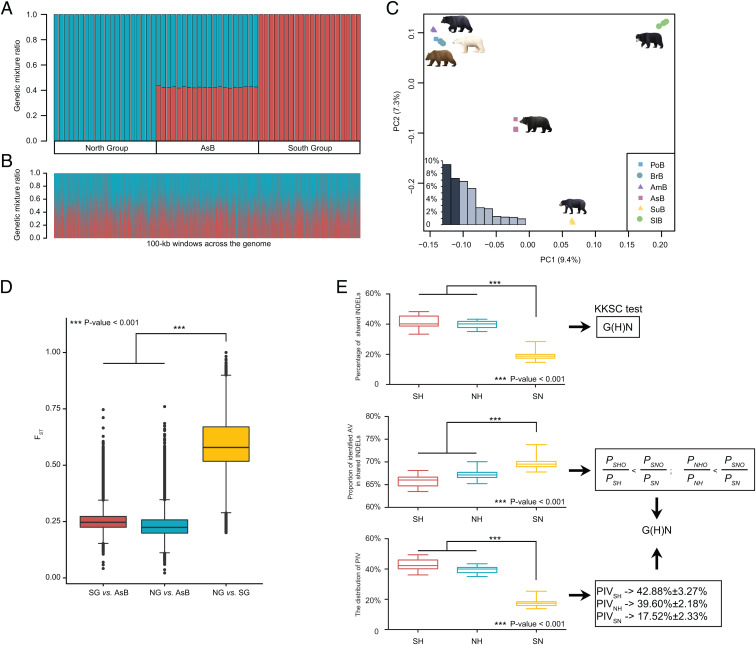
Fig. 2.
The hybrid origin of the Asiatic black bear based on HyDe, PCA, FST, and shared variations analyses. (A) HyDe analysis. AsB represents Asiatic black bear. The y axis indicates the proportion of genetic mixture for each individual; blue represents genetic contribution (γ) from the North Group and red represents genetic contribution (1 − γ) from the South Group. (B) HyDe scans with 100-kb sliding windows across the genome of the Asiatic black bear. (C) PCA results for 60 bears representing six Ursinae species. The inset bar plot shows the values of the 10 primary components, of which PC1 and PC2 explained the greatest proportions of the variance. (D) Distributions and comparisons of genomic 100-kb windowed FST for the three population pairs (SG vs. AsB, NG vs. AsB, and NG vs. SG). (E) Detection of hybrid speciation based on shared INDELs (S: South Group; H: Hybrid species Asiatic black bear; N: North Group; O: Outgroup spectacled bear). (Top) Percentages of shared INDELs in SH (South Group and Hybrid species Asiatic black bear: red bars), NH (North Group and Hybrid species Asiatic black bear: blue bars), and SN (South Group and North Group: yellow bars). A KKSC test supports the hybrid speciation model G(H)N. (Middle) Proportion of AVs. The values of SH, NH, and SN were calculated based on the numbers of shared INDELs in SHO/SH, NHO/NH, and SNO/SN, respectively. A Student’s t test of these data supported . (Bottom) Percentages of shared phylogenetically informative variations in SH, NH, and SN.
Principal component analyses (PCA) and FST analyses recapitulated the HyDe results. Our PCA plot (PC1 and 2) showed that all Asiatic black bears clustered together in a position intermediate between the North and South groups (Fig. 2C). FST analyses showed that population differentiation was significantly higher between the North Group and the South Group (FST = 0.6 ± 0.12) than between Asiatic black bear and either the North Group (FST = 0.23 ± 0.05) or the South Group (FST = 0.25 ± 0.05) (both P < 0.001) (Fig. 2D).
The Hybrid Origin of Asiatic Black Bears Based on Shared Variations.
We further used a recently developed test for detecting hybrid speciation based on shared variations, which can differentiate between hybrid speciation and ancestral subdivision (16). We considered two types of shared variations (insertion/deletions [INDELs] and SNPs) from three bear lineages, Asiatic black bear and its two presumed parental lineages, i.e., the North Group and the South Group. Analyses of ∼14.52 Mb of shared INDELs and ∼100.96 Mb of shared SNPs produced similar results (Fig. 2E and SI Appendix, Fig. S3). First, based on the shared variations, we could see that the North Group (N) and South Group (S) lineages are more closely related to the hybrid species Asiatic black bear (H) than to each other. A KKSC significance test (17) supported a hybrid speciation model for Asiatic black bear (P < 1.0E-16). To distinguish hybridization from ancestral subdivision, we then used the spectacled bear genome as outgroup (O) to identify ancestral variations (AVs). We found that the shared AV proportions of SNO (S, N, and O lineages) in SN (S and N lineages) were significantly higher than the proportions of SHO (S, H, and O lineages) in SH (S and H lineages) and NHO (N, H, and O lineages) in NH (N and H lineages). In addition, phylogenetically informative variations (PIVs) were more shared in both NH and SH than in SN. Such patterns of distribution of AVs and PIVs were to be expected from a hybrid speciation model (16). These findings support the hybrid origin of Asiatic black bears.
Evolutionary Simulations of the Hybrid Origin of Asiatic Black Bears.
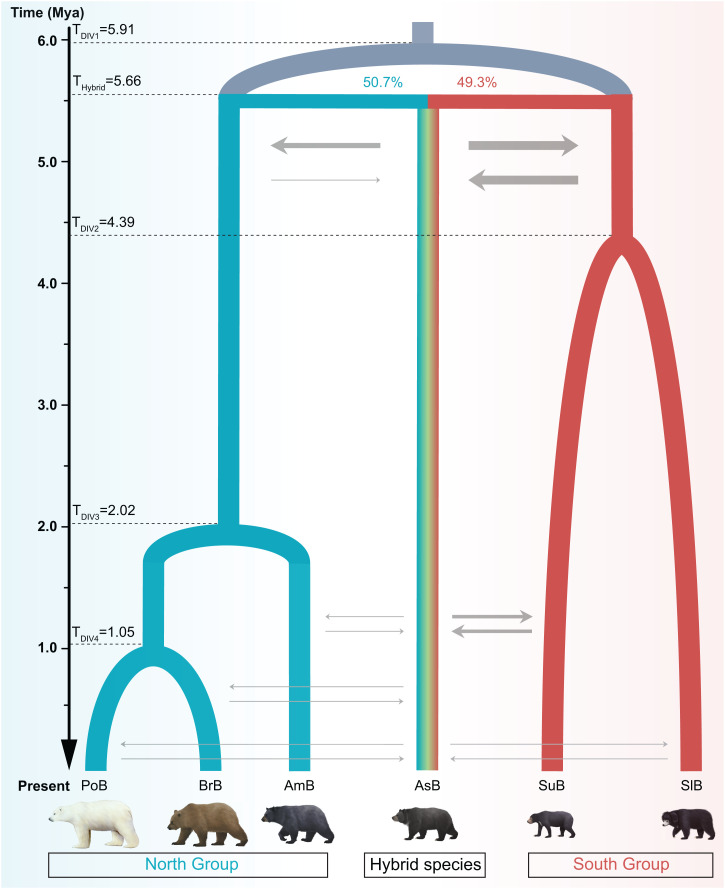
To further test the hybrid origin of Asiatic black bears, we used fastsimcoal2 to simulate and infer the most likely evolutionary scenario. Model comparisons showed that the model of hybrid speciation, i.e., hybridization after the split of the North and South groups followed by secondary gene flow, fitted better than those in which bifurcating speciation was followed by introgression (SI Appendix, Fig. S4 and Table S6). This best-fitting model (Fig. 3 and SI Appendix, Tables S6 and S7) indicated that the two parental lineages of Asiatic black bears, i.e., the ancestral North Group and the ancestral South Group, diverged at ∼5.91 Ma (with a 95% confidence interval of 4.57 to 5.94 Ma). Shortly after this split (∼0.25 My later, corresponding to 25,000 generations later, i.e., ∼5.66 Ma with a 95% confidence interval of 3.60 to 5.84 Ma), the two lineages hybridized, making almost equal contributions (50.7% from the North Group and 49.3% from the South Group), to establish the ancestral lineage of Asiatic black bears. After that, bidirectional gene flow between the hybrid population and two parental lineages occurred, but the gene flow between the hybrid population and the ancestral South Group was greater than that between it and the ancestral North Group (SI Appendix, Table S7), which may explain the grouping of Asiatic black bears with the South Group observed in the species tree (Fig. 1B). Subsequently, alongside the formation of the other bear species since ∼4.39 Ma (with a 95% confidence interval of 2.47 to 5.53 Ma), there was still substantial gene flow between Asian black bears and other extant bear species (Fig. 3 and SI Appendix, Table S7).
Fig. 3.
Evolutionary scenario simulations of the hybrid origin of the Asiatic black bear. TDIV1 represents the divergence time of the ancestor of subfamily Ursinae; THybrid represents the time of the hybrid origin of the Asiatic black bear; TDIV2 represents the divergence time of the ancestor of the South Group; TDIV3 represents the divergence time of the ancestor of the North Group; TDIV4 represents the divergence time of the ancestor of polar bears and brown bears. Gray arrows represent introgressions between the Asiatic black bear and other bear species before and after species divergence. The thickness of each of these arrows is proportional to the corresponding intensity of gene flow.
In order to better understand the mechanisms by which these species hybridized, we reconstructed an mt genome and a Y chromosome gene tree (SI Appendix, Fig. S5). As was previously shown, based on a smaller number of Ursidae bear individuals than in the present study (3, 4), the Y chromosome gene tree was identical to the species tree (SI Appendix, Fig. S5A), while the mt genome tree clustered Asiatic black bears with American black bears in the North Group, and sun bears in the South Group was closely related to them, leaving the sloth bear of the South Group at the base of the Ursinae tree (SI Appendix, Fig. S5B). Gene flow analyses of mt genomes based on MIGRATE-N (SI Appendix, Table S8) indicated strong gene flow from Asian black bears to both brown bears and American black bears of the North Group. This signal, however, was likely diluted in brown bears due to high levels of gene flow from polar bears, leading to the clustering of American black bears as a sister group to Asiatic black bears. Gene flow analyses of mt genomes based on MIGRATE-N (SI Appendix, Table S8) further suggested that gene flow from the North Group and Asiatic black bears to sun bears was stronger than that to sloth bears. This result could explain the mt genome tree topology, in which sun bears clustered with the North Group and Asiatic black bears rather than sloth bears. This observation is also consistent with the fact that the effective population size for the mt genome is substantially smaller than that for the nuclear genome (18), which would facilitate the fixation of an introgressed mt genome.
Ursinae species exhibit the behavioral traits of male-biased dispersal and female philopatry (14, 19, 20). Combined with the mt genome results, in which all Asiatic black bears were clustered with the North Group and also showed gene flow to North Group bears, we suggest that Asiatic black bears may have originated from sex-biased hybridization through migration from males of the ancestral South Group to the females of the ancestral North Group, followed by matrilineal introgression.
Genetic Basis of the Intermediate Body Size in Asiatic Black Bears.
The Asiatic black bear possesses morphological features, particularly body size, that are intermediate between those of the two assumed parental lineages (13, 14), potentially as a result of its hybrid origin (21). To test this hypothesis, we performed genomic scans using a modified HKA test (22) and a method to detect positively selected genes (PSGs) that had diverged between parents before hybridization and were then inherited from one parent by the hybrid species (23). We identified 539 PSGs that were inherited by Asiatic black bears from the North Group and 702 PSGs from the South Group (Fig. 4 A and B).
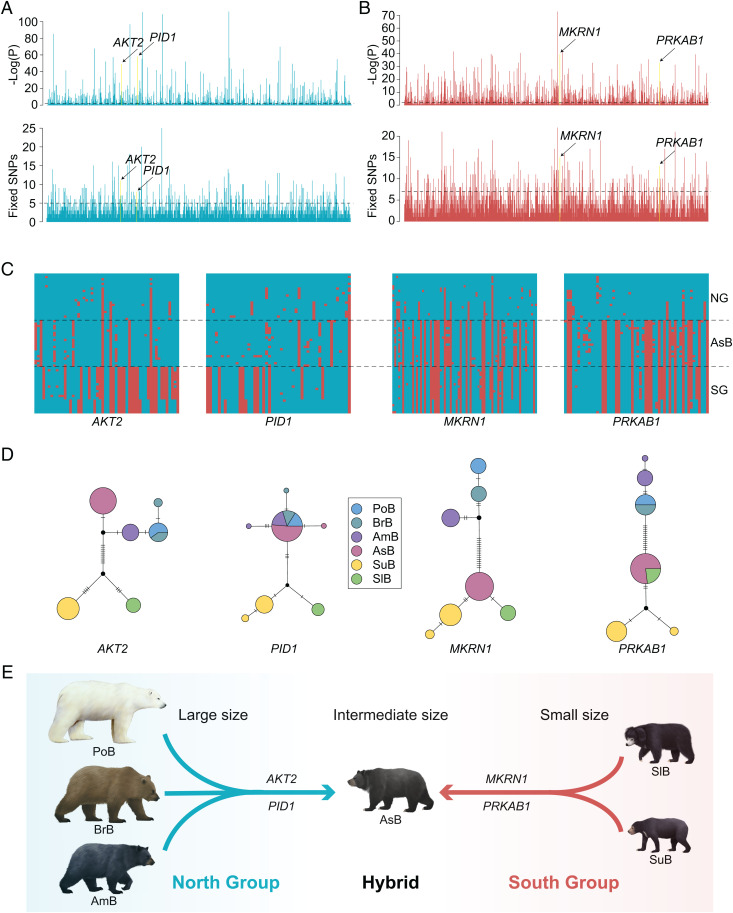
Fig. 4.
Genetic basis of the intermediate body size of Asiatic black bears. (A) Genomic selective scans of HKA test and population-specific fixed mutation calculation for the merged populations of AsB and NG. Two candidate PSGs in the top 20 are shown. (B) Genomic selective scans of HKA test and population-specific fixed mutation calculation for the merged populations of AsB and SG. Two candidate PSGs in the top 20 are shown. (C) Haplotype clustering analysis of the four candidate PSGs. (D) Haplotype networks for the four candidate PSGs. (E) Schematic illustration of the genetic basis of the intermediate body size in the Asiatic black bear relative to its parental lineages.
Gene Ontology (GO) analyses revealed that 111 PSGs out of these two sets were significantly enriched for biological processes related to body weight (GO: 0006869: lipid transport; GO: 0045834: positive regulation of lipid metabolic process; GO: 0032787: monocarboxylic acid metabolic process; GO: 0008289: lipid binding; GO: 0070542: response to fatty acid) (SI Appendix, Fig. S6). Among the top 20 PSGs that were shared between Asiatic black bears and each of the two parental lineages, respectively (SI Appendix, Tables S9 and S10), four of them (AKT2, PID1, MKRN1, and PRKAB1) (Fig. 4 A and B) have been reported to be related to body weight loss or gain in humans and mice (24–27).
Haplotype network analyses showed that the Asiatic black bear haplotypes for the AKT2 and PID1 genes clustered within those of large-sized North Group bears, while the Asiatic black bear haplotypes for the MKRN1 and PRKAB1 clustered within those of small-sized South Group bears (Fig. 4 C and 4D). Previous functional studies in mice indicated that AKT2 deficiency resulted in weight loss (27), while PID1 overexpression resulted in weight gain (25). The MKRN1 and PRKAB1 genes, on the other hand, are both involved in glucose and lipid metabolisms, and their deficiency led to glucose consumption and lipid accumulation, and ultimately weight gain (24, 26).
SNPs under selection in these four genes (SI Appendix, Table S11) were located in either the promotor region or the coding sequence region, possibly leading to functional changes by regulating gene expression or altering amino acid sequences (SI Appendix, Fig. S7). The SNPs in AKT2 (NW_007907093.1: 1827300, G > T), PID1 (NW_007907114.1: 9950344, T > C), and PRKAB1 (NW_007907289.1: 3048277, A > G), which were fixed in both Asiatic black bears and one parental lineage but showed extreme divergence from the other parental lineage (FST = 0.98 or 1.0), were all located in the promotor regions, and moreover they were in transcription factor binding sites (SI Appendix, Fig. S7A). The SNP (NW_007907159.1: 7227213, C > G) in MKRN1, which was fixed in both Asiatic black bears and the South Group where it was highly divergent from that in the North Group (FST = 1.0) (SI Appendix, Fig. S7B), was located in the coding sequence and caused an amino acid alteration from nonpolar Ala to polar Gly, leading to changes in the tertiary structure of the protein (SI Appendix, Fig. S7B). Taken together, our results suggest that these genes identified from the two different parental lineages likely contributed to the establishment of the intermediate body size observed in Asiatic black bears (Fig. 4E).
Development of RI between the Hybrid Species Asiatic Black Bear and Its Two Parental Lineages.
The initial hybrid population of Asiatic black bears had to develop RI barriers in order to evolve independently (23). The intermediate body size of Asiatic black bears due to alternate inheritance of related genes from each parent may have resulted in premating isolation through sexual selection. Asiatic black bears may have inherited more diverged genes from each of the two parental lineages, especially those giving rise to Bateson–Dobzhansky–Muller (BDM) incompatibility between two parents. If such genes were alternately inherited, postmating RI could have arisen due to the new hybrid combination of BDM incompatibility loci (28, 29). Among 1,200 PSGs which had diverged between the parents and were alternately inherited by Asiatic black bears (Fig. 4 A and B), we found that 16 genes (SI Appendix, Fig. S8 and Table S12) were involved in reproductive processes, including male fertility, folliculogenesis, fertilization, sperm development, and meiosis (https://www.genecards.org/). If diverged alleles at these loci were involved in BDM incompatibility between the two parents, their recombination in Asiatic black bears due to alternate inheritance could immediately cause postmating RI (28, 29). In addition, we found that Asiatic black bears had developed a high level of genetic divergence from both parental lineages at numerous loci, and 12 of these were involved in the reproductive process (SI Appendix, Fig. S9 and Table S13). These genes that evolved rapidly since the origin of Asiatic black bears may have further reinforced its postmating RI from the two parental lineages.
Discussion
Phylogenetic discordance in Asiatic black bears has been reported previously (3, 4) and generally attributed to hybridization, but the evolutionary process, that is, whether it was introgression or hybrid speciation that occurred, has not hitherto been explicitly explored and tested. Here, we revisit this paradigm on a much finer scale. Our analyses provide evidence supporting the hybrid origin of the Asiatic black bear, which derives ∼50.7% of its ancestry from the ancestral North Group (polar, black and brown bears) and ∼49.3% from the ancestral South Group (sun and sloth bears). We show that this ancient hybridization took place 5.66 Ma (3.60 to 5.84 Ma), shortly after the divergence of the South and North Groups (5.91 Ma; 4.57 to 5.94 Ma). According to paleontological records, the ancestor of the Ursinae started to diversify in Eurasia near the Miocene–Pliocene boundary (30), so we speculated that the hybridization that established the Asiatic black bear occurred in Eurasia, which is consistent with the fossil record from Moldova (an Eastern European country) of the Asian black bear in the early Pliocene (31). This hybridization and the establishment of the Asiatic black bear might have been triggered by climate-driven population expansion, migration, and contraction during the early Pliocene (30), given that it has been suggested that the dramatic Miocene–Pliocene climatic changes linked with glaciations and the opening of the Bering Strait promoted a major wave of Ursinae species burst that occurred during this period (32).
Interestingly, our analyses reveal frequent gene flow between Asiatic black bears and species belonging to both North and South Groups following the first ancient hybridization event (Fig. 3), which is likely to have been associated with the existence of a substantial overlap in geographic range between them. In addition, it has been suggested that the Bering land bridge connected eastern Asia and North America several times during the Pleistocene, thus providing the opportunity for Asiatic black bears to make contact with American black bears (3, 33). Thus, Asiatic black bears might have experienced gene flow with these bears when in contact with them throughout the early Pliocene into the late Pleistocene, as suggested by introgression of Asiatic black bears with them during this period (Fig. 3).
As a result of this ancient hybridization, Asiatic black bears could have inherited highly diverged alleles at many loci from each parental lineage alternately. Our analyses show that many of these genes may be involved in both premating and postmating RI. The alternate fixation of alleles from each parental species also likely led to the intermediate state of phenotypic traits found in Asiatic black bears, particularly body size (34). Body size in bears (and other mammals) is positively correlated with sexual dimorphism (35), which in turn is strongly associated with the polygynous breeding system (14, 36) (SI Appendix, Table S1). It is therefore possible that the intermediate body size of Asiatic black bears also promoted the establishment of premating RI from its two parental lineages. Interestingly, a direct effect of differences in body size on mate choice or assortative mating has been reported in Canis species (37) and stickleback populations (38), supporting the existence of a relationship between body size and premating RI. In addition, many of the alternately inherited genes (SI Appendix, Fig. S8 and Table S12) are involved in the reproductive process, suggesting that hybridization is likely to have played a role in directly creating postmating RI due to BDM incompatibility RI between this hybrid species and its two parents (28, 29). Overall, these findings reveal the hybrid origin of Asiatic black bears because alternate inheritance of diverged alleles at numerous loci through hybridization not only resulted in its body size, intermediate between the other two groups of species, but also directly contributed to both premating and postmating RI from its two parents, establishing it as an independent lineage.
Our discovery has profound implications for the understanding of mammalian speciation. Up to now, most reports on mammals have been about postspeciation introgression, while explicit examples of new species having formed through the process of hybrid speciation are very scarce, as opposed to the situation in plants which can accommodate polyploidy (39, 40), making the evolutionary role of hybridization in mammals as yet poorly understood. The present results indicate that the Asiatic black bear was established through a hybrid speciation scenario. The increasing number of genomic studies being carried out will be expected to potentially reveal more evidence of speciation via hybridization and document the importance of hybridization as an effective catalyst of new species formation and phenotypic evolution in mammals.
Materials and Methods
Sample Collection and Genome Sequencing.
For genomic DNA sequencing, 43 bears, consisting of 18 Asiatic black bears, 5 American black bears, 14 sun bears, 5 sloth bears, and 1 spectacled bear, were collected from the Animal Branch of the Germplasm Bank of Wild Species of the Chinese Academy of Science and from the San Diego Zoo (SI Appendix, Table S2). Genomic DNA was extracted following the standard phenol/chloroform protocol. Paired-end 125- or 150-bp libraries were sequenced using the Illumina HiSEq. 2500 or 4000 sequencing platforms.
In addition, short-read library reads were collected from previously published genomic sequences for 18 bears: 2 Asiatic black bears, 3 American black bears, 1 sloth bear, 6 polar bears, and 6 brown bears (1, 4, 10, 41–43) (SI Appendix, Supplementary Text and Table S2). In total, 60 bears representing all six Ursinae bear species, comprising 20 Asiatic black bears, 8 American black bears, 14 sun bears, 6 sloth bears, 6 polar bears, and 6 brown bears, and 1 spectacled bear outgroup, were used for population genomic analyses (SI Appendix, Supplementary Text and Table S2).
Resequencing Data Processing and SNP Calling.
All the Illumina paired-end reads were trimmed using filtering criteria to obtain high-quality paired-end reads (SI Appendix, Supplementary Text). We used BWA-MEM version 0.78 (44) to align the trimmed paired-end reads from all the bears to the available de novo assembled polar bear reference genome (UrsMar_1.0) (41). Binary sequence alignment files were generated and sorted using SAMtools version 0.1.18 (45). Duplicate reads were removed using Picard version 1.87 (broadinstitute.github.io/picard). Realignment around INDEL (insert and deletion) regions was performed using the IndelRealigner algorithm with the default settings (GATK version 3.7) (46, 47).
Genome-wide SNPs were identified using GATK version 3.7 (46, 47). The raw SNPs were filtered to obtain high-quality variants using a custom script (SI Appendix, Supplementary Text). To obtain autosomal SNPs for subsequent analyses, we excluded SNPs (∼2.27 million) in scaffolds which were identified as being linked to the X and Y chromosomes (8, 10).
Mt Genomes and Y Chromosome Sequence Data Identification.
The mt genomes of all bears were determined according to consensus sequences between the aligned short reads and the corresponding published 16-kb mt genomes of seven bear species (2, 48) using BWA-MEM (44).
The Y chromosome sequences of all male bears were determined according to consensus sequences between the aligned short reads and the polar bear Y chromosomes (∼1.14 Mb in length) using BWA-MEM. For bears for which sex information was unavailable, we compared genomic coverage on the X chromosome and Y chromosome with genomic coverage on autosomal chromosomes in order to identify their sex (SI Appendix, Supplementary Text). The reliability of the results was confirmed by applying the same approach to other bears for which the sex was known, and consistent results were obtained. Finally, Y chromosome sequences were determined for a total of 42 male bears.
Based on BAM files, the consensus mt genomes and Y chromosome sequences were filtered using the mpileup module of SAMtools (45) and aligned using MAFFT version 7.486 (49). Ambiguous sites were removed using Gblocks version 0.91 (gap = all) (50).
Phylogenetic Analyses.
The spectacled bear was used as the outgroup in all phylogenetic analyses. We used two species tree estimation methods to reconstruct the bear phylogeny based on our dataset. One method was MP-EST (51) analyses based on all 100-kb window sequences and protein-coding gene sequences, respectively. To overcome the computation time limitation, one individual with a high level of sequencing coverage (>20-fold) from each bear species was used. The corresponding six genomic consensus sequences were extracted from the BAM file following the GATK pipeline. With the six genomic consensus sequences of Ursinae species and one spectacled bear, windowed sequences with different window-size gradients (50, 100, and 200 kb) and all protein-coding gene sequences were generated. 22,105 genomic windowed trees (100 kb) and 13,529 protein-coding gene trees were first reconstructed using RAxML version 8.2.11 (52) with the GTRGAMMAI model and 100 bootstrap replicates, and then the species trees were estimated using MP-EST. Five independent runs and five searches for each run were performed to check the consistency of the results.
The other method was SVDquartets (53) analysis implemented in PAUP4a168 (54) based on all autosomal SNPs from the 61 bears. The autosomal SNPs were thinned with a 10-kb window size. The resulting 217,935 SNPs were retained for the species tree analysis.
Phylogenetic network analyses were performed using SplitsTree version 5 (55) based on 22,105 genomic windowed trees (under 100-kb window size) with various threshold settings (5%, 10%, 20%, and 30%) to explore phylogenetic conflict among the bear species.
Mt genome and Y chromosome sequence analyses were also performed for the ML tree estimation as described above.
Genome-wide D-Statistics (ABBA-BABA) Test.
The 217,935 thinned autosomal SNPs from 61 bears were used for genome-wide D-statistics (ABBA-BABA test) (56, 57). The qpDstat tool implemented in the ADMIXTOOLS package was applied to calculate D-statistics and Z-scores based on weighted block jackknife tests (block size of 5 Mb) (56, 57). The D-statistics/ABBA-BABA test was conducted based on the topology of a given species tree, such as “(((P1, P2), P3), O)” in which P1, P2, and P3 represent the three ingroup species and “O” is the outgroup (56, 57). To detect whether the ancestral North Group had contributed genetic ancestry to the hybrid species Asiatic black bear, on the basis of the bear species tree topology, the outgroup spectacled bear was set as “O.” Asiatic black bear was set as “P1.” All South Group bear species as a whole were set as “P2.” Each of the three bear species in North Group, including American black bears, polar bears, and brown bears, and all North Group bear species as a whole, were set to “P3.” Thus, there were four strategies, as shown in SI Appendix, Table S3. An absolute value of Z-score greater than 3 indicated a significant hybridization signal.
HyDe, PCA, and FST Analyses.
Based on 225,196 thinned SNPs (from autosomes and sex chromosomes) for the 61 bears, the HyDe program (58) was used to detect hybridization; this software implements a site-pattern probabilities method to detect population(s) that have arisen via hybrid speciation as well as their putative parental populations and estimates the inheritance parameter, γ, to quantify the genomic contributions of the parents to the hybrid (SI Appendix, Supplementary Text). PCA was carried out using smartPCA from EIGENSOFT v7.2.0 (59) based on 172,516 thinned SNPs from noncoding regions (on autosomes and sex chromosomes) from 60 bears of the six Ursinae bear species. The distribution of fixed differences along the genome was measured by FST across all 100-kb windows (with a 50-kb step) in three pairs of taxa (Asiatic black bear vs. North Group, Asiatic black bear vs. South Group, and North Group vs. South Group) using VCFtools version 0.1.14 (60).
Detection of Hybridization Based on Shared Variations.
An approach for detecting hybridization recently developed by Jiang et al. (16) was used; it can differentiate between hybridization and ancestral subdivision (16). In this study, differences in the patterns of distribution of AVs and PIVs in Asiatic black bear and its parental lineages were used to infer the hybrid speciation of Asiatic black bears (SI Appendix, Supplementary Text). We applied this analysis based on the high-quality INDELs dataset (∼14.52 million) and SNPs (∼100.96 million) (SI Appendix, Supplementary Text).
A random sampling strategy with 100 independent repeats was applied in this analysis, in which one individual from each of the three lineages (e.g., North Group, South Group, and Asiatic black bears) was used. Based on the averaged shared INDELs/SNPs numbers of three lineage pairs, i.e., South Group and hybrid species Asiatic black bear (SH), North Group and hybrid species Asiatic black bear (NH), South Group and North Group (SN), KKSC test (17) was firstly used for checking the robustness of hybrid speciation scenario of Asiatic black bears, and then with the results of 100 independent repeats, the significant differences in genomic distributions of shared AVs or PIVs among the three lineage pairs were evaluated by Student’s t test (implemented in R), which can be further used to test the Non-HHS model, i.e., (North Group, (South Group, Asiatic black bear)), and the HHS model, i.e., (South Group (Asiatic black bear) North Group).
Evolutionary Simulations of the Hybrid Origin of Asiatic Black Bears.
Based on 132,157 thinned autosomal SNPs in noncoding regions from 60 bears of the six Ursinae bear species, we used the maximum-composite-likelihood approach of two-dimensional unfolded site frequency spectra (2D-unfoldSFSs) implemented in fastsimcoal2 v26 (61) to assess the fit of various models and to infer the optimal evolutionary scenario for the origin of Asiatic black bears (SI Appendix, Fig. S4).
Genomic Selection Scans of the Asiatic Black Bear and Its Parental Lineages.
We applied an HKA test (22) and population-specific fixed mutations (23) to identify PSGs inherited by the Asiatic black bear from each of its parental lineages (SI Appendix, Supplementary Text). Genes with a significant P value (<0.01) in the HKA test, and with the number of fixed differences ranked ≥95th quantile in genomic population-specific fixed mutation calculation, were considered to be candidate PSGs. Functional enrichment analyses for these genes were performed using the METASCAPE resource (62). For the candidate genes in which we were interested, haplotypes were constructed with DnaSP version 6.12.03 (63). Haplotype network analyses and haplotype clustering were performed using PopART version 1.7 (64) and the TCS method (65) was applied to infer the genetic origin of each gene. The functional effects of positively selected SNPs in promotor regions were assessed by scanning the JASPAR database (jaspar.genereg.net). The protein structures of the positively selected nonsynonymous substitution SNPs were predicted by I-TASSER (66).
Identifying Genes Highly Diverged between Asiatic Black Bear and Both of Its Parental Lineages.
We applied PBS statistics (67) using the following formula: PBSAsB = 1/2(TAsB_NG + TAsB_SG − TNG_SG) (SI Appendix, Supplementary Text). We took the top 1% of windows in PBSAsB genomic scans as regions with significant signals, and the annotated genes were defined as cases of extreme genetic divergence between the Asiatic black bear and its two parental lineages.
Supplementary Material
Acknowledgments
We thank native English speakers Dr. John Blackwell and his colleagues for polishing the English of the manuscript. We thank Oliver Ryder, who provided helpful comments on the manuscript. This project was supported by grants from the National Natural Science Foundation of China to Y.L. (31925006) and Strategic Priority Research Program of Chinese Academy of Sciences to J.L. (XDB31000000). This work was supported by the Animal Branch of the Germplasm Bank of Wild Species, Chinese Academy of Sciences.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120307119/-/DCSupplemental.
Data Availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa/) under accession number CRA005022 (68).
References
- 1.Miller W., et al. , Polar and brown bear genomes reveal ancient admixture and demographic footprints of past climate change. Proc. Natl. Acad. Sci. U.S.A. 109, E2382–E2390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L., Li Y. W., Ryder O. A., Zhang Y. P., Analysis of complete mitochondrial genome sequences increases phylogenetic resolution of bears (Ursidae), a mammalian family that experienced rapid speciation. BMC Evol. Biol. 7, 198 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutschera V. E., et al. , Bears in a forest of gene trees: Phylogenetic inference is complicated by incomplete lineage sorting and gene flow. Mol. Biol. Evol. 31, 2004–2017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar V., et al. , The evolutionary history of bears is characterized by gene flow across species. Sci. Rep. 7, 46487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preuß A., Gansloßer U., Purschke G., Magiera U., Bear-hybrids: Behaviour and phenotype. Zool Garten N F 78, 204–220 (2009). [Google Scholar]
- 6.S. I, Polar Bears: The Natural History of a Threatened Species (Fitzhenry and Whiteside, Brighton, MA, 2011). [Google Scholar]
- 7.Hailer F., et al. , Nuclear genomic sequences reveal that polar bears are an old and distinct bear lineage. Science 336, 344–347 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Bidon T., et al. , Brown and polar bear Y chromosomes reveal extensive male-biased gene flow within brother lineages. Mol. Biol. Evol. 31, 1353–1363 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Edwards C. J., et al. , Ancient hybridization and an Irish origin for the modern polar bear matriline. Curr. Biol. 21, 1251–1258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahill J. A., et al. , Genomic evidence for island population conversion resolves conflicting theories of polar bear evolution. PLoS Genet. 9, e1003345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahill J. A., et al. , Genomic evidence of geographically widespread effect of gene flow from polar bears into brown bears. Mol. Ecol. 24, 1205–1217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow A., et al. , Partial genomic survival of cave bears in living brown bears. Nat. Ecol. Evol. 2, 1563–1570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak R. M., Paradiso J. L., Walker’s Mammals of the World (Johns Hopkins University Press, 1983), Vol. 18. [Google Scholar]
- 14.Stirling I. D., Derocher A. E., “Factors affecting the evolution and behavioral ecology of the modern bears” in Bears: Their Biology and Management (International Association of Bear Research and Management, 1990), vol. 8, pp. 189-204. [Google Scholar]
- 15.Wu J., et al. , Phylogeographic and demographic analysis of the Asian Black Bear (Ursus thibetanus) based on Mitochondrial DNA. PLoS One 10, e0136398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y., et al. , Differentiating homoploid hybridization from ancestral subdivision in evaluating the origin of the D lineage in wheat. New Phytol. 228, 409–414 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Kuritzin A., Kischka T., Schmitz J., Churakov G., Incomplete lineage sorting and hybridization statistics for large-scale retroposon insertion data. PLOS Comput. Biol. 12, e1004812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballard J. W., Whitlock M. C., The incomplete natural history of mitochondria. Mol. Ecol. 13, 729–744 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Nakagome S., Pecon-Slattery J., Masuda R., Unequal rates of Y chromosome gene divergence during speciation of the family Ursidae. Mol. Biol. Evol. 25, 1344–1356 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Nakagome S., Mano S., Hasegawa M., Ancestral polymorphisms and sex-biased migration shaped the demographic history of brown bears and polar bears. PLoS One 8, e78813 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavárez J., Linares M., Homoploid hybrid speciation in animals. Mol. Ecol. 17, 4181–4185 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Hudson R. R. K., Kreitman M., Aguadé M., A test of neutral molecular evolution based on nucleotide data. Genetics 116, 153–159 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z., et al. , Hybrid speciation via inheritance of alternate alleles of parental isolating genes. Mol. Plant 14, 208–222 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Gruzman A., Babai G., Sasson S., Adenosine monophosphate-activated protein kinase (AMPK) as a new target for antidiabetic drugs: A review on metabolic, pharmacological and chemical considerations. Rev. Diabet. Stud. 6, 13–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L., et al. , PID1 in adipocytes modulates whole-body glucose homeostasis. Biochim. Biophys. Acta. Gene Regul. Mech. 1861, 125–132 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lee M. S., et al. , Loss of the E3 ubiquitin ligase MKRN1 represses diet-induced metabolic syndrome through AMPK activation. Nat. Commun. 9, 3404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Gurmaches J., Martinez Calejman C., Jung S. M., Li H., Guertin D. A., Brown fat organogenesis and maintenance requires AKT1 and AKT2. Mol. Metab. 23, 60–74 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermansen J. S., et al. , Hybrid speciation through sorting of parental incompatibilities in Italian sparrows. Mol. Ecol. 23, 5831–5842 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Schumer M., Cui R., Rosenthal G. G., Andolfatto P., Reproductive isolation of hybrid populations driven by genetic incompatibilities. PLoS Genet. 11, e1005041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLellan B. R., Reiner D. C., A review of bear evolution. Int. Conf. Bear Res. Manage. 9, 85-96 (1994). [Google Scholar]
- 31.Baryshnikov G. F., Zakharov D. S., Early Pliocene bear Ursus thibetanus (Mammalia, Carnivora) from Priozernoe Locality in the Dniester Basin (Moldova Republic). Proc. Zool. Inst. RAS 317, 3-10 (2013). [Google Scholar]
- 32.Krause J., et al. , Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary. BMC Evol. Biol. 8, 220 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffecker E. S., The Human Ecology of Beringia (Columbia University Press, New York, 2007). [Google Scholar]
- 34.Yakimowski S. B., Rieseberg L. H., The role of homoploid hybridization in evolution: A century of studies synthesizing genetics and ecology. Am. J. Bot. 101, 1247–1258 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Clutton-Brock T. H., Harvey P. H., Rudder B., Sexual dimorphism, socionomic sex ratio and body weight in primates. Nature 269, 797–800 (1977). [DOI] [PubMed] [Google Scholar]
- 36.Trivers R. L., “Parental investment and sexual selection” in Sexual Selection and the Descent of Man, 1871-1971, Campbell B., Ed. (Aldine, Chicago, 1972), pp. 136–179. [Google Scholar]
- 37.Hinton J. W., Gittleman J. L., van Manen F. T., Chamberlain M. J., Size-assortative choice and mate availability influences hybridization between red wolves (Canis rufus) and coyotes (Canis latrans). Ecol. Evol. 8, 3927–3940 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinnon S. M., Mori S., Blackman B. K., David L., Kingsley D. M., Jamieson L., Chou J., Schluter D., Evidence for ecology’s role in speciation. Nature 429, 292–298 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Mallet J., Hybrid speciation. Nature 446, 279–283 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Larsen P. A., Marchán-Rivadeneira M. R., Baker R. J., Natural hybridization generates mammalian lineage with species characteristics. Proc. Natl. Acad. Sci. U.S.A. 107, 11447–11452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S., et al. , Population genomics reveal recent speciation and rapid evolutionary adaptation in polar bears. Cell 157, 785–794 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava A., et al. , Genome assembly and gene expression in the American black bear provides new insights into the renal response to hibernation. DNA Res. 26, 37–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu C., et al. , Draft genome assembly for the Tibetan black bear (Ursus thibetanus thibetanus). Front. Genet. 11, 231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H., Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., et al. ; 1000 Genome Project Data Processing Subgroup, The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna A., et al. , The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DePristo M. A., et al. , A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delisle I., Strobeck C., Conserved primers for rapid sequencing of the complete mitochondrial genome from carnivores, applied to three species of bears. Mol. Biol. Evol. 19, 357–361 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Katoh K., Misawa K., Kuma K., Miyata T., MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castresana J., Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Liu L., Yu L., Edwards S. V., A maximum pseudo-likelihood approach for estimating species trees under the coalescent model. BMC Evol. Biol. 10, 302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamatakis A., RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chifman J., Kubatko L., Quartet inference from SNP data under the coalescent model. Bioinformatics 30, 3317–3324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronquist F., Teslenko M., Vandermark P., Ayres D., Sowfford D., PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods). Version 4 (Sinauer Associates, Sunderland, MA, 2002). [Google Scholar]
- 55.Huson D. H., Bryant D., Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Green R. E., et al. , A draft sequence of the Neandertal genome. Science 328, 710–722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand E. Y., Patterson N., Reich D., Slatkin M., Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 28, 2239–2252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blischak P. D., Chifman J., Wolfe A. D., Kubatko L. S., HyDe: A python package for genome-scale hybridization detection. Syst. Biol. 67, 821–829 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patterson N., Price A. L., Reich D., Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danecek P., et al. ; 1000 Genomes Project Analysis Group, The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Excoffier L., Dupanloup I., Huerta-Sánchez E., Sousa V. C., Foll M., Robust demographic inference from genomic and SNP data. PLoS Genet. 9, e1003905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y., et al. , Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozas J., et al. , DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 34, 3299–3302 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Leigh J. W., Bryant D., Nakagawa S., popart: Full‐feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015). [Google Scholar]
- 65.Clement M., Posada D., Crandall K. A., TCS: A computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Roy A., Kucukural A., Zhang Y., I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yi X., et al. , Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou T., et al. , Uncovering the enigmatic evolution of bears in greater depth: The hybrid origin of the Asiatic black bear. Genome Sequence Archive: CRA005022. https://ngdc.cncb.ac.cn/gsa/. Deposited 14 September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa/) under accession number CRA005022 (68).