Abstract
Introduction
Ultrasound is the examination of choice for the diagnosis of hypertrophic pyloric stenosis (HPS). A correct diagnosis is dependent on the technique and measurement accuracy. However, in the world literature there is a wide range of values suggested for the diagnosis of this condition. The current minimum measurements used to diagnose HPS seem excessively large, and therefore, we set out to redefine these values.
Methods
A retrospective study was performed on 607 patients (615 scans) being investigated for HPS. The length and transverse diameter of the pyloric canal, and thickness of the pyloric muscle were measured. All results were correlated with clinical and surgical findings.
Results
In this study, the muscle thickness in the normal group was <2.0 mm than in HPS infants having a muscle thickness of 2.0–5.0 mm. All the pyloric canal lengths in the normal group were <5.0 mm than in those with HPS having a length of 10.0–24.0 mm. The transverse diameters ranged from 6.0 to 11.0 mm in the normal group compared with those with HPS having a diameter between 8.0 and 16.0 mm.
Conclusions
The current criteria for sonographic diagnosis of HPS should be redefined. The canal length is the single most important discriminator, with a clear separation between normal and abnormal. The commonly used 16.0‐mm measurement is too long and should be reduced to 10.0 mm (without the risk of false positives). In many cases, the muscle thickness in those with HPS is as low as 2.0 mm, considerably less than the 3.0 mm that is currently used. The transverse diameter is not a useful discriminator for HPS. The use of current values will delay the diagnosis and timely treatment of this condition.
Keywords: children, hypertrophic pyloric stenosis, ultrasound
Introduction
Teele and Smith first described the use of ultrasound in the diagnosis of hypertrophic pyloric stenosis (HPS) in five infants in 1977. 1 In this landmark study, the authors found the anteroposterior diameter of the pylorus in children with HPS to be between 18.0 and 28.0 mm. Since that time, ultrasound has been the examination of choice for the diagnosis of HPS, and in most institutions, it has completely replaced contrast radiography for this purpose. The obvious advantage of using ultrasound is the avoidance of an ionising radiation dose. A review of the literature reveals that the diagnostic criteria for this condition have not changed significantly in the last 30 years (Table 1), with wide discrepancies in values from different studies. However, the authors have been diagnosing HPS (with surgical confirmation) using sonographic measurements that are far smaller than those widely published in the literature, for many years. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 We believe that the published values are no longer applicable and therefore undertook an analysis of cases at our hospital, with a threefold purpose: to establish which measurements are most diagnostic of HPS; to establish more realistic minimum measurements for confident diagnosis of HPS; and to avoid a wide range of values for any one measurement criterion.
Table 1.
Literature review of HPS measurements. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
| References | Year | Pylorus length (mm) | Muscle thickness (mm) | Pylorus diameter (mm) | Data set (n) |
|---|---|---|---|---|---|
| Wilson et al. | 1984 | 20.0 | 4.0 | 13.0 | 29 |
| Haller et al. | 1986 | 18.0 | 4.0 | 25.0 | – |
| Stunden et al. | 1986 | 16.0 | 2.5 | 11.0 | 112 |
| Blumhagen et al. | 1988 | 17.8 | 4.8 | 107 | |
| Okeeffe et al. | 1991 | 3.0 | 40 | ||
| Hernanz‐Schulman et al. | 1994 | 4.0 | 13.0 | 66 | |
| Neilson et al. | 1994 | 16.0 | 2.5 | 11.0 | 66 |
| Lowe et al. | 1999 | 3.0 | 87 | ||
| Leaphart et al. | 2008 | 16.0 | 3.5 | 200 | |
| Huang et al. | 2011 | 14.0 | 4.0 | 214 | |
| Niedzielski et al. | 2011 | 17.0 | 3.0 | 12.0 | 96 |
| Said et al. | 2012 | 15.0 | 3.0 | 189 | |
| Iqbal et al. | 2012 | 15.0 | 3.0 | 67 | |
| Sivitz et al. | 2013 | 17.0 | 3.0 | 10 | |
| Cascio et al. | 2013 | 16.0 | 3.0 | 42 | |
| Khan et al. | 2014 | 16.0 | 3.0 | 14.0 | 50 |
| Ayaz et al. | 2015 | 16.0 | 3.5 | 10.0 | 20 |
| Vinycomb et al. | 2021 | 14.5 | 3.0 | 288 |
HPS, hypertrophic pyloric stenosis.
Methods
We conducted a retrospective study evaluating the pyloric measurements in 615 ultrasound scans (607 patients) performed for suspected HPS from 2000 to 2019. To our knowledge, this is the largest set of data (combined normal and patients with HPS) that have been analysed for suspected HPS.
At our hospital, to allow for proper evaluation of the pylorus and duodenum, we routinely ask that the baby be fasted for 2 h prior to the appointment time. A nasogastric tube (NGT) is inserted by a registered nurse either in emergency or in radiology shortly before the appointment time. Consent for NGT insertion is undertaken by referring medical staff. To confirm that the NGT is in the stomach, an aspirate is tested with a litmus paper. In cases where there is no clear aspirate, we check the NGT position by scanning the gastric cardia. Fluid is only injected after a brief scan has been performed to ensure that there are no contraindications such as an already full stomach or a volvulus. As much as warm sterile water of 15 mL in the stomach is necessary as the starting point to achieve clear visualisation of the antrum, pyloric canal and duodenal cap, and as much as 60 mL may be necessary to obtain the necessary views. This allows control of stomach filling throughout the study and promotes peristalsis, thereby allowing dynamic assessment of the stomach and pylorus. The sterile water is kept in a heater at 37.5°C, to ensure safe and comfortable delivery. The echo‐free water provides a high degree of contrast, which delineates structures of interest and allows accurate measurement of pyloric canal length. In cases when the air in the stomach obscures a view of the pylorus with the patient supine, the examination can be performed with the patient rolled onto the right side so that water fills the antrum, displacing air and acting as an acoustic window. This usually allows clear observation of the activity of the stomach as it pushes water through the canal. The transducer is aligned with the longitudinal axis of the pylorus, thereby allowing assessment of pyloric opening and measurement of canal length. This view also shows whether fluid is passing freely into the duodenum, which can be assessed for evidence of a more distal obstruction such as a duodenal web.
Apart from facilitating accurate measurement of pyloric canal length, the fluid bolus delivered by NGT also allows the duodenal course to be tracked 19 in patients with a normal pylorus and who might otherwise subsequently have a barium contrast study to exclude malrotation as a cause of vomiting. The NGT is removed by the nurse if the pylorus is normal but is left in situ in cases of HPS. We do not usually aspirate any fluid at the completion of the examination.
While the insertion of a NGT tube is relatively easy in a hospital setting, successful filling of the stomach may also be achieved by the drinking water from a bottle (preferably after a 2 h fast). However, it is not uncommon for babies to be reluctant to drink enough water to achieve adequate imaging. While milk can be used instead of water, it is isoechoic with the mucosa and makes accurate measurement of the pyloric canal more difficult. In addition, poor stomach filling is likely to result in reduced peristalsis, making functional assessment more difficult and increasing the likelihood that a collapsed antrum will be misinterpreted as an elongated canal. The authors have seen several cases where a collapsed antrum has been misdiagnosed as pyloric stenosis in a non‐paediatric imaging department. Rarely, the study will be performed without a NGT, such as in cases where the patient age is well outside of the typical age for HPS. We have not experienced any issues with carer or clinician hesitancy with regard to the use of a NGT to ensure an adequate diagnostic study.
The transducer of choice for evaluation of the upper gastrointestinal tract is a small, tightly curved array. The small footprint, curved surface and divergent field of view are useful features, particularly when scanning small babies. Almost all patients in this study were scanned with a tightly curved array transducer (Philips C8‐5, Bothell, WA, USA). The curved surface minimises patient discomfort when increased pressure is required to displace bowel gas. The sector field of view allows good images to be obtained even when the acoustic window is limited by bowel gas. A higher frequency linear array L17‐5 MHz transducer was sometimes used in smaller patients.
Three measurements of the pylorus were obtained (length, diameter and muscle thickness). The length of the canal was measured with the transducer aligned with the longitudinal axis of the pylorus (Figure 1). In HPS cases with a curved elongated canal, a curved measurement tool is used. When the pylorus is normal, endpoints for exact measurement of length are subjective because of the rounded ends of the canal. This is of no consequence, however, because of the clear difference between normal and abnormal canal lengths. The overall diameter and muscle thickness of the pylorus were measured in a transverse view (Figure 2). Measurements in cases of HPS are shown in Figure 3. Measurement accuracy is the best when measured along the axis of the ultrasound beam. Sometimes, however, anisotropy causes the circular muscle to be echogenic anteriorly and posteriorly (where the beams approach the fibres at 90°), making the muscle margins more difficult to see. In these cases, the muscle thickness can be done from side to side (Figure 3c). Care must be taken to avoid misinterpretation of a collapsed antrum as an elongated canal (Figure 4).
Figure 1.
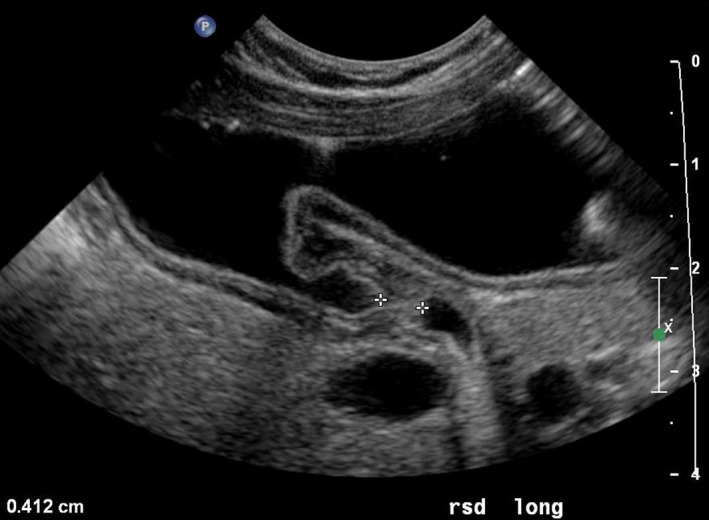
Longitudinal scan (long) of the normal pylorus (transverse section of the abdomen) with the patient in a right lateral decubitus position for exact measurement of the length of the canal. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
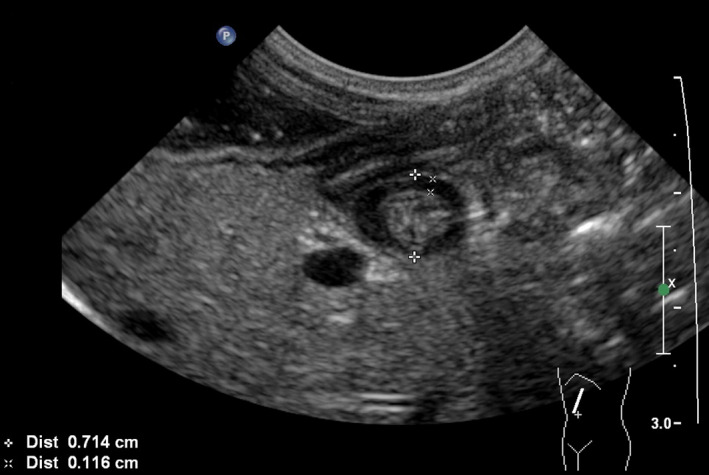
Transverse scan of the normal pylorus (sagittal section of the abdomen) with the patient lying right side down for measurement of the pyloric diameter (7.1 mm) and muscle thickness (1.2 mm). [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
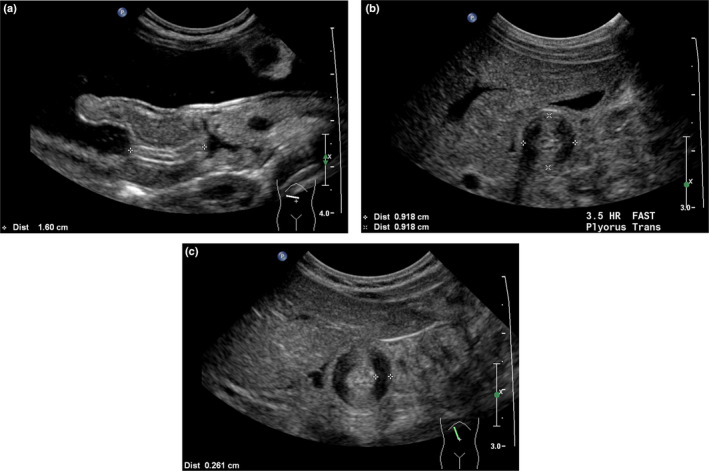
(a) Longitudinal scan of hypertrophic pyloric stenosis (HPS) (transverse section of the abdomen) with the patient lying supine for measurement of the length of the elongated pyloric canal (16.0 mm). (b) Transverse scan of HPS (sagittal section of the abdomen) with the patient supine for measurement of the pyloric diameter (9.2 mm). (c) Transverse axis view of HPS (sagittal section of the abdomen), with the patient supine, showing anisotropy obscuring anterior and posterior muscle margins and side‐to‐side measurement of muscle thickness (2.6 mm). [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
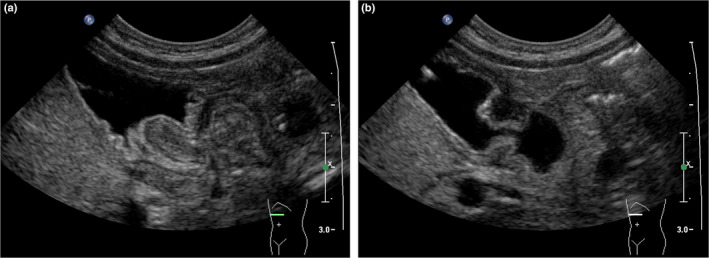
(a) Image of a collapsed distal antrum, taken during the passage of a peristaltic wave simulating an elongated canal, suggestive of hypertrophic pyloric stenosis (HPS). (b) Same patient as in (a). After passage of the wave, the antrum opens and the pylorus is clearly seen to be normal. Image (b) acquired 20 sec after image (a). [Colour figure can be viewed at wileyonlinelibrary.com]
The presence of gastric foveolar cell hyperplasia (FCH) was also recorded. Ultrasound examination of these children demonstrates a polypoid or redundant mucosal fold in the pyloric canal, which often protrudes into the gastric antrum.
All patients diagnosed with HPS in the ultrasound study had the condition confirmed at surgery and underwent a pyloromyotomy. All patients who did not have a diagnosis of HPS at ultrasound were followed up to ensure that they did not present later with HPS or have a pyloromyotomy at our hospital. As the only tertiary paediatric surgery centre in the state of South Australia where this condition is managed, it is highly unlikely any of these patients went elsewhere for a subsequent diagnosis of HPS.
The data were retrospectively analysed to determine whether there is a significant difference between the normal and the HPS patients for all three measurement parameters.
The statistical package used was Systat (Inpixon, Palo Alto, CA, USA). The statistical analysis was performed with regression statistics with a confidence interval of 95%. Microsoft Excel spreadsheet software program was used for the production of graphs.
Results
Age
Of the normal group, 95% of the patients were aged between 0 and 100 days with a mean age of 47.9 days (Table 2). There was no statistical correlation between the age of patients and the pylorus dimensions (length, muscle and diameter), all with a coefficient of correlation (R2) < 0.01. In patients with HPS, 95% were between the ages of 8 and 69 days, with a mean age of 38.4 days. Also, there was no statistical correlation between the age of patients and the pylorus dimensions (R2 < 0.02 for all parameters).
Table 2.
Statistical results of the parameters length, muscle diameter and age for normal and HPS studies.
| Normal | HPS | |||||||
|---|---|---|---|---|---|---|---|---|
| Length (mm) | Muscle (mm) | Diameter (mm) | Age (days) | Length (mm) | Muscle (mm) | Diameter (mm) | Age (days) | |
| Mean | 2.8 | 1.3 | 8.2 | 47.9 | 16.3 | 2.9 | 11.6 | 38.4 |
| Standard deviation | 0.6 | 0.3 | 1.1 | 26.8 | 2.3 | 0.5 | 1.6 | 18.5 |
| Minimum | 1.2 | 0.6 | 6.0 | 0 | 10.0 | 2.0 | 8.0 | 12 |
| Maximum | 4.6 | 2.0 | 11.0 | 154 | 24.0 | 5.0 | 16.0 | 155 |
| Count | 321 | 321 | 321 | 321 | 286 | 286 | 286 | 286 |
HPS, hypertrophic pyloric stenosis.
Length of pyloric canal
The difference in canal length between normal patients and those with HPS is clear, with the normal and HPS distributions being completely separate (Figure 5). The maximum length for the normal group is 4.6 mm, while the minimum length for the HPS group is 10.0 mm (Table 2). The mean and standard deviation (SD) of the distribution of the normal data are 2.8 mm and 0.6 mm, respectively, while the mean and SD of the HPS data are 16.3 and 2.3 mm, respectively. Thus, the mean of the HPS data is 22.5 SD apart from the mean of the normal data, and the minimum length of the HPS group is 9 SD apart from the maximum normal measurement. Given that a length greater than 4.6 mm (+3 SD) establishes 99.7% accuracy for HPS, it is reasonable to accept any length greater than 10.0 mm as 100% diagnostic for HPS. As mentioned earlier, care must be taken that the measurement is of canal length only, not including collapsed antrum.
Figure 5.
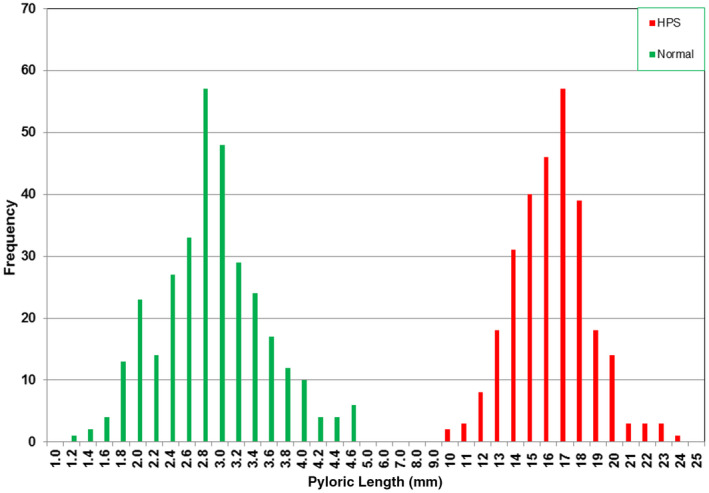
Histogram of the pyloric lengths of the normal and hypertrophic pyloric stenosis groups. [Colour figure can be viewed at wileyonlinelibrary.com]
Muscle thickness
The histograms of the normal and HPS values of muscle thickness are shown in Figure 6. There is a small overlap between the two distributions, with the maximum normal value (2.0 mm) being the same as the minimum HPS value (Table 2). To determine whether the two distributions were significantly different and also to determine the false‐negative and the false‐positive values caused by their overlaps, Gaussian functions were fitted to these distributions. The fitted Gaussian functions are shown in Figure 7. Statistical analysis of the normal and HPS data shows that the difference in the means of the two distributions is significant to <5% level (probability, P > 95%) and that the false‐negative and false‐positive values as shown in Figure 7 were small, 2.9% and 1.8%, respectively. The overlap between normal and abnormal is compounded by the inherent inaccuracy of measurements of fractions of a millimetre when using ultrasound and taking into account the variability of scan technique and measurement accuracy. However, it is clear that many patients in our study with HPS, proven at surgery, had muscle thicknesses much less than the 3.0‐mm threshold referred to in the current literature.
Figure 6.
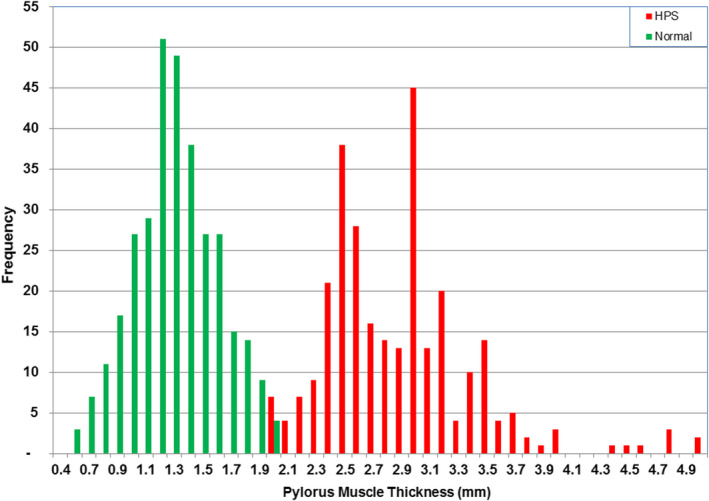
Histogram of muscle thickness of the normal and hypertrophic pyloric stenosis groups. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 7.
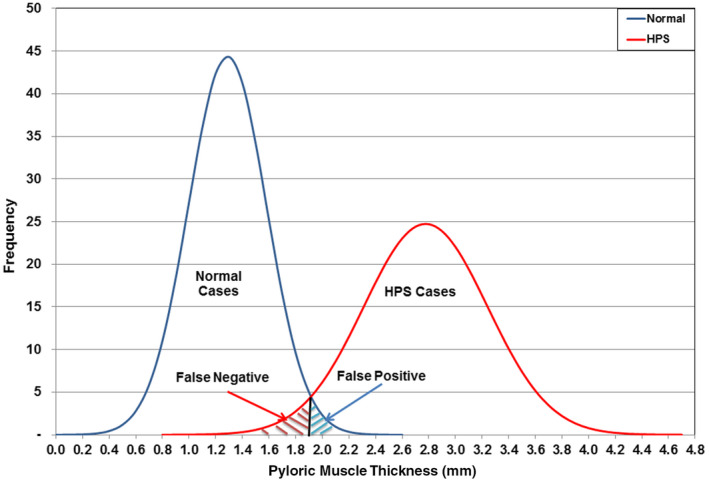
Muscle thickness of the normal and hypertrophic pyloric stenosis groups showing false negatives and false positives. [Colour figure can be viewed at wileyonlinelibrary.com]
Diameter of pylorus
Distributions of the pylorus diameter for the normal and HPS data are shown together in Figure 8. The HPS data (mean 11.6 mm and SD 1.6 mm) have significant overlap with the normal data (mean 8.2 mm and SD 1.1 mm; Table 1). The difference in the two distributions is not significant at the 5% level. The overlap between the two distributions would contribute to 11% false‐negative and 32% false‐positive cases. The measurement of the pylorus diameter in this dataset woudl not accurately determine the presence of HPS.
Figure 8.
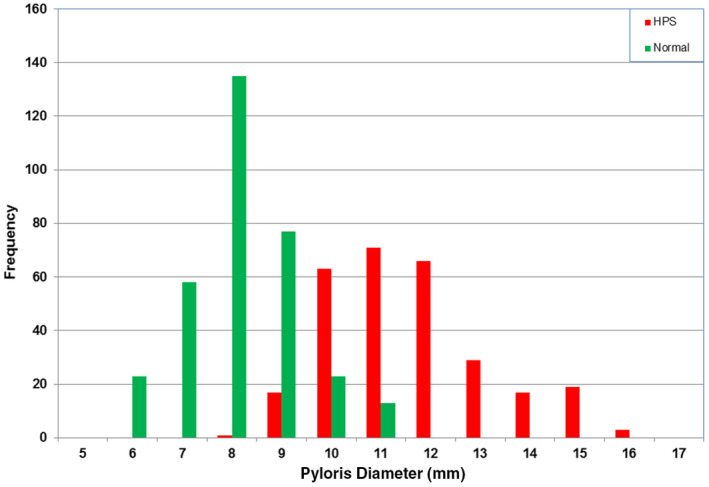
Histogram of the pylorus diameter of the normal and hypertrophic pyloric stenosis groups. [Colour figure can be viewed at wileyonlinelibrary.com]
Correlations
In the normal group, no correlation was found between length and muscle thickness (R2 = 0.01), length and pyloric diameter (R2 = 0.012), and diameter and muscle thickness (R2 = 0.1051). In the HPS group, there was also no correlation between canal length and muscle thickness (R2 = 0.03) or between length and diameter (R2 = 0.04). There is a weak correlation (R2 = 0.40) between muscle thickness and overall diameter of the pylorus. As expected, the diameter increased slightly with muscle thickness.
Gastric foveolar cell hyperplasia
Another finding in the HPS group was the presence of gastric FCH, also known as focal foveolar hyperplasia (FFH), in 29 of 286 (10.1%) patients. The appearance is one of a sausage‐shaped mass beginning in the wall of the distal antrum and extending along the canal, compromising the canal lumen, which appears crescentic in cross section (Figure 9). In patients without HPS, FCH was shown in four cases only (1.2%), one of whom subsequently developed HPS 4 weeks later.
Figure 9.
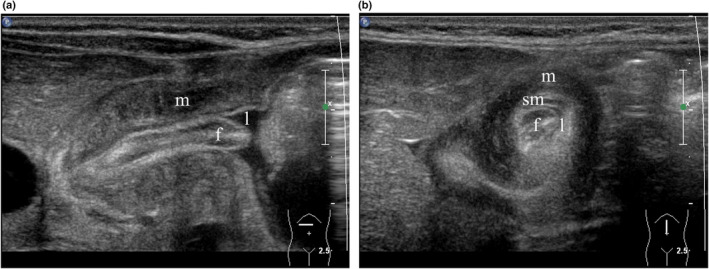
(a) Longitudinal scan of hypertrophic pyloric stenosis (HPS) (transverse section of the abdomen) with the patient supine demonstrating a sausage‐shaped mass representing foveolar cell hyperplasia (FCH) (f) in the pylorus eccentrically narrowing the lumen (l). Note hypertrophied muscle (m). (b) Transverse scan of HPS (sagittal section of the abdomen) with the patient supine. From internal to external, note FCH (f), the eccentric crescentic lumen (l), the submucosa (sm) and the hypertrophied muscle (m). [Colour figure can be viewed at wileyonlinelibrary.com]
Other pathologies
Of the 329 patients who were found to have a normal pylorus, unexpected other pathology was found in 13 cases (4.0%). These included five cases of pelviureteric junction obstruction; single cases of vesicoureteric junction obstruction, medullary nephrocalcinosis, choledochal cyst, hepatoblastoma, malrotation, ileoileal intussusception caused by Meckel's diverticulum and single kidney; and one case showing a small scarred kidney (subsequently shown to have grade 5 vesicoureteric reflux).
Of the HPS group, unexpected other pathology was found in nine of the 286 cases (3.1%). These included two cases of adrenal neuroblastoma; four cases of pelviureteric junction obstruction; and single cases of dominant polycystic kidney disease, duplex kidney with lower pole reflux nephropathy and vesicoureteric junction obstruction.
Discussion
Hypertrophic pyloric stenosis also known as idiopathic HPS but usually abbreviated to pyloric stenosis, is a condition in which there is a lengthening of the pylorus and hypertrophy of its circular and, to a lesser degree, longitudinal muscle fibres, 20 resulting in the compromise of the pyloric canal and outlet obstruction of the stomach. These patients typically present at approximately 5 weeks of age, with a history of increasing projectile vomiting after feeds. There is often a history of poor weight gain or even weight loss. It is well documented that pyloric stenosis develops after birth. A vomiting baby with an unequivocally normal pyloric ultrasound at 3 weeks of age may subsequently develop pyloric stenosis representing the crossover of children with other causes of vomiting who are increasingly referred to the ultrasound department for a probable HPS. Diagnosis after the age of 10 weeks is uncommon but does occur. At presentation, patients with HPS often have a metabolic alkalosis resulting from profuse vomiting with excessive loss of gastric secretions including hydrochloric acid, and this must be corrected prior to surgery.
The original ultrasonic criteria for the diagnosis of HPS were based solely on static images and have not changed in four decades. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 The current consensus in the literature requires a pyloric canal length of at least 16.0 mm and a muscle thickness of at least 3.0 mm for a diagnosis of HPS. However, for many years now, the authors of this paper have been accurately diagnosing HPS using significantly lower measurements.
In this study, the authors have determined (with surgical confirmation) that pyloric lengths as low as 10.0 mm and muscle thicknesses as small as 2.0 mm are diagnostic of HPS, with 102 of 286 (35.7%) HPS cases having a length of <16.0 mm and 157 of 286 (54.9%) HPS cases having a muscle thickness of <3.0 mm minimum used in the literature.
The salient feature of these data is that the length of the pylorus is the single discriminating criterion that distinguishes normal patients from those with HPS (Figure 5), with a clear separation between the two groups. All patients with lengths of 10.0 mm or more were surgically proven to have HPS. Had the 16.0 mm value been used as the minimum diagnostic length, delay in diagnosis would have occurred in 102 of 286 (35.7%) cases. Although delayed diagnosis is not likely to have a long‐term adverse outcome for the patient, it is of benefit to families that a cause for the vomiting is found and corrected sooner rather than later, saving hospital visits and alleviating parental anxiety. Early diagnosis may also reduce the incidence of severe electrolyte imbalance, which needs to be corrected prior to surgery.
Another salient aspect of this study is the discrepancy between normal canal lengths and values previously published. In this study, the maximum length for a normal pylorus was 4.6 mm. Lengths much greater than this are described in the literature as normal, with three articles reporting normal values of >20.0 mm. 4 , 8 , 13 The authors believe that this discrepancy can be partly explained by poorer resolution of older ultrasound systems and by inadvertent inclusion of collapsed antrum when measuring canal length. As stated earlier, this pitfall can be avoided when water is used as a contrast agent, providing clear endpoints for measurement. Measurements should also be made just after the passage of a peristaltic wave so that no collapsed antrum is included.
Current literature suggests a minimum thickness of muscle of 3.0 mm is necessary for the diagnosis of HPS, but this study shows some surgically proven cases with 2.0‐mm thickness, with 16.8% of HPS cases having a thickness of <2.5 mm. There is a paucity of images showing the methodology for measuring muscle thickness in most published articles. This makes it difficult to validate the 3.0‐mm threshold in use to diagnose HPS and to be sure that measurements in this study replicate those from earlier studies. The improvement in spatial resolution since the original measurement criteria were established has been profound, but measurements of fractions of a millimetre are nevertheless unreliable, with absolute values being affected by transducer frequency, gain settings, dynamic range, spatial compounding, technique, plane of section and mensuration accuracy. Given that canal length alone distinguishes normal from abnormal, it seems that absolute muscle thickness is not essential to make the diagnosis of HPS. However, if muscle thickness is used as an indicator of HPS, it is safe to use 2.2 mm rather than 3.0 mm. The measurement of muscle thickness is very useful in the rare cases when pylorospasm is present, potentially resulting in an erroneous canal length. In these cases, the normal muscle thickness will avoid a false‐positive diagnosis of HPS.
Difficulty in obtaining reliable and accurate details about the duration of vomiting retrospectively from carers and case notes meant it was not possible to correlate pylorus length with this variable. Some of the longest canal lengths from this study were in patients said to have had vomiting for only 1 or 2 days. Subjectively, clinicians seem to be requesting an ultrasound for possible HPS with a shorter and shorter suggestive history (even after a single projectile vomit), possibly because of the accuracy of ultrasound and the fact that it does not involve ionising radiation. One might then expect to see shorter canal lengths in the latter part of the study, with the diagnosis being made earlier than previously. This was not the case, however, with the spread of lengths in the HPS group being statistically the same (R2 < 0.01) throughout the 18 years of the study. The constancy of the spread of lengths over the duration of the study is an indicator of the standardised scanning and measurement technique used throughout data acquisition.
Measurements of the overall diameter of the pylorus show considerable overlap between normal and abnormal and therefore have no diagnostic relevance to the diagnosis of HPS.
When scanning the pylorus, it is important to remember that its shape, position and orientation are variable and change significantly with the activity of the stomach. Therefore, for accurate assessment, the pylorus must be scanned in real time, taking time to watch the peristaltic activity of the stomach and the opening and closing of the pyloric canal. When HPS is present, the canal rarely opens as peristaltic waves pass through it. It is not uncommon, however, to see small amounts of fluid pass into the duodenum in patients with HPS as the muscular hypertrophy does not usually cause total obstruction.
Obtaining images without clear endpoints for measurement of the pyloric canal length and pyloric muscle thickness can result in false‐positive diagnoses of HPS. Another cause for a false‐positive diagnosis of HPS is a collapsed antrum and pylorus.
The former can be avoided by being diligent with endpoint placement for measurements, and the latter, by scanning during several peristaltic waves that would result in the antrum and canal opening and allowing antegrade passage of fluid. Failure to recognise both these aspects is likely to result in a false‐positive diagnosis.
In the early years of the data set for this study, nine patients had a repeat scan 2 or 3 days after an initial scan because the measurements were below the accepted values used to diagnose HPS. The reports for each of these initial scans described the findings as ‘consistent with an evolving HPS’, but with measurements below those required to make a definite diagnosis. In all nine cases, measurements taken in the second scan satisfied the HPS diagnostic values. Using the criteria, the authors are suggesting from this study, all of these patients would have had a positive diagnosis at the time of the first scan, and proceeded to pyloromyotomy thereafter. The need for repeat scans was avoided after lengths much less than 16.0 mm were accepted as being indicative of HPS.
While the authors used the instillation of warm water via a NGT tube to achieve adequate filling and contrast of the stomach, we acknowledge that this may be impractical in a non‐hospital setting. In such cases, encouraging the drinking of water via a bottle in the first instance should be attempted to provide better contrast than milk. If this proves unsuccessful, then a bottle of milk or a breastfeed can be used for filling of the stomach. Foveolar cell hyperplasia (FCH) also known as FFH was identified in 10.1% of children with HPS. 21 In this condition, a redundant mucosal fold arises at the distal end of the antrum and extends along the length of the pyloric canal. At ultrasound, it appears as a sausage‐shaped mucosal fold that protrudes into the canal along its full length, significantly compromising the lumen, which is crescentic in cross section. This is best appreciated in a transverse view, which demonstrates the crescentic lumen surrounding the mucosal fold (Figure 9b). FCH is known to occur in isolation but is more commonly associated with HPS. One patient in this series had FCH in an initial ultrasound at 8 days of age and went on to develop HPS, diagnosed at 5 weeks. Another three patients showed FCH but did not re‐present with HPS. One patient, predating the time of this study, had FCH without HPS, and his vomiting ceased after excision of the redundant fold of mucosa. 22
While the aetiologies of HPS and FCH are unknown, large studies have shown that babies with HPS also have an increased incidence of FCH, suggesting that in some children, this is a precursor to the development of HPS, perhaps by compromising the gastric outlet. 6 , 21 , 22 , 23 There is no consensus about the relevance or need for treatment of FCH. It has been suggested that when FCH is present with HPS, the pyloromyotomy incision should be extended onto the stomach antrum to reduce any obstructive effect of the FCH, separate from the narrowing caused by the HPS. 21 Cases of HPS with FCH from this series and preceding 2009 are dealt with in the article by Tan et al. 21
Prompt, reliable ultrasound diagnosis of HPS allows the surgeon to proceed confidently to surgery, whereas in the past the clinician was obliged to make a diagnosis on clinical examination by palpation of the pyloric tumour. Sometimes, this involved repeated clinical examinations where the pyloric tumour was difficult to palpate, with consequent delay in operative treatment.
The treatment of HPS is pyloromyotomy (Ramstedt's operation), in which the pyloric muscularis is incised and spread open along the length of the canal, but without breaching the mucosal layer of the pylorus.
Conclusions
Current data for ultrasound diagnosis of hypertrophic pyloric stenosis are widely discrepant for a variety of possible reasons including the type of equipment, technique and mensuration accuracy. The consensus minimum length of 16.0 mm and muscle thickness of 3.0 mm currently used to diagnose hypertrophic pyloric stenosis are excessive, and their use is likely to delay diagnosis unnecessarily. Measurements from this study indicate that the criteria for diagnosing hypertrophic pyloric stenosis can be safely redefined. An accurately measured pyloric length >10.0 mm is pathognomonic of hypertrophic pyloric stenosis. Muscle thickness >2.2 mm is diagnostic of hypertrophic pyloric stenosis, but given the potential for inaccuracy in measurements of fractions of a millimetre, there is greater potential for error. The length of the canal is the single most useful criterion to use, with a clear separation of normal and hypertrophic pyloric stenosis values. The overall pyloric diameter has no diagnostic value for hypertrophic pyloric stenosis. The scanning technique is important. The use of water as a contrast medium allows very accurate measurement of canal length. Ensuring measurements are made after the passage of a peristaltic wave avoids the pitfall of misinterpreting collapsed antrum as part of the canal.
Ethics statement
The Human Research Ethics Committee of our hospital waived the informed consent requirement for the purpose of this review.
Authorship declaration
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Funding
No funding information is provided.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
Lino Piotto: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal). Roger Gent: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal). Ajay Taranath: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal). Giovanni Bibbo: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal). Day Way Goh: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal).
References
- 1. Stunden RJ, LeQuesne GW, Little KET. The improved ultrasound diagnosis of hypertrophic pyloric stenosis. Pediatr Radiol 1986; 16: 200–5. [DOI] [PubMed] [Google Scholar]
- 2. Wilson DA, Vanhoutte JJ. The reliable sonographic diagnosis of hypertrophic pyloric stenosis. J Clin Ultrasound 1984; 12: 201–4. [DOI] [PubMed] [Google Scholar]
- 3. Haller JO, Cohen HL. Hypertrophic pyloric stenosis: diagnosis using US. Radiology 1986; 161: 335–9. [DOI] [PubMed] [Google Scholar]
- 4. Blumhagen JD, Maclin L, Krauter D, Rosenbaum DM, Weinberger E. Sonographic diagnosis of hypertrophic pyloric stenosis. AJR 1988; 150: 1367–70. [DOI] [PubMed] [Google Scholar]
- 5. O'Keeffe FN, Stansberry SD, Swischuk LE, Hayden CK Jr. Antropyloric muscle thickness at US in infants: what is normal? Radiology 1991; 178(3): 827–30. [DOI] [PubMed] [Google Scholar]
- 6. Hernanz‐Schulman M, Lowe LH, Johnson J, Neblett WW, Polk DB, Perez R Jr, et al. In vivo visualization of pyloric mucosal hypertrophy in infants with hypertrophic pyloric stenosis: is there an etiologic role? AJR 2001; 177: 843–8. [DOI] [PubMed] [Google Scholar]
- 7. Neilson D, Hollman AS. The ultrasonic diagnosis of infantile hypertrophic pyloric stenosis: technique and accuracy. Clin Radiol 1994; 49: 246–7. [DOI] [PubMed] [Google Scholar]
- 8. Lowe LH, Banks WJ, Shyr Y. Pyloric ratio: efficacy in the diagnosis of hypertrophic pyloric Stenosis. J Ultrasound Med 1999; 18: 773–7. [DOI] [PubMed] [Google Scholar]
- 9. Leaphart CL, Borland K, Kane TD, Hackam DJ. Hypertrophic pyloric stenosis in newborns younger than 21 days: remodeling the path of surgical intervention. J Pediatr Surg 2008; 43(6): 998–1001. [DOI] [PubMed] [Google Scholar]
- 10. Huang IF, Tiao MM, Chiou CC, Shih HH, Hu HH, Ruiz JP. Infantile hypertrophic pyloric stenosis before 3 weeks of age in infants and preterm babies. Pediatr Int 2011; 53: 18–23. [DOI] [PubMed] [Google Scholar]
- 11. Niedzielski J, Kobielski A, Sokal J, Krakós M. Accuracy of sonographic criteria in the decision for surgical treatment in infantile hypertrophic pyloric stenosis. Arch Med Sci 2011; 7(3): 508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Said M, Shaul DB, Fujimoto M, Radner G, Sydorak RM, Applebaum H. Ultrasound measurements in hypertrophic pyloric stenosis: don't let the numbers fool you. Perm J 2012; 16(3): 25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iqbal CW, Rivard DC, Mortellaro VE, Sharp SW, St. Peter SD. Evaluation of ultrasonographic parameters in the diagnosis of pyloric stenosis relative to patient age and size. J Pediatr Surg 2012; 47(8): 1542–7. [DOI] [PubMed] [Google Scholar]
- 14. Sivitz AB, Tejani C, Cohen SG. Evaluation of hypertrophic pyloric stenosis by pediatric emergency physician sonography. Acad Emerg Med 2013; 20: 646–51. [DOI] [PubMed] [Google Scholar]
- 15. Cascio S, Steven M, Livingstone H, Young D, Carachi R. Hypertrophic pyloric stenosis in premature infants: evaluation of sonographic criteria and short‐term outcomes. Pediatr Surg Int 2013; 29(7): 697–702. [DOI] [PubMed] [Google Scholar]
- 16. Khan AA, Yousaf MA, Ashraf M. Role of ultrasonography in early diagnosis of infantile hypertrophic pyloric stenosis. J Ayub Med Coll Abbottabad 2014; 26(3): 316–9. [PubMed] [Google Scholar]
- 17. Ayaz ÜY, Döğen ME, Dilli A, Ayaz S, Api A. The use of ultrasonography in infantile hypertrophic pyloric stenosis: do the patient's age and weight affect pyloric size and pyloric ratio? Med Ultrason 2015; 17(1): 28–33. [DOI] [PubMed] [Google Scholar]
- 18. Vinycomb T, Vanhaltren K, Pacilli M, Ditchfield M, Nataraja RM. Ev evaluating the validity of ultrasound in diagnosing hypertrophic pyloric stenosis: a cross‐sectional diagnostic accuracy study. ANZ J Surg 2021; 91(11): 2507–13. [DOI] [PubMed] [Google Scholar]
- 19. Hennessey I, John R, Gent R, Goh D. Utility of sonographic assessment of the position of the third part of the duodenum using water instillation in intestinal malrotation: a single‐center retrospective audit. Pediatr Radiol 2014; 44(4): 387–91. [DOI] [PubMed] [Google Scholar]
- 20. Gilbert‐Barness E, Kapur RP, Oligny LL, Siebert JR (Eds). Potter's pathology of the fetus, infant and child, Vols 1 and 2, 2nd ed, 2444 pp (with illus, with CD‐ROM). Philadelphia, PA, USA: Mosby; 2007. [Google Scholar]
- 21. Tan HL, Blythe A, Kirby CP, Gent R. Gastric foveolar cell hyperplasia and its role in postoperative vomiting in patients with infantile hypertrophic pyloric stenosis. Eur J Pediatr Surg 2009; 19(2): 76–8. [DOI] [PubMed] [Google Scholar]
- 22. Holland AJ, Freeman JK, Le Quesne GW, Khong TY. Idiopathic focal foveolar hyperplasia in infants. Pediatr Surg 1997; 12(7): 497–500. [DOI] [PubMed] [Google Scholar]
- 23. Callahan MJ, McCauley RG, Patel H, Hijazi ZM. The development of hypertrophic pyloric stenosis in a patient with prostaglandin‐induced foveolar hyperplasia. Pediatr Radiol 1999; 29(10): 748–51. [DOI] [PubMed] [Google Scholar]


