Significance
Acute respiratory distress syndrome is characterized by aberrant inflammatory responses, including polymorphonuclear neutrophil granulocyte dysfunction and hyperactive Toll-like receptor signaling. Timely resolution of bacterial infections depends on efficient removal of neutrophils from the inflamed tissue. Here we show that the antiviral cytokine interferon-β is essential for the resolution of neutrophil-driven airway inflammation by countering Toll-like receptor 9–mediated suppression of phagocytosis, neutrophil apoptosis, and uptake by macrophages. We also report that the beneficial effects of interferon-β are, in part, mediated by production of proresolving lipid mediators, such as 15-epi-lipoxin A4 and resolvin D1, which act through the lipoxin receptor ALX/FPR2. These findings uncover an interferon-β–initiated ALX/FPR2-centered resolution program as a potential target for facilitating the resolution of airway inflammation.
Keywords: resolution of inflammation, interferon-β, neutrophils, phagocytosis, specialized proresolving lipid mediators
Abstract
Aberrant immune responses, including hyperresponsiveness to Toll-like receptor (TLR) ligands, underlie acute respiratory distress syndrome (ARDS). Type I interferons confer antiviral activities and could also regulate the inflammatory response, whereas little is known about their actions to resolve aberrant inflammation. Here we report that interferon-β (IFN-β) exerts partially overlapping, but also cooperative actions with aspirin-triggered 15-epi-lipoxin A4 (15-epi-LXA4) and 17-epi-resolvin D1 to counter TLR9-generated cues to regulate neutrophil apoptosis and phagocytosis in human neutrophils. In mice, TLR9 activation impairs bacterial clearance, prolongs Escherichia coli–evoked lung injury, and suppresses production of IFN-β and the proresolving lipid mediators 15-epi-LXA4 and resolvin D1 (RvD1) in the lung. Neutralization of endogenous IFN-β delays pulmonary clearance of E. coli and aggravates mucosal injury. Conversely, treatment of mice with IFN-β accelerates clearance of bacteria, restores neutrophil phagocytosis, promotes neutrophil apoptosis and efferocytosis, and accelerates resolution of airway inflammation with concomitant increases in 15-epi-LXA4 and RvD1 production in the lungs. Pharmacological blockade of the lipoxin receptor ALX/FPR2 partially prevents IFN-β–mediated resolution. These findings point to a pivotal role of IFN-β in orchestrating timely resolution of neutrophil and TLR9 activation–driven airway inflammation and uncover an IFN-β–initiated resolution program, activation of an ALX/FPR2-centered, proresolving lipids-mediated circuit, for ARDS.
Acute respiratory distress syndrome (ARDS) is a common syndrome associated with high mortality in patients admitted to intensive care units (1). ARDS is characterized by diffuse alveolar damage that develops in patients with known risk factors, most commonly pneumonia, sepsis, or trauma (2, 3). The initial alveolar damage leads to recruitment of neutrophils and monocytes, which further aggravate injury (3). Treatment of the underlying cause and lung-protective ventilation are the main elements of supportive therapy (4, 5). Importantly, no therapies are available to resolve the aberrant immune responses underlying ARDS.
Type I interferons, IFN-α and IFN-β, are well established to confer antiviral activities to host cells and could also regulate the inflammatory response. A delayed type I interferon response triggers the generation of proinflammatory cytokines and facilitates the recruitment of monocytes to the lung, resulting in lethal pneumonia in mice infected with SARS-CoV-1 (6) or SARS-CoV-2 (7). Type I interferons break TNF-induced tolerance to Toll-like receptor (TLR) signals on monocytes/macrophages, rendering them hyperresponsive to additional TLR signals concurrent with inflammatory activation (8). For instance, bacterial DNA (CpG DNA) or mitochondrial DNA through TLR9 impairs neutrophil phagocytosis, delays neutrophil apoptosis, and perpetuates inflammation (9, 10). In contrast, IFN-β protects against lethal polymicrobial sepsis through inhibiting IL-1 production and/or induction of IL-10 (11–13). IFN-β produced by macrophages during resolution of bacterial pneumonia facilitates removal of neutrophils from inflamed tissues and reprograms macrophages to a proresolving phenotype, thereby driving inflammatory resolution in mice (14). However, the underlying mechanisms are incompletely understood; albeit these would be essential for implementing precision treatment with IFN-β.
Resolution of inflammation is an active process governed by specialized proresolving lipid and protein mediators (SPMs) (15–19). These mediators converge on select receptors, including the pleiotropic lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2) (20). ALX/FPR2 plays critical roles in host defense and orchestrating inflammatory resolution (20–23). ALX/FPR2 binds multiple lipid ligands, including aspirin-triggered 15-epi-lipoxin A4 (15-epi-LXA4) and 17-epi-resolvin D1 (17-epi-RvD1), generated within the inflammatory microenvironment (17, 18). SPMs inhibit neutrophil recruitment, promote neutrophil apoptosis and efferocytosis, and facilitate tissue repair and return to homeostasis (17, 18, 24). Activation of ALX/FPR2 with 15-epi-LXA4 or 17-epi-RvD1 counters TLR9-generated cues, restores impaired neutrophil function, and enhances timely resolution of airway bacterial infections (9). Since resolution of inflammation is skewed toward a proresolving lipid profile (18, 25, 26), we investigated whether IFN-β can modulate ALX/FPR2-based resolution mechanisms. Here, we report that IFN-β exerts partially overlapping, but also cooperative actions with 17-epi-RvD1 to counter TLR9-generated signals to regulate neutrophil phagocytosis and apoptosis in vitro. In mice, IFN-β facilitates clearance of bacteria, neutrophil apoptosis and efferocytosis, and promotes the resolution of acute airway inflammation, in part, by stimulating generation of proresolving lipids and activation of ALX/FPR2-centered proresolving circuits. Our results uncover a hitherto unrecognized effector mechanism by which IFN-β may facilitate resolution of ARDS.
Results
IFN-β Acts in Concert with 15-Epi-LXA4 and 17-Epi-RvD1 to Overcome TLR9-Mediated Impaired Apoptosis in Human Neutrophils.
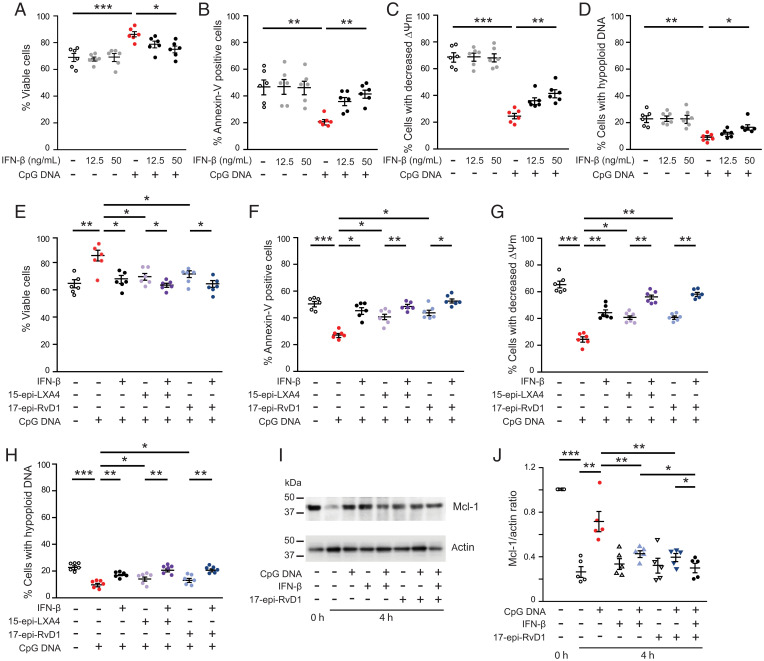
Confirming our previous results (9, 10), CpG DNA prolonged neutrophil survival by delaying intrinsic apoptosis as indicated by reduced staining for annexin-V, prevention of the collapse of mitochondrial transmembrane potential, and hypoploid nuclei (Fig. 1 A–D). Pretreatment of neutrophils with IFN-β countered the survival cue from CpG DNA in a concentration-dependent fashion (Fig. 1 A–D). The inhibitory action was still detectable when neutrophils were treated with IFN-β at 60 min post-CpG DNA (SI Appendix, Fig. S1). Because ligation of ALX/FPR2 with proresolving lipid mediators, including 15-epi-LXA4 and 17-epi-RvD1, can efficiently counter the prosurvival action of CpG DNA (9), we compared the effects of IFN-β, 15-epi-LXA4, and 17-epi-RvD1. Pretreatment of neutrophils with either IFN-β, 15-epi-LXA4, or 17-epi-RvD1 resulted in reversal of the prosurvival action of CpG DNA, and the effects of IFN-β and 15-epi-LXA4 or 17-epi-RvD1 were additive (Fig. 1 E–H). Both IL-1β and 17-epi-RvD1 attenuated CpG DNA preservation of Mcl-1 expression, a central regulator of life span of human neutrophils (27), with more pronounced decreases in the presence of both IL-1β and 17-epi-RvD1 (Fig. 1 I and J). IFN-β signals through IFNα/βR in neutrophils (14), and pharmacological blockade of formyl peptide receptors did not alter its proapoptosis action (SI Appendix, Fig. S2).
Fig. 1.
IFN-β and 17-epi-RvD1 act in concert to reverse CpG DNA suppression of neutrophil apoptosis. Human neutrophils (5 × 106 cells/mL) were cultured with IFN-β (12.5 or 50 ng/mL) and then challenged with CpG DNA (1.6 μg/mL) (A–D), or pretreated with IFN-β (50 ng/mL) plus 15-epi-LXA4 (1 μM) or 17-epi-RvD1 (200 nM) for 10 min and then challenged with CpG DNA (E–H). Cell viability (propidium iodide staining) (A and E), annexin-V staining (B and F), mitochondrial transmembrane potential (Δψm, CMXRos staining) (C and G), and nuclear DNA content (D and H) were analyzed after 24 h of culture. Data are means ± SEM (n = 6 different blood donors). *P < 0.05, **P < 0.01, ***P < 0.001 (Dunn’s multiple contrast hypothesis test). (I and J) Mcl-1 expression. Neutrophils were lysed after isolation (t0) or after culture with CpG DNA (1.6 μg/mL) with or without 17-epi-RvD1 (200 nM) plus IFN-β (50 ng/mL) for 4 h. Proteins were subjected to immunoblotting with antibodies to Mcl-1 or actin. Representative blots (I) and densitometry analysis show five independent experiments with different blood donors (J). *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test).
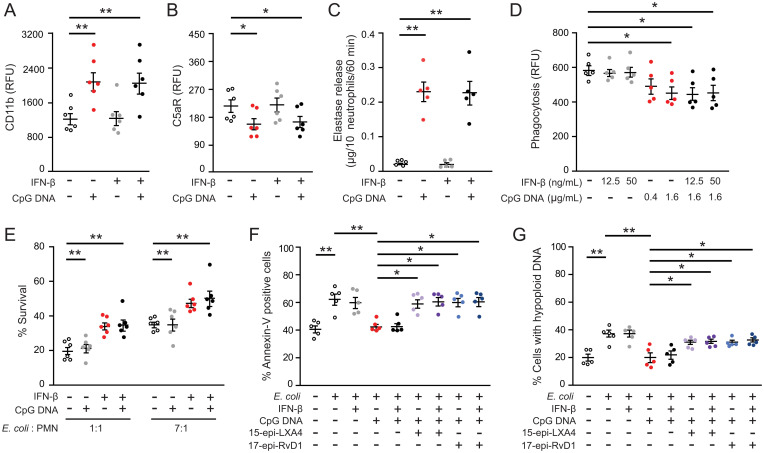
Consistent with previous results (9), CpG DNA impaired phagocytosis of bacteria and phagocytosis-induced neutrophil apoptosis via neutrophil elastase–mediated cleavage of C5aR (CD88) (Fig. 2 A–D). IFN-β did not affect these responses to CpG DNA (Fig. 2 A–D), and consequently did not restore impaired killing of Escherichia coli by CpG DNA (Fig. 2E). By contrast, 15-epi-LXA4 and 17-epi-RvD1 reversed CpG DNA suppression of phagocytosis-induced death in neutrophils at 24 h of culture as assessed by staining for annexin-V and hypoploid DNA (Fig. 2 F and G). Both 15-epi-LXA4 and 17-epi-RvD1 exerted similar actions in the absence and presence of IFN-β (Fig. 2 F and G).
Fig. 2.
17-epi-RvD1, but not IFN-β restores impaired phagocytosis-induced neutrophil apoptosis. (A–C) Human polymorphonuclear neutrophil granulocytes (PMNs (5 × 106 cells/mL) were cultured for 10 min with IFN-β (50 ng/mL) and then with CpG DNA (1.6 μg/mL) for 60 min. Surface expression of CD11b (A) and complement 5a receptor (C5aR, CD88) (B) was assessed by flow cytometry; neutrophil elastase activity in cell-free culture media was measured with a colorimetric assay using N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide as a substrate (C). Results are means ± SEM (n = 5 or 6 different blood donors). *P < 0.05, **P < 0.01. (D) Neutrophils were cultured with IFN-β for 10 min and CpG DNA for 60 min and then with opsonized FITC-labeled E. coli (seven bacteria per neutrophil) for 30 min. Extracellular fluorescence was quenched with 0.2% trypan blue and intracellular fluorescence was analyzed with flow cytometry. Results are means ± SEM (n = 5 different blood donors). *P < 0.05. (E) Neutrophils were cultured with IFN-β (50 ng/mL) for 10 min CpG DNA and then mixed with E. coli at a ratio of 1:1 or 1:7 for 3 h. Bacteria levels in culture media were assessed by growth on tryptic agarose. (F and G) Neutrophils were cultured with IFN-β (50 ng/mL), 15-epi-LXA4 (1 μM), or 17-epi-RvD1 (200 nM) for 10 min, then with CpG DNA (1.6 μg/mL) and opsonized FITC-labeled E. coli (seven bacteria per neutrophil) for 24 h. Apoptosis was assessed by annexin-V staining (F) and nuclear DNA content (G) with flow cytometry. Results are means ± SEM (n = 5 different blood donors). *P < 0.05, **P < 0.01, ***P < 0.001 (Dunn’s multiple contrast hypothesis test).
Since human neutrophils express 5-lipoxygenase and can express cyclooxygenase-2 (17, 18), we next examined whether IFN-β could directly stimulate 15-epi-LXA4 and RvD1 production. Challenging neutrophils with IFN-β for 10 min or 4 h did not produce statistically significant increases in the levels of these SPMs, not even in the presence of CpG DNA (SI Appendix, Fig. S3 A and B). Phagocytosis of E. coli resulted in significant increases in 15-epi-LXA4 and RvD1 secretion, which was markedly suppressed by CpG DNA (SI Appendix, Fig. S3 C and D). IFN-β evoked increases in 15-epi-LXA4 and RvD1 secretion by phagocytosing neutrophils in the presence of CpG DNA, though these changes did not reach statistical significance (SI Appendix, Fig. S3 C and D).
IFN-β Restores TLR9-Impaired Bacterial Clearance, Regulates Cytokine and Lipid Mediators, and Facilitates Resolution of Airway Inflammation.
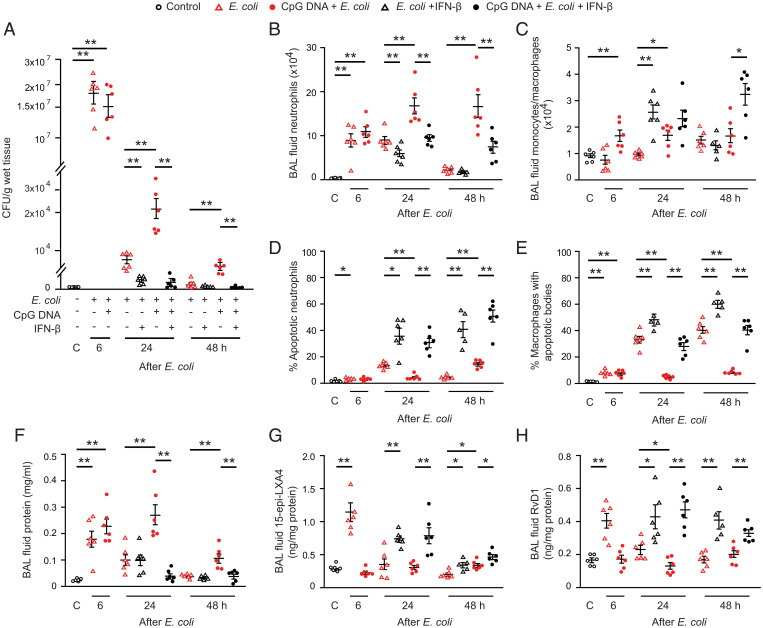
Having characterized IFN-β regulation of apoptosis in human neutrophils in vitro, we investigated the effects of IFN-β on the resolution of inflammation in mice. We used models of lung inflammation evoked by intratracheal instillation of live E. coli, which resolves within 48 h without treatment and can be prolonged by simultaneous administration of CpG DNA (9). As anticipated, wild-type mice rapidly cleared E. coli (Fig. 3A) parallel with rapid dissipation of inflammatory infiltrates in the lung (Fig. 3 B–F and SI Appendix, Fig. S4). By contrast, lung inflammation persisted at least 48 h in mice that received E. coli plus CpG DNA. This is reflected by delayed bacterial clearance (Fig. 3A), massive neutrophil accumulation (Fig. 3B and SI Appendix, Fig. S4B), reduced neutrophil apoptosis (Fig. 3D) and efferocytosis (Fig. 3E), and edema formation (Fig. 3F and SI Appendix, Fig. S4C). CpG DNA significantly reduced lavage fluid levels of 15-epi-LXA4 (Fig. 3G), RvD1 (Fig. 3H), and IL-10 (SI Appendix, Fig. S4J) at 24 h and 48 h, whereas it evoked marked and prolonged increases in lavage fluid levels of proinflammatory cytokines (SI Appendix, Fig. S4 D–I).
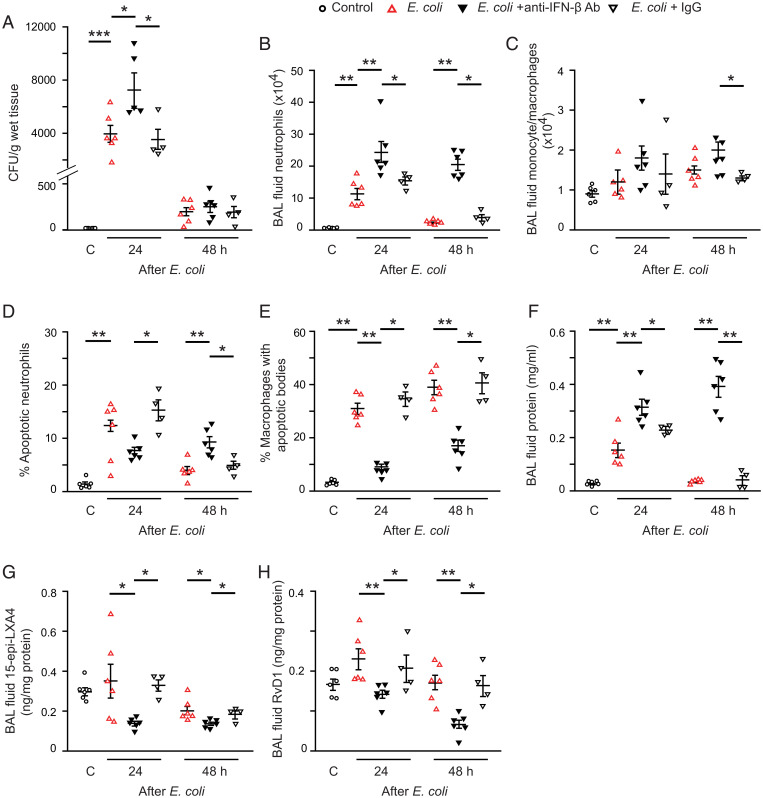
Fig. 3.
IFN-β facilitates bacterial clearance, neutrophil apoptosis, and resolution of lung inflammation. Acute lung inflammation was induced in female C57BL/6 mice by intratracheal instillation of 5 × 106 live E. coli with or without simultaneous injection of CpG DNA (1 μg/g body weight [b.w]., i.p.). At 6 h post E. coli, mice were treated with IFN-β (2.5 ng/g b.w., i.p.). Mice were killed at the indicated times, and bronchoalveolar lavage was performed or the lungs were processed for analysis without lavage. Lung tissue bacterial content (A), lavage fluid neutrophil number (B), and monocyte/macrophage number (C), the percentage of annexin-V positive neutrophils (identified as Ly6G-positive cells) in lavage fluid (D), the percentage of macrophages with apoptotic bodies (E), lavage fluid protein concentration (F), 15-epi-LXA4 levels (G), and RvD1 levels (H) were determined. Results are means ± SEM (n = 6 mice per group). *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test).
Treatment with IFN-β at 6 h post E. coli, near the peak of inflammation, accelerated clearance of bacteria in mice that either received E. coli or E. coli plus CpG DNA (Fig. 3A). Thus, no bacteria were detectable in lung homogenates at 48 h. Antiinflammatory and proresolving actions were also evident as IFN-β markedly reduced lavage fluid total leukocyte (SI Appendix, Fig. S4A) and neutrophil counts (Fig. 3B), and tissue myeloperoxidase (MPO) content (SI Appendix, Fig. S4B) with concomitant increases in the number of monocytes/macrophages (Fig. 3C). Decreased neutrophil accumulation occurred parallel with increases in the percentage of apoptotic neutrophils (Fig. 3D) and the percentage of macrophages containing apoptotic bodies at 24 and 48 h (Fig. 3E). Treatment with IFN-β also attenuated edema formation (Fig. 3F and SI Appendix, Fig. S4C) and produced increases in lavage fluid levels of 15-epi-LXA4 (Fig. 3G) and RvD1 (Fig. 3H). IFN-β reduced lavage fluid levels of IL-6, TNF-α, IL-1β, G-CSF, KC, and IL-17A to near control values at 24 h and 48 h, whereas it evoked increases in IL-10, which persisted at 48 h (SI Appendix, Fig. S4 D–J). The effects of IFN-β were comparable in the two models of lung injury, underscoring its therapeutic potential.
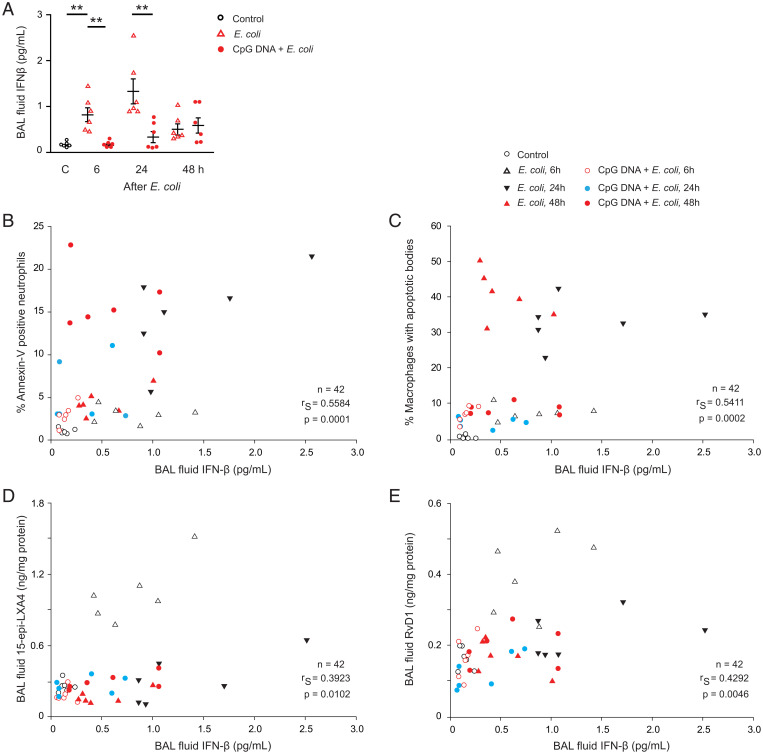
Spontaneous resolution of E. coli infection was associated with marked increases in IFN-β level, which was evident at 6 h post E. coli and peaked around 24 h (Fig. 4A). By contrast, no increases in lavage fluid IFN-β levels, not even at 48 h, were detectable in mice that had received E. coli plus CpG DNA (Fig. 4A). We also detected strong positive correlations of lavage fluid IFN-β levels with the percentage of apoptotic neutrophils (Fig. 4B), percentage of macrophages with apoptotic bodies (Fig. 4C), and lavage fluid levels of 15-epi-LXA4 (Fig. 4D) and RvD1 (Fig. 4E).
Fig. 4.
Bronchoalveolar lavage fluid levels of IFN-β correlates with neutrophil apoptosis, efferocytosis, and lavage fluid levels of 15-epi-LXA4 and RvD1. Acute lung inflammation was induced in female C57BL/6 mice by intratracheal instillation of 5 × 106 live E. coli with or without simultaneous injection of CpG DNA (1 μg/g b.w., i.p.). Mice were killed at the indicated times and bronchoalveolar lavage was performed. (A) Lavage fluid levels of IFN-β. Results are means ± SEM (n = 6 mice per group). *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test). Lavage fluid IFN-β levels positively correlate with the percentage of annexin-V positive neutrophils (gated as Ly6G-positive cells) in lavage fluid (B), the percentage of macrophages containing apoptotic bodies (C), lavage fluid concentrations of 15-epi-LXA4 (D), and RvD1 (E) (Spearman correlation analysis).
Having shown the beneficial actions of IFN-β treatment in spontaneously resolving and more severe prolonged lung injury models, we investigated the impact of neutralizing endogenous IFN-β. Pretreatment of mice with an anti–IFN-β antibody impaired E. coli clearance at 24 h (Fig. 5A) while enhancing and prolonging E. coli-evoked lung inflammation (Fig. 5 B–F and SI Appendix, Fig. S5). Thus, neutralizing IFN-β enhanced lavage fluid neutrophil (Fig. 5B) and monocyte counts (Fig. 5C), lung myeloperoxidase content (SI Appendix, Fig. S5B) and edema formation (Fig. 5F and SI Appendix, Fig. S5C) compared with IgG control. By contrast, blocking INF-β significantly reduced the percentage of apoptotic neutrophils (Fig. 5D) and macrophages with apoptotic bodies (Fig. 5E), and lavage fluid levels of 15-epi-LXA4 (Fig. 5G) and RvD1 (Fig. 5H) at both 24 h and 48 h post E. coli. Neutralizing IFN-β increased lavage fluid levels of IL-6, TNF-α, IL-1β, G-CSF, KC, and IL-17A at 24 h and 48 h (SI Appendix, Fig. S5 D–I), while it prevented E. coli-evoked increases in IL-10 at 24 h (SI Appendix, Fig. S5J).
Fig. 5.
Blockade of endogenous IFN-β dampens bacterial clearance, reduces generation of proresolving lipid mediators, and delays the resolution of lung inflammation. Female C57BL/6 mice were first injected with a rat anti-mouse IFN-β mAb or rat IgG (both at 50 ng/g b.w., i.p.) followed by intratracheal instillation of 5 × 106 live E. coli. Mice were killed at the indicated times and lung tissue E. coli content (A), lavage fluid neutrophil number (B), and monocyte/macrophage number (C), the percentage of annexin-V positive neutrophils (identified as Ly6G-positive cells) (D), the percentage of macrophages with apoptotic bodies (E), lavage fluid protein (F), 15-epi-LXA4 (G), and RvD1 levels (H) were determined. Results are means ± SEM (n = 4 mice for E. coli plus IgG; n = 6 mice per group for the other groups). *P < 0.05, **P < 0.01, ***P < 0.001 (Dunn’s multiple contrast hypothesis test).
IFN-β Activates ALX/FPR2-Based Proresolution Circuits.
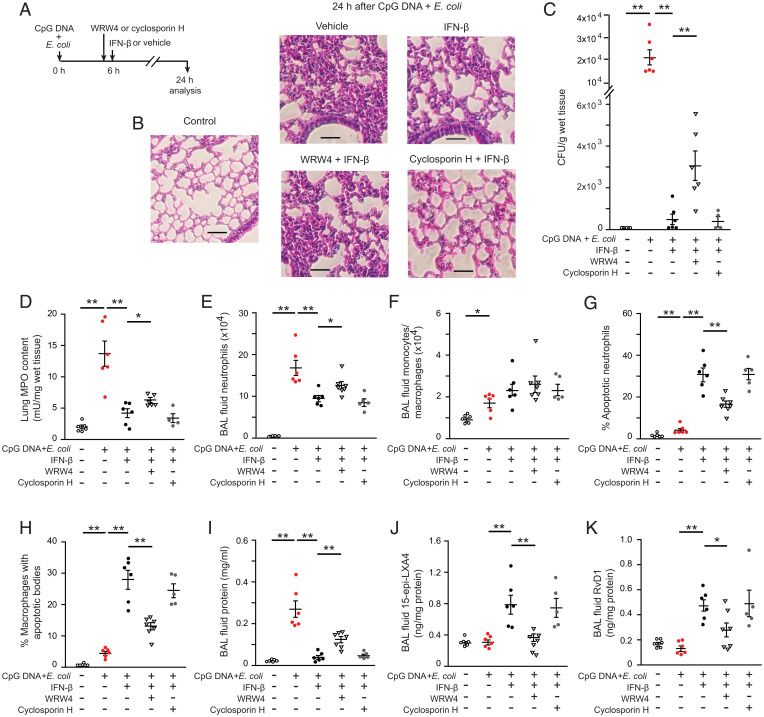
Next, we investigated the role of 15-epi-LXA4 and RvD1 in mediating IFN-β enhanced resolution of lung inflammation. As these lipids act through ALX/FPR2 (17, 18), mice were injected with the selective FPR1 antagonist cyclosporin H or the FPR2/ALX inhibitor WRW4 (20, 28) before treatment with IFN-β at 6 h post E. coli plus CpG DNA (Fig. 6A). While cyclosporin H did not interfere with the proresolving actions of IFN-β (Fig. 6 B–K), administration of WRW4 partially countered IFN-β dissipation of inflammation, as evidenced by persisting inflammatory cell infiltration and tissue injury (Fig. 6B) and delayed clearance of E. coli (Fig. 6C). Consistently, lung MPO content (Fig. 6D), lavage fluid neutrophil (Fig. 6E), and total leukocyte counts (SI Appendix, Fig. S6A) were significantly lower in WRW4 plus IFN-β–treated mice than in mice treated with IFN-β only. The numbers of monocytes/macrophages did not differ significantly in these groups (Fig. 6F), whereas the percentage of apoptotic neutrophils (Fig. 6G) and the percentage of macrophages containing apoptotic bodies (Fig. 6H) were markedly reduced. Lavage fluid protein levels were significantly higher (Fig. 6I) in mice that received WRW4 plus IFN-β compared to IFN-β treatment alone. Furthermore, WRW4 drastically reduced IFN-β–evoked increases in lavage fluid levels of 15-epi-LXA4 (Fig. 6J), RvD1 (Fig. 6K), and IL-10 (SI Appendix, Fig. S6I). Conversely, lavage fluid concentrations of IL-6, TNF-α, IL-1β, GC-SF, KC, and IL-17A were significantly higher in the WRW4 plus IFN-β group than in mice treated with IFN-β only (SI Appendix, Fig. S6 C–H).
Fig. 6.
Pharmacological blockade of ALX/FPR2 attenuates the proresolving actions of IFN-β. Acute lung inflammation was induced in female C57BL/6 mice by intratracheal instillation of 5 × 106 live E. coli plus CpG DNA (1 μg/g b.w., i.p.). At 6 h post E. coli, mice were first injected intraperitoneally with WRW4 (1 μg/g b.w.) or cyclosporin H (5 μg/g b.w.) and 10 min later were treated with IFN-β (2.5 ng/g b.w., i.p.) (A). Mice were killed at 24 h post E. coli, and bronchoalveolar lavage was performed or lungs were processed for analysis without lavage. (B) Lung tissue sections from naïve mice (control) and vehicle or IFN-β ± WRW4 or cyclosporin H–treated mice. Hematoxylin and eosin stain was used. (Scale bars: 100 μm.) (C) Lung tissue of E. coli content, (D) lung tissue of MPO content, (E) lavage fluid neutrophil number, (F) lavage fluid monocyte/macrophage number, (G) the percentage of annexin-V positive neutrophils (identified as Ly6G-positive cells) in lavage fluid, (H) the percentage of macrophages with apoptotic bodies, (I) lavage fluid protein concentration, (J) lavage fluid 15-epi-LXA4, and (K) RvD1 levels. Results are means ± SEM (n = 5 to 7 mice per group). *P < 0.05, **P < 0.01 (Dunn’s multiple contrast hypothesis test).
Because selective ALX/FPR2 blockade reduced lavage fluid levels of 15-epi-LXA4 and RvD1, we studied the time course of changes in these lipids following treatment of mice with either 15-epi-LXA4 or 17-epi-RvD1, at doses that accelerated resolution of inflammation (9), at 6 h post CpG DNA plus E. coli. We used 17-epi-RvD1 instead of RvD1 because it was shown to be longer acting yet acting on the same receptor (17, 18). As anticipated, treatment with either 15-epi-LXA4 or 17-epi-RvD1 markedly reduced lavage fluid neutrophil numbers (SI Appendix, Fig. S7A) and protein levels (SI Appendix, Fig. S7B). Intriguingly, bolus injection of either 15-epi-LXA4 or 17-epi-RvD1 resulted in significantly elevated lavage fluid levels of 15-epi-LXA4 at 24 h post CpG DNA plus E. coli, returning to baseline (control) levels at 48 h (SI Appendix, Fig. S7C). Likewise, treatment with 15-epi-LXA4 or 17-epi-RvD1 led to significantly higher lavage fluid RvD1 levels at 24 h, and RvD1 levels remained elevated at 48 h in mice treated with 15-epi-LXA4 (SI Appendix, Fig. S7D).
Discussion
Here, we provide evidence that IFN-β is essential for limiting airway inflammation and demonstrate a complex interplay between IFN-β and proresolving lipid mediators. IFN-β acted in concert with 17-epi-RvD1 to promote neutrophil apoptosis, but did not restore TLR9-impaired phagocytosis in vitro. Treatment with IFN-β accelerated clearance of bacteria, restored neutrophil phagocytosis, and facilitated phagocytosis-induced apoptosis and efferocytosis, consequently dampening inflammation and accelerating resolution of E. coli plus TLR9 activation–evoked airway inflammation in vivo. Antibody neutralization experiments confirmed IFN-β as an important driver of resolution in these models. IFN-β also evoked generation of the SPMs, 17-epi-LXA4 and RvD1, parallel with enhanced neutrophil apoptosis and efferocytosis. Moreover, pharmacological blockade of ALX/FPR2 partially prevented IFN-β–mediated resolution. Thus, activation of ALX/FPR2-centered resolution circuits by IFN-β may function as a downstream mechanism for its proresolving actions in mucosal inflammation.
IFN-β, produced by nonphagocytic resolution phase macrophages, mediates both feedback and bidirectional cross-talk between macrophages and neutrophils to facilitate removal of neutrophils from the inflamed tissue, critical for the timely resolution of inflammation (14). Neutrophil apoptosis is required for their removal by macrophages via efferocytosis, and hence it is one of the checkpoints that determine the outcome of inflammation (24, 29, 30). CpG DNA through TLR9 generates survival cues for neutrophils (10) and impairs phagocytosis and phagocytosis-induced apoptosis (9). IFN-β overrode the antiapoptosis signal from CpG DNA, thus redirecting human neutrophils to apoptosis. The proapoptotic action of IFN-β was dominant over CpG DNA–mediated effects even when it was added as a treatment, highlighting its therapeutic potential. IFN-β did not restore CpG DNA–impaired phagocytosis and subsequently phagocytosis-induced neutrophil death in vitro. In contrast, IFN-β accelerated bacterial clearance in mice, indicating activation of additional counterregulatory signaling. Of note, IFN-β acted in concert with 15-epi-LXA4 and 17-epi-RvD1 to overcome the antiapoptosis signal from CpG DNA by preventing the CpG DNA preservation of Mcl-1 expression, a key regulator of apoptosis in human neutrophils (27). Confirming previous results (9), both 15-epi-LXA4 and 17-epi-RvD1 restored TLR9-mediated impaired phagocytosis in vitro and this was not affected by IFN-β.
The mouse models were chosen for their clinical relevance (31, 32) and because of the self-resolving nature of E. coli–induced inflammation that can be aggravated and prolonged by simultaneous administration of CpG DNA (9). CpG DNA has been detected in the blood of critically ill patients (33) and in the airways (34). CpG DNA promotes neutrophil accumulation and neutrophil-mediated lung injury (9, 10, 34, 35), leading to persisting inflammation. After intratracheal E. coli challenge, there were spontaneous increases in bronchoalveolar lavage (BAL) fluid IFN-β parallel with resolution of inflammation. Antibody neutralization experiments confirmed that IFN-β is indispensable for resolution of airway inflammation in this setting. Mice that received CpG DNA were less efficient in clearing E. coli than vehicle-treated controls and failed to increase IFN-β and resolve mucosal inflammation within 48 h. We suggest that suppression of IFN-β production by CpG DNA released from proliferating bacteria represents a mechanism by which microbes may evade host defense.
Lavage fluid levels of IFN-β were positively correlated with the percentage of apoptotic neutrophils and macrophage efferocytosis in the lavage fluid as well as with levels of the proresolving lipid mediators 15-epi-LXA4 and RvD1. Moreover, selective blockade of ALX/FPR2 with WRW4 partially blocked IFN-β–mediated resolution, leading to impaired bacterial clearance and persisting lung injury. WRW4 also attenuated IFN-β–stimulated increases in 15-epi-LXA4 and RvD1 parallel with increased neutrophil accumulation and edema formation and reduced neutrophil apoptosis and efferocytosis, hallmarks of prolonged lung injury (24, 26, 30). Moreover, WRW4 reversed IFN-β suppression of proinflammatory cytokines, such as IL-6, TNFα, and KC, and prevented IFN-β–stimulated increases in IL-10, consistent with prolonging airway inflammation and delaying IFN-β–facilitated resolution. TNF-α displays a tolerizing action on monocytes and macrophages, causing hyperresponsiveness to TLR signals (8). However, IFN-β efficiently countered TLR9-mediated delay of neutrophil apoptosis both in vitro and in vivo despite reduced TNF-α generation, indicating cell type–specific modulation of TLR9 signaling. Since IL-10 promotes efferocytosis (36) and limits the severity of bacterial infections (37, 38), it may act downstream to IFN-β. In contrast to WRW4, the FPR1-selective inhibitor cyclosporin H did not affect the proresolving action of IFN-β. Furthermore, as WRW4 did not modify the proapoptosis action of IFN-β in human neutrophils, IFN-β partially promoted resolution via ALX/FPR2-based mechanisms. ALX/FPR2 is a cognate receptor for 15-epi-LXA4 and is expressed on several immune cell types, including neutrophils (20). Fpr2-deficient mice, in models of ischemia–reperfusion, sepsis, atherosclerosis, and obesity, exhibit profound nonresolving inflammation, indicating that this receptor is important for counterregulatory signaling (39–42). Together our findings bolster the idea that IFN-β activates ALX/FPR2-mediated resolution circuits through induction of SPMs acting on this receptor.
Of note, treatment of mice with either 15-epi-LXA4 or 17-epi-RvD1 resulted in marked elevations of lavage fluid levels of 15-epi-LXA4 and RvD1 that persisted for 24 h and 48 h, respectively. It is unlikely that these reflect pulmonary accumulation of the injected lipids, because of their short half-life in the circulation (43); rather these findings underscore the intricate interplay among proresolving lipid mediators in orchestrating resolution (44). SPMs have partially overlapping and synergistic effector functions and could induce generation of other SPMs. For instance, resolvin E1 was shown to stimulate LXA4 synthesis during the resolution of allergic airway inflammation (45) and ligation of ALX/FPR2 triggers LXA4 synthesis, setting in motion integrated actions underpinning resolution (23).
IFN-β also increased BAL fluid RvD1 levels. As RvD1 signals through both ALX/FPR2 and GPR32 to facilitate resolution (25, 46), IFN-β likely promoted additional counterregulatory mechanisms distinct from ALX/FPR2-centered ones, which would require further investigations. Together, these findings uncover a “fingerprint” of selective mediator regulation by IFN-β to promote resolution. The observations that IFN-β did not stimulate SPM secretion from human neutrophils and only partially reversed CpG DNA suppression of phagocytosis-stimulated production of 15-epi-LXA4 and RvD1 would indicate that neutrophils were not a major source of IFN-β–induced generation of these SPMs in vivo. While our study did not address the cellular sources of SPMs, IFN-β reprogramming of resolution-phase macrophages, including increased expression of the SPM-producing enzyme 12/15-lipoxygenase (14, 17, 18) and their high capacity to produce SPMs (47) would imply these cells as potential sources of SPMs detected in our models of airway inflammation.
The present and previous studies underscore the potent antiinflammatory, proresolving actions of IFN-β in addition to its broad antiviral activities. These findings would suggest the therapeutic potential of IFN-β to dampen aberrant immune responses underlying ARDS of bacterial and viral origin. Since mitochondrial DNA fully mimics the actions of CpG DNA (9), our findings may have relevance to airway injury evoked by viral infections. Of note, IFN-β was reported to reduce SARS-CoV-1–evoked ARDS (48) and ongoing trials are testing its efficacies in SARS-CoV-2–infected patients (49). Given that IFN-α and IFN-β have overlapping functions, bind to the same receptor IFNα/βR (50), and exhibit similar therapeutic benefits (48), it seems unlikely that the association of IFN-β with SPMs is specific for this interferon. Whether type II interferons, which share some common mechanisms with type I interferons to elicit immune responses, could also stimulate SPMs remains to be investigated. Although there are limitations in extrapolating from our short-term assays to chronic conditions, our data indicate that IFN-β and SPMs restore impaired neutrophil function, rather than simply reduce neutrophil survival, which could lead to immunosuppression. However, this possibility seems unlikely as SPMs are not immunosuppressive (18) and IFN-β itself did not affect neutrophil survival (ref. 14 and the present study).
Collectively, our results indicate that IFN-β is essential for the timely resolution of neutrophil-driven airway inflammation. To promote resolution, IFN-β restored impaired phagocytosis, accelerated clearance of bacteria, redirected neutrophils to apoptosis and enhanced efferocytosis, and decreased production of proinflammatory cytokines, which were largely mediated by IFN-β–stimulated generation of proresolving lipid mediators that signal through ALX/FPR2. These findings also imply the therapeutic potential of harnessing the interplay between IFN-β and ALX/FPR2-centered circuits for promoting the resolution of ARDS.
Materials and Methods
A brief description of the materials and methods can be found below, whereas an expanded methods section can be found in SI Appendix.
Neutrophil Isolation and Culture.
Neutrophils were isolated (51) from the venous blood of healthy volunteers. The Clinical Research Committee at the Maisonneuve-Rosemont Hospital approved the experimental protocols (permit No. 99097) and each blood donor gave written informed consent. Neutrophils (5 × 106 cells/mL, purity >96%, viability >98%) were cultured in RPMI medium 1640 supplemented with 10% autologous serum, IFN-β (12.5 to 50 ng/mL; Peprotech), 15-epi-LXA4 (1 μM; Cayman Chemical) or 17-epi-RvD1 (200 nM; Cayman Chemical), and then challenged with CpG DNA (E. coli strain B, 0.4 to 1.6 μg/mL). In some experiments, neutrophils were cultured with the FPR1 inhibitor cyclosporin H (1 μM, Tocris) or the ALX/FPR2 inhibitors N-t-Boc-Phe-Leu-Phe-Leu-Phe (Boc-2, 50 μM; MP Biomedicals) or Trp-Arg-Trp-Trp-Trp (WRW4, 5 μM; Tocris) (20), and then challenged with IFN-β and CpG DNA.
Apoptosis.
Apoptosis in human neutrophils was assessed by annexin-V staining, nuclear DNA content, and mitochondrial transmembrane potential with flow cytometry (9).
CD11b and CD88 Expression.
Neutrophil surface expression of CD11b and CD88 was assessed using R-phycoerythrin-conjugated anti-CD11b antibody (BD Biosciences) and fluorescein isothiocyanate (FITC)-labeled anti-CD88 antibody (BioLegend), respectively, with flow cytometry (9).
Phagocytosis and Phagocytosis-Induced Cell Death.
For quantitative analysis of phagocytosis, neutrophils were mixed with FITC-labeled E. coli (Invitrogen) for 30 min and intracellular fluorescence was analyzed with flow cytometry (9). For assessment of cell death, neutrophils were cultured for 24 h with live E. coli (American Type Tissue Culture) with or without IFN-β. Apoptosis was assessed by annexin-V staining and mitochondrial transmembrane potential with flow cytometry.
Bacterial Killing.
Neutrophils were cultured with IFN-β and CpG DNA and then mixed with E. coli for 3 h. Bacteria levels in culture media were assessed by growth on tryptic agarose.
In Vivo Lung Inflammation.
Female C57BL/6 mice (aged 8 to 14 wk, Charles River Laboratories) were housed in pathogen-free conditions. The Animal Care Committee of the Maisonneuve-Rosemont Hospital approved the protocols (permit Nos. 2015-31 and 2019-1765). Mice were injected intratracheally with 5 × 106 live E. coli with or without simultaneous injection of CpG DNA (1 μg/g, intraperitoneally [i.p.]) (9). At 6 h postinfection, mice were treated with IFN-β (BioLegend) (2.5 ng/g, i.p.), 15-epi-LXA4 (125 ng/g, i.p.), or 17-epi-RvD1 (25 ng/g, i.p.), as informed from previous studies (9, 51, 52). Some mice received cyclosporin H (5 μg/g, i.p.) or WRW4 (1 μg/g, i.p.) (28) 10 min before IFN-β or were injected with an anti–IFN-β antibody (1 μg/20 g, i.p., Abcam) or IgG1 before intratracheal challenge with E. coli.
Assessment of Inflammation.
At the indicated times, mice were killed and the lungs were lavaged. Lavage fluid protein, leukocyte counts, percentage of apoptotic neutrophils and macrophages containing apoptotic bodies, IFN-β, 15-epi-LXA4, RvD1, and cytokine levels were determined (9, 14). In separate experiments, lungs were removed without lavage and processed for histological evaluation, determination of dry-to-wet weight ratio, tissue myeloperoxidase activity, and bacterium contents (9, 51).
Statistical Analysis.
Results are expressed as means ± SEM. Statistical comparisons were made by analysis of variance using ranks (Kruskal–Wallis test) followed by Dunn’s multiple contrast hypothesis test to identify differences between various treatments. Correlations were analyzed by the Spearman test. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Lucie Boutin (Clinical Research Unit, Maisonneuve-Rosemont Hospital) for managing blood donors and blood collection and Julie Dubeau (Animal Care Facility, Maisonneuve-Rosemont Hospital) for help with mouse experiments. This work was supported by grants from Canadian Institutes of Health Research (MOP-97742 and MOP-102619) (to J.G.F.). M.S. is in receipt of a doctoral research award from the Faculty of Medicine, University of Montreal.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. C.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201146119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Bellani G., et al. ; LUNG SAFE Investigators; ESICM Trials Group, Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315, 788–800 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Ferguson N. D., et al. , The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 38, 1573–1582 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Thompson B. T., Chambers R. C., Liu K. D., Acute respiratory distress syndrome. N. Engl. J. Med. 377, 562–572 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A., et al. , Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 45, 486–552 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Slutsky A. S., Ranieri V. M., Ventilator-induced lung injury. N. Engl. J. Med. 369, 2126–2136 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Channappanavar R., et al. , Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19, 181–193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israelow B., et al. , Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 217, e20201241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S. H., et al. , Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 18, 1104–1116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekheri M., El Kebir D., Edner N., Filep J. G., 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc. Natl. Acad. Sci. U.S.A. 117, 7971–7980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.József L., Khreiss T., Filep J. G., CpG motifs in bacterial DNA delay apoptosis of neutrophil granulocytes. FASEB J. 18, 1776–1778 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Karaghiosoff M., et al. , Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 4, 471–477 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kelly-Scumpia K. M., et al. , Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J. Exp. Med. 207, 319–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarda G., et al. , Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Kumaran Satyanarayanan S., et al. , IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat. Commun. 10, 3471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan C. N., et al. , Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilroy D. W., Lawrence T., Perretti M., Rossi A. G., Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Serhan C. N., Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan C. N., Levy B. D., Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perretti M., D’Acquisto F., Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Ye R. D., et al. , International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooray S. N., et al. , Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. U.S.A. 110, 18232–18237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filep J. G., Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc. Natl. Acad. Sci. U.S.A. 110, 18033–18034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perretti M., Godson C., Formyl peptide receptor type 2 agonists to kick-start resolution pharmacology. Br. J. Pharmacol. 177, 4595–4600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filep J. G., Ariel A., Neutrophil heterogeneity and fate in inflamed tissues: Implications for the resolution of inflammation. Am. J. Physiol. Cell Physiol. 319, C510–C532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang N., et al. , Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnamoorthy N., Abdulnour R. E., Walker K. H., Engstrom B. D., Levy B. D., Specialized proresolving mediators in innate and adaptive immune responses in airway diseases. Physiol. Rev. 98, 1335–1370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzhagalov I., St John A., He Y. W., The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenceslau C. F., McCarthy C. G., Szasz T., Goulopoulou S., Webb R. C., Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am. J. Physiol. Heart Circ. Physiol. 308, H768–H777 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A. G., et al. , Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Potey P. M., Rossi A. G., Lucas C. D., Dorward D. A., Neutrophils in the initiation and resolution of acute pulmonary inflammation: Understanding biological function and therapeutic potential. J. Pathol. 247, 672–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong Y., Shimoda S., Shimono N., Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 61, 185–188 (2018). [DOI] [PubMed] [Google Scholar]
- 32.La Combe B., et al. ; COLOCOLI group, Pneumonia-specific Escherichia coli with distinct phylogenetic and virulence profiles, France, 2012-2014. Emerg. Infect. Dis. 25, 710–718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratanarat R., et al. , Usefulness of a molecular strategy for the detection of bacterial DNA in patients with severe sepsis undergoing continuous renal replacement therapy. Blood Purif. 25, 106–111 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Schwartz D. A., et al. , CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J. Clin. Invest. 100, 68–73 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knuefermann P., et al. , CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir. Res. 8, 72 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campana L., et al. , The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury. J. Immunol. 200, 1169–1187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins D. J., et al. , Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production. PLoS Pathog. 9, e1003479 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eshleman E. M., Lenz L. L., Type I interferons in bacterial infections: Taming of myeloid cells and possible implications for autoimmunity. Front. Immunol. 5, 431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dufton N., et al. , Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 184, 2611–2619 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gobbetti T., et al. , Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc. Natl. Acad. Sci. U.S.A. 111, 18685–18690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petri M. H., et al. , The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 105, 65–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X., et al. , Fpr2 deficiency alleviates diet-induced insulin resistance through reducing body weight gain and inhibiting inflammation mediated by macrophage chemotaxis and M1 polarization. Diabetes 68, 1130–1142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lahoz-Beneytez J., et al. , Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 127, 3431–3438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serhan C. N., et al. , The Atlas of Inflammation Resolution (AIR). Mol. Aspects Med. 74, 100894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haworth O., Cernadas M., Yang R., Serhan C. N., Levy B. D., Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 9, 873–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamoorthy S., et al. , Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 1660–1665 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T., et al. , HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 116, 23254–23263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christian M. D., Poutanen S. M., Loutfy M. R., Muller M. P., Low D. E., Severe acute respiratory syndrome. Clin. Infect. Dis. 38, 1420–1427 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J. S., Shin E.-C., The type I interferon response in COVID-19: Implications for treatment. Nat. Rev. Immunol. 20, 585–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchieri G., Type I interferon: Friend or foe? J. Exp. Med. 207, 2053–2063 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Kebir D., et al. , 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am. J. Respir. Crit. Care Med. 180, 311–319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brancaleone V., et al. , A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood 122, 608–617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.