ABSTRACT
The intestinal microbiota produces β-glucuronidase that plays an essential role in the metabolism of the immunosuppressant mycophenolate mofetil (MMF). This drug is commonly used in organ and hematopoietic cell transplantation (HCT), with variations in dosing across transplant types. We hypothesized that β-glucuronidase activity differs between transplant types, which may account for differences in dosing requirements. We evaluated fecal β-glucuronidase activity in patients receiving MMF post-allogeneic HCT and post-kidney transplant. Kidney transplant patients had significantly greater β-glucuronidase activity (8.48 ± 6.21 nmol/hr/g) than HCT patients (3.50 ± 3.29 nmol/hr/g; P = .001). Microbially mediated β-glucuronidase activity may be a critical determinant in the amount of mycophenolate entering the systemic circulation and an important factor to consider for precision dosing of MMF.
KEYWORDS: Beta-glucuronidase, immunosuppression, intestinal microbiota, mycophenolate, MMF, transplantation
To the editor
The intestinal microbiota is associated with human health and performs many functions for the body including protective, metabolic, and structural functions.1 Antibiotic and immunosuppressive treatments, however, adversely affect the composition and functions of the microbiota.2–4 Recently, the microbiota has received attention as a collection of agents that can affect the efficacy of drugs, including immunosuppressants (IS), by altering normal metabolism and/or transport, or by facilitating drug bioaccumulation in cells.4–8 Mycophenolate mofetil (MMF) is an IS prodrug that is widely used in combination with tacrolimus (TAC) for post-allogeneic hematopoietic cell transplantation (HCT) and kidney transplantation. The active form of MMF, mycophenolic acid (MPA), inhibits the proliferation of T and B lymphocytes thereby reducing the risk of graft-versus-host disease (GVHD), improves stem cell engraftment after HCT, and reduces the risk of allograft rejection after kidney transplant.9 Blood MPA concentrations are influenced by enterohepatic recycling (EHR) of MPA due to the de-glucuronidation of MPA glucuronide (MPAG, major inactive metabolite of MMF) by microbial β-glucuronidase post-biliary excretion.4,10 We recently showed that therapeutic concentrations of MPA in the blood of HCT patients were associated with the microbiome composition measured in their fecal samples, although the mechanism for this finding remains unknown.4 We observed that individuals with therapeutic MPA concentrations were more likely to have β-glucuronidase-producing bacteria in their stool and significantly greater EHR and reformation of MPA.
Despite the importance of MMF in facilitating donor engraftment and preventing GVHD, 50% of patients suffer from GVHD and associated non-relapse mortality,4,11 and 5–10% of kidney transplant recipients with have an acute rejection event leading to a higher risk of kidney graft loss.12 Variability in MPA plasma concentrations among patients affects the potential for adverse clinical outcomes. Lower concentrations increase the risk of GVHD and rejection, while higher concentrations are associated with toxicity.10,13,14 There is a well-known difference in the doses of MMF needed to achieve therapeutic concentrations in HCT and kidney transplant recipients. Lower MPA exposure is observed in HCT recipients compared to kidney transplant recipients receiving a similar MMF dose.15–17 Reasons for this difference are not understood but may be due to lower β-glucuronidase activity in the stool of HCT recipients, resulting in lower enterohepatic recirculation and reformation of MPA, lower MPA exposure in the blood, and the need for higher MMF doses. Understanding microbially mediated processes that affect MPA exposure is required to inform precision MMF dosing and for other drugs that undergo β-glucuronidase-mediated enterohepatic recirculation (e.g. irinotecan).18
We compared β-glucuronidase activity from stool samples of patients receiving MMF and tacrolimus post-HCT or kidney transplant. We hypothesized that, based on observed differences in therapeutic dosing regimens between transplantation types, β-glucuronidase activity would differ between transplant types. We observed that fecal β-glucuronidase activity was over two-fold lower in HCT patients (3.50 ± 3.29 nmol/h/g) than in kidney transplant patients (8.48 ± 6.21 nmol/h/g, P = .001; Figure 1). Previous studies have found an association of distinct intestinal microbiota with clinical outcomes including infectious complications in HCT recipients19–21 and dosing of the IS drug tacrolimus (TAC) in kidney transplant recipients.22 In addition, greater abundances of the genera Blautia and Enterococcus, and decreasing abundances of clostridia have been associated with a lower incidence of GVHD-related mortality in HCT patients.19–21 In kidney transplant patients, Lee, et al.22 hypothesized that the abundance of Faecalibacterium prausnitzii was associated with a healthy and diverse colon and found a positive correlation between TAC dosage and F. prausnitzii. This hypothesis was tested in vitro by Guo, et al.23 with results suggesting involvement of F. prausnitzii in metabolism of TAC. Taken together, these results suggest that improved understanding of the role of the microbiota in biotransformation and transporting IS drugs will be critical to inform precision therapy to maximize efficacy.
Figure 1.
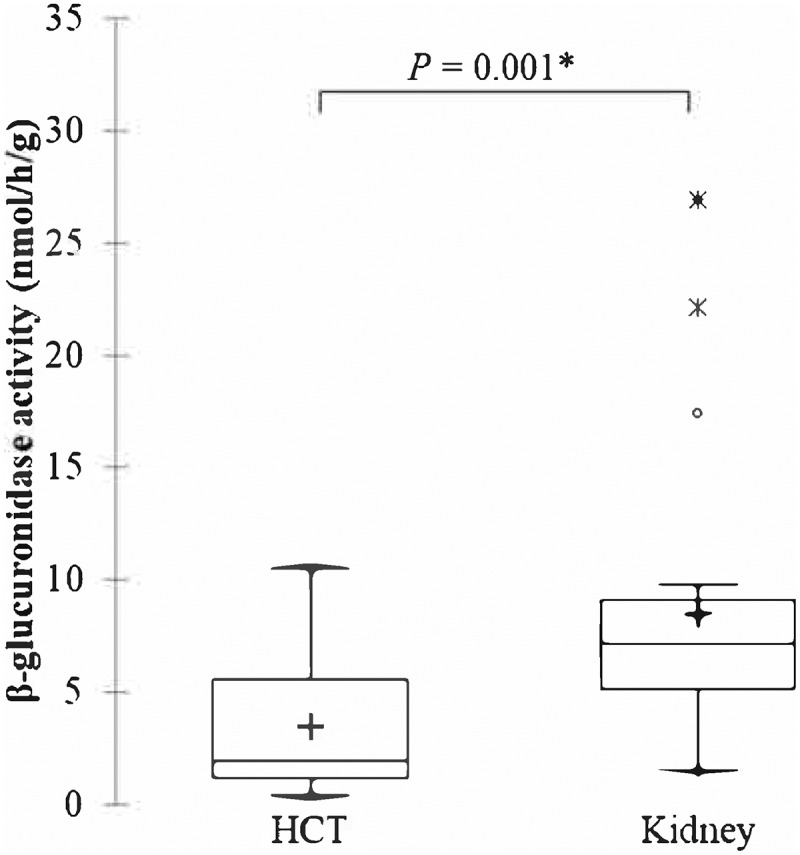
Β-glucuronidase activity observed in fecal samples from patients in the HCT and MISSION studies. Boxes show median and interquartile ranges, with mean denoted by +.
There have been few studies that have similarly assessed fecal β-glucuronidase activity in human studies, and results are difficult to compare due to the various methodologies reported.24 A recent study of patients with celiac disease, non-celiac gluten sensitivity, and healthy controls found similar levels of fecal β-glucuronidase activity in all groups (mean ± standard deviation: 30.0 ± 15.0, 25.9 ± 15.0, and 29.9 ± 18.0 U g−1 wet weight, respectively).25 Similarly, an earlier study of children with inflammatory bowel disease (IBD), compared to healthy controls, found that while there was a non-significant difference in fecal β-glucuronidase activity between groups, the activity from children with IBD (mean 15.86, range 0.12–81.63 mM phenolphthalein/mg protein/h) was less than half that of controls (44.86, 5.82–141.13 mM/mg/h). In a study of kidney transplantation patients who received MMF, no differences in fecal β-glucuronidase activity were observed among patients who had diarrhea vs. those who did not (median 4.6 vs. 4.4 mg free phenolphthalein/mg protein/h, P = .78).26 A significantly greater percentage of diarrheal patients with high β-glucuronidase activity (91%) had a prolonged course (≥7 days) of diarrhea, while only 40% of low β-glucuronidase-activity patients had a prolonged course.27 In a murine study, exposure to MMF resulted in an altered microbial community composition with increased β-glucuronidase activity that was ablated when vancomycin was given.28 Results of these studies highlight the role of microbial β-glucuronidase in MMF-related toxicity and adverse side effects.
Several factors have been associated with variability in MPA concentrations including kidney function, albumin, weight, diabetes and time post-transplant, although they have not been consistently observed in all studies.17,29,30 There are also several important drug interactions with MPA. The combination of TAC with MPA results in higher MPA plasma concentrations and all of our patients received TAC. Antibiotics such as norfloxacin and metronidazole, ciprofloxacin, amoxicillin-clavulanic acid result in significantly lower MPA concentrations.31–33 These interactions have been hypothesized to be due to antimicrobial-related alterations in gut microbiota that change enterohepatic recirculation and reabsorption of MPA. HCT patients receive chemotherapy and more anti-infective agents than kidney transplant recipients in the early post-transplant period. Our observation that β-glucuronidase activity is significantly lower in HCT recipients is highly consistent with the consequences of high antibacterial drug pressure and elimination of β-glucuronidase producing bacteria.
In our HCT population, 20 adult (18–75 years) participants undergoing allogeneic transplant for hematologic malignancies were enrolled and are described in our previous study.4 The HCT study protocol was IRB approved (Study#00005621) and all patients provided written, informed consent. All HCT participants received prophylactic MMF (Cellcept or generic) and TAC (Prograf or generic) at time of stool collection on day +7. Mycophenolate mofetil was administered 1 g every 8 h intravenously (IV) over 2 h, every 8 hours beginning on day +5. Tacrolimus (0.03 mg/kg/day) was administered IV beginning at post-transplant day 5 to promote stem cell engraftment and to prevent GVHD. In our kidney transplant study, patients were enrolled in the Microbiome and Immunosuppression (MISSION) study (NCT04953715). This study was IRB approved (Study#00032309) and patients provided written, informed consent. Twenty-two adult patients (≥18 years) were studied and the stool collected a median of 69 days of receiving a living or deceased donor kidney transplant. Patients were receiving MMF (Cellcept or generic) 500 mg/g twice a day and TAC (Prograf or generic) orally as maintenance IS.
Stool samples were collected in single-use specimen collector pans and transferred to 30 mL polystyrene tubes for storage. Single samples were collected from each patient. Samples were refrigerated immediately and transferred to the lab frozen (−20°C) and stored at −80°C prior to assay. Samples from patients in both the HCT and kidney-transplantation studies were treated identically for lab analysis. β-glucuronidase activity was measured in stool samples using a fluorometric assay kit (ab234625; Abcam, Cambridge, United Kingdom) according to the manufacturer’s instructions. Briefly, 100 µl of β-glucuronidase assay buffer (BGA) was added to a 10 mg (wet weight) stool sample. The sample was homogenized at room temperature for 10 min using an ultrasonic bath (2 A, 40 kHz constant frequency; Thermo Fisher Scientific, Waltham, MA, United States). The lysates were then centrifuged at 10,000 × g for 5 min at 4°C. The supernatant volume was adjusted to 90 µl with BGA before adding 10 µl of proprietary substrate (provided with the ab234625 kit). All reactions were performed in Microfluor™ 96-well black plates with flat bottoms (Thermo Fisher Scientific). Fluorescence measurement (Ex/Em = 330/450 nm) was done on a Biotek ELISA reader (Agilent Technologies, Santa Clara, CA, United States) immediately after adding substrate for 0–60 mins at 37°C. A background (no substrate) control, positive control and standard (4-methylumberlliferone; 4-MU) dilutions were also included while performing the assay. Enzyme activity was expressed as nmol 4-MU hr−1 gm−1 sample. Differences in β-glucuronidase activity were evaluated using the non-parametric Kruskal Wallis test. Statistical analyses were performed using XLSTAT software version 2020.3.1 (Addinsoft, Belmont, MA) at α = 0.05.
Funding Statement
The HCT study was supported by a University of Minnesota, Masonic Cancer Center Chainbreaker award, NIH grant P30CA077598, and the National Center for Advancing Translational Sciences of the National Institutes of Health P30CA077598, UL1-TR00249, R01AI140303 Award Number UL1-TR00249. The MISSION study was supported by NIH R01AI140303 from the NIAID. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health; Masonic Cancer Center, University of Minnesota;
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
There are no conflicts of interest to report.
Author contributions
MHK and GCO received samples and performed β-glucuronidase assays. MHK and CS performed data analysis and drafted the manuscript. AI and PAJ oversaw study design and patient recruitment. GCO, AR, SGH, AK, AI, and PAJ critically reviewed and enhanced the manuscript. All authors have read and approved the final manuscript.
References
- 1.Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashidi A, Kaiser T, Graiziger C, Holtan SG, Rehman TU, Weisdorf DJ, Dunny GM, Khoruts A, Staley C.. Gut dysbiosis during antileukemia chemotherapy versus allogeneic hematopoietic cell transplantation. Cancer. 2020;126:1434–1447. doi: 10.1002/cncr.32641. [DOI] [PubMed] [Google Scholar]
- 4.Saqr A, Carlson B, Staley C, Rashidi A, Al-Kofahi M, Kaiser T, Holtan S, MacMillan M, Young J-A, El JN, et al. Reduced enterohepatic recirculation of mycophenolate and lower blood concentrations are associated with the stool bacterial microbiome after hematopoietic cell transplantation. Transplant Cell Ther. 2022. doi: 10.1016/j.jtct.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klünemann M, Andrejev S, Blasche S, Mateus A, Phapale P, Devendran S, Vappiani J, Simon B, Scott TA, Kafkia E, et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021;597:533–538. doi: 10.1038/s41586-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmassry MM, Kim S, Busby B. Predicting drug-metagenome interactions: variation in the microbial β-glucuronidase level in the human gut metagenomes. PLoS One. 2021;16:e0244876. doi: 10.1371/journal.pone.0244876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JS, Sze C, Barbar T, Lee JR. New insights into the microbiome in kidney transplantation. Curr Opin Organ Transplant. 2021;26:582–586. doi: 10.1097/MOT.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88:1351–1389. doi: 10.1007/s00204-014-1247-1. [DOI] [PubMed] [Google Scholar]
- 10.Okour M, Jacobson PA, Ahmed MA, Israni AK, Brundage RC. Mycophenolic acid and its metabolites in kidney transplant recipients: a semimechanistic enterohepatic circulation model to improve estimating exposure. J Clin Pharmacol. 2018;58:628–639. doi: 10.1002/jcph.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adom D, Rowan C, Adeniyan T, Yang J, Paczesny S. Biomarkers for allogeneic HCT outcomes. Front Immunol. 2020;11:673. doi: 10.3389/fimmu.2020.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narouie B, Saatchi M. Short term and long term survival rate and risk factors of graft rejection after deceased donor kidney transplantation: a systematic review and meta-analysis. Transl Res Urol. 2021;3:95–114. [Google Scholar]
- 13.Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, Tan Y, Long J, Remmel R, Sawchuk R, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78:486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Harnicar S, Ponce DM, Hilden P, Zheng J, Devlin SM, Lubin M, Pozotrigo M, Mathew S, Adel N, Kernan NA, et al. Intensified mycophenolate mofetil dosing and higher mycophenolic acid trough levels reduce severe acute graft-versus-host disease after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2015;21:920–925. doi: 10.1016/j.bbmt.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, Petersdorf E, McSweeney P, Pulsipher M, Woolfrey A, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 16.Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, Cole S, Nash RA, Storb RF, Georges GE. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106:4381–4388. doi: 10.1182/blood-2005-06-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Pellock SJ, Redinbo MR. Glucuronides in the gut: sugar-driven symbioses between microbe and host. J Biol Chem. 2017;292:8569–8576. doi: 10.1074/jbc.R116.767434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, Zhu W, Sporrer D, Hehlgans T, Kreutz M, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:640–645. doi: 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, Kim M, Zhan X, Greenberg DE, Xie Y, et al. Antibiotic-induced depletion of anti-inflammatory clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol Blood Marrow Transplant. 2017;23:820–829. doi: 10.1016/j.bbmt.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee JR, Muthukumar T, Dadhania D, Taur Y, Jenq RR, Toussaint NC, Ling L, Pamer E, Suthanthiran M. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS One. 2015;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Crnkovic C, Won KJ, Lee JR, Orjala J, Lee H, Jeong H. Commensal gut bacteria convert the immunosuppressant tacrolimus to less potent metabolites. Drug Metab Dispos. 2019;47:194–202. doi: 10.1124/dmd.118.084772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T, Li G, Su Z, Liu J, Wang P. Recent advances of sensing strategies for the detection of β-glucuronidase activity. Anal Bioanal Chem. 2022;414:2935–2951. doi: 10.1007/s00216-022-03921-y. [DOI] [PubMed] [Google Scholar]
- 25.Nylund L, Hakkola S, Lahti L, Salminen S, Kalliomäki M, Yang B, Linderborg KM. Diet, perceived intestinal well-being and compositions of fecal microbiota and short chain fatty acids in oat-using subjects with celiac disease or gluten sensitivity. Nutrients. 2020;12:2570. doi: 10.3390/nu12092570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang LT, Westblade LF, Iqbal F, Taylor MR, Chung A, Satlin MJ, Magruder M, Edusei E, Albakry S, Botticelli B, et al. Gut microbiota profiles and fecal beta-glucuronidase activity in kidney transplant recipients with and without post-transplant diarrhea. Clin Transplant. 2021;35:e14260. doi: 10.1111/ctr.14260. [DOI] [PubMed] [Google Scholar]
- 27.Mroczyńska M, Gałȩcka M, Szachta P, Kamoda D, Libudzisz Z, Roszak D. β-glucuronidase and β-glucosidase activity in stool specimens of children with inflammatory bowel disease. Polish J Microbiol. 2013;62:319–325. doi: 10.33073/pjm-2013-043. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MR, Flannigan KL, Rahim H, Mohamud A, Lewis IA, Hirota SA, Greenway SC. Vancomycin relieves mycophenolate mofetil–induced gastrointestinal toxicity by eliminating gut bacterial -glucuronidase activity. Sci Adv. 2019;5:eaax2358. doi: 10.1126/sciadv.aax2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherwin CMT, Fukuda T, Brunner HI, Goebel J, Vinks AA. The evolution of population pharmacokinetic models to describe the enterohepatic recycling of mycophenolic acid in solid organ transplantation and autoimmune disease. Clin Pharmacokinet. 2012;50:1–24. doi: 10.2165/11536640-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Qin X, Wang Y, Huang Z, Li X. Controlled-dose versus fixed-dose mycophenolate mofetil for kidney transplant recipients: a systematic review and meta-analysis of randomized controlled trials. Transplantation. 2013;96:361–367. doi: 10.1097/TP.0b013e31828c6dc7. [DOI] [PubMed] [Google Scholar]
- 31.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66:487–495. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 32.Ilett EE, Jørgensen M, Noguera-Julian M, Nørgaard JC, Daugaard G, Helleberg M, Paredes R, Murray DD, Lundgren J, MacPherson C, et al. Associations of the gut microbiome and clinical factors with acute GVHD in allogeneic HSCT recipients. Blood Adv. 2020;4:5797–5809. doi: 10.1182/bloodadvances.2020002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashidi A, Kaiser T, Holtan SG, Rehman TU, Weisdorf DJ, Khoruts A, Staley C. Levaquin gets a pass: levofloxacin and dysbiosis during intensive therapy. Biol Blood Marrow Transplant. 2020;26:778–781. doi: 10.1016/j.bbmt.2019.12.722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


