Abstract
Autism Spectrum Disorders (ASD) and Williams Syndrome (WS) are frequently characterized as mirror conditions in the socio-cognitive domain, with ASD entailing restrictive social interests and with WS exhibiting hypersociability. In this review paper, we examine in detail the strong points and deficits of people with ASD or WS in the socio-cognitive domain and show that both conditions also share some common features. Moreover, we explore the neurobiological basis of the social profile of ASD and WS and found a similar mixture of common affected areas and condition-specific impaired regions. We discuss these findings under the hypothesis of a continuum of the socio-cognitive abilities in humans.
Keywords: Autism spectrum disorders, Williams syndrome, social cognition, social behavior, neurobiology, human evolution
Introduction
Williams syndrome (WS) is a complex clinical condition which is defined on an etiological basis, as most cases result from the deletion of 1.5 to 1.8 Mb in one of the chromosomes 7, affecting nearly 30 genes in the on 7q11.23 region (Korenberg et al. 2000, Pober 2010). WS presents with a set of distinctive physical, cognitive, and behavioral features, including altered growth patterns, craniofacial anomalies, heart problems, gastrointestinal and genitourinary disease, skin defects, intellectual disability, and impaired visuospatial cognition, but with quite spared sociability, notable musical abilities, and substantially preserved language (Morris et al. 2003, Mervis and Becerra 2007, Martens et al. 2008, Pober 2010). By contrast, Autism Spectrum Disorders (ASD) is a cover term for a set of pervasive neurodevelopmental disorders mostly defined on a symptomatic basis, with all of them exhibiting language and communication problems, repetitive and stereotypical behaviour, and problems with social interaction (American Psychiatric Association 2013). Contrary to WS, ASD has a complex, unclear etiology, as many genes have been associated with this condition, but also several environmental factors (Geschwind and State 2015, Bölte et al. 2019, Gyawali and Patra 2019).
At first sight, ASD and WS can be viewed as opposite conditions in the domain of social cognition and behavior. Individuals with ASD are normally characterized as withdrawn, difficult to engage in social interaction, struggling to understand social norms, and generally uninterested in social relationships with others (for a general review, see Newschaffer et al. 2007). This hyposocial phenotype starkly contrasts with the hypersocial phenotype exhibited by people with WS, who are usually characterized as overly friendly, gregarious, and eager to interact with others, sometimes to an excessive degree (for a general review, see Bellugi et al. 2000, Jones et al. 2000, Doyle et al. 2004, Martens et al. 2008, Järvinen et al. 2013). However, this is just a rough picture that deserves a closer examination, particularly at a time when studies about social cognition and behavior in these two conditions have grown exponentially. It is now clear that the management of the social context by affected people can be crucial for understanding the different clinical presentation of ASD and WS, considering that in terms of general cognition, both conditions are quite similar when social motivation is removed. For instance, Vivanti and colleagues (2016) found that when the objective of a task is learning and not social interaction, both ASD and WS participants are equally able to imitate and learn in social situations (see Ingersoll et al. 2013. Berger and Ingersoll 2015 for similar findings).
A second reason that makes this comparative study interesting is that ASD and WS are thought to share genetic determinants (Newschaffer et al 2007, Jawaid et al 2012) so a common genetic basis for their respective (even opposite) social deficits (and strong points) can be hypothesized. Supporting this view, in a recent paper we have found a significant overlap between the genes that are abnormally expressed in ASD and WS compared to neurotypical controls, with most of them exhibiting a similar trend of dysregulation in both conditions. Accordingly, they are usually found either upregulated or downregulated in both ASD and WS, although fold-changes are usually greater in WS than in ASD. Not surprisingly, most of these genes are involved in aspects of brain development and function (particularly, dendritogenesis) and are expressed in brain areas (particularly, the cerebellum, the thalamus and the striatum) of relevance for both the ASD and the WS etiopathogenesis (Niego and Benítez-Burraco 2020, see Tebbenkamp et al. (2014)for a more general review of how developmental transcriptome data can be used to improve our understanding of the etiology of complex neurodevelopmental disorders, particularly, ASD and WS). For instance, the gene EPHB1, which encodes a receptor for ephrin-B family members, is found downregulated in the blood of people with ASD and WS. A polymorphism of EPHB1 has been associated to attentive behavior to faces (Yang et al. 2016). Interestingly, EphB1 knocked-out mice show aberrant thalamic-cortical axon guidance (Robichaux et al. 2014), which, as discussed below (Section 4), is a common feature of both ASD and WS. Recent findings about two other purportedly mirror conditions, namely, ASD and schizophrenia (SZ), suggest that the same genes can be a risk factor for both conditions (Zhou et al. 2016, Zarrei et al. 2019), whereas common biological mechanisms (i.e. synaptic plasticity, brain connectivity) are implicated in the aetiology of both SZ and ASD regardless of their (partially) different clinical profiles and onset times (Liu et al. 2017). Overall, this evidence supports the view of a continuum for the human socio-cognitive phenotype, with neurodevelopmental disorders resulting from selective damage of specific biological mechanisms involved in brain development and maturation, mostly during sensitive and critical periods of brain growth (see Meredith 2015 for details).
A final reason is that exploring these overlaps and divergences between ASD and WS in the socio-cognitive domain is expected to reveal interesting findings about the evolution of human social cognition. An important reason for this is the deep link that exists between abnormal ontogeny and evolution, with human-specific cognitive abilities arising to a great extent from changes in preexisting neural circuits, but with these human-specific brain features being implicated, as noted, in neurodevelopmental and neurodegenerative disease risk (see Pattabiraman et al. 2020 for discussion). In turn, this is seemingly due to the circumstance that recently-evolved aspects of human cognition and behavior are more sensitive to ontogenetic damage because of their reduced resilience (see Toro et al. 2010 for ASD).
In this paper we examine in detail the similarities and differences between the socio-cognitive deficits (and strong points) exhibited by participants with ASD and WS. In doing so, we have relied on available repositories of technical papers, particularly, PubMed (https://pubmed.ncbi.nlm.nih.gov/). Whenever possible, we have made use of meta-analyses and review papers. We have mostly focused on papers published from 2000 to the present. Additionally, we provide a detailed discussion regarding the neurobiological basis of the highlighted deficits and strong points. Overall, we reveal an intricate profile of similarities and differences, which we discuss under the hypothesis of a continuum for the human socio-cognitive phenotype.
Socio-cognitive similarities between ASD and WS
In spite of people with WS being labeled as ‘overly friendly’ and ‘hypersocial’, they exhibit many difficulties in the social arena that overlap in part with ASD. Accordingly, parents of children with WS often report that they have poor social skills, difficulties with understanding important social cues or information, and difficulty maintaining friendships (Mervis et al. 2001, Sullivan et al. 2003, Stojanovik 2006, Klein-Tasman et al. 2009, Järvinen et al. 2015). Included in these similarities are “…social isolation, and other types of social impairment, distractibility, inflexibility, ritualism, obsessiveness, and pragmatic deficits” (Gillberg and Rasmussen 1994). An important study illustrating these parallels was conducted by Järvinen and colleagues (2015). The study aimed at directly comparing responses to emotional stimuli in participants with ASD and WS, to see if there were unique profiles of behavioral responses between or across groups. 52 children participated: 12 with WS and 17 with ASD, and 20 typically-developing (TD) controls. The experimental portion required participants to identify emotions depicted in different pictures (after a ‘passive task’ that required them to simply look at the pictures carefully). There were social tasks, wherein the images depicting emotion included photos of readily identifiable facial expressions (i.e. ‘happy’, ‘angry’ etc), and non-social tasks, wherein the images did not include human faces, but instead more neutral images of nature and sometimes animals. The study found that both participants with ASD and WS had a similar overall degree of social dysfunction when it comes to identifying emotions, both with social and non- social prompts, although it was noted that participants with WS exhibited more variability in their impairment across social domains.
Interestingly too, both groups also show difficulties with the typical boundaries of personal space (Lough et al. 2015). These shared social difficulties make them more vulnerable socially, prone to disadvantages when it comes to forming productive relationships and social connections. Social vulnerability is thought to be the source of some of the anxiety documented in both groups, which report higher levels compared to neurotypical people (Dykens 2003, Graham et al. 2005, Jawaid et al. 2012). Still, notable differences between conditions can be found. Hence, prevalence rates for social anxiety in WS are approximately 1% whereas it rises to approximately 30% in ASD (Simonoff et al. 2008, Royston et al. 2017). This elevated anxiety is coupled in many cases with restricted and repetitive behaviors (Rodgers et al. 2012).
To an important extent, these problems in the social sphere are expected to arise from deeper cognitive and behavioral dysfunction. People with WS exhibit a mean IQ about 55 (Pober 2010), with intellectual functioning remaining stable across adolescence and adulthood, but with adaptive functioning declining over time (Fisher et al. 2016), whereas participants with ASD have more variable IQ levels, with more severe forms being associated with lower scores suggestive of intellectual disability (Dykens and Lense 2011). More specifically, young children with ASD or WS show delays in pointing behaviors and joint attention, and underperform in theory of mind (ToM) tasks (i.e. tasks aimed at evaluating o evaluate the participant’s ability to attribute mental states to others), like the false belief test (Baron-Cohen et al. 1985, Charman et al. 1997, Klein-Tasman et al. 2009, Vivanti et al. 2016). Also documented are difficulties in inhibitory control and shifting attention (Rhodes et al. 2010, Riby et al. 2011). Sparaci and colleagues found that both participants with ASD and WS had difficulty specifying the ‘why’ of an experimenter’s actions. That is, in a task where they were asked to observe a hand-object action (e.g. grasping a mug to drink tea or touching the handle to put it in a cupboard), both groups had difficulty specifying why they were doing so (i.e. they couldn’t say they were grasping the object to put it away). According to Sparaci and colleagues, this difficulty with predicting others’ intentions and actions can be hypothesized to arise from deficits in predicting physical actions. Indeed, motor impairments have been widely documented in both ASD (e.g. Teitelbaum et al. 1998, Jansiewicz et al. 2006, Dewey et al. 2007) and WS (see Trauner et al. 1989, Elliott et al. 2006, Gagliardi et al. 2007). In contrast to their similarities in ‘why (mis)understanding’, participants with ASD showed superior ability in specifying the ‘what’ of an experimenter’s actions. That is, they were better at specifying that the individual was grasping a mug vs. simply touching it, while those with WS showed more difficulty with this task. As Sparaci and colleagues (2015) point out, this difference in ‘what’ ability coupled with a similarity in ‘why’ ability is indicative of the fact that certain low level abilities may shape higher level processes in unexpected ways (Sparaci et al. 2015).
Finally, the socio-cognitive problems exhibited by people with ASD or WS are expected to have an impact on (and result in part from) their language deficits. Language development is generally delayed in both conditions, although it tends to follow the typical progression (Asada and Itakura 2012). In both ASD and WS, grammar was originally thought to be relatively spared, although more recent research has highlighted the fact that it is more impaired than it appears at the surface (see Perovic et al. 2013, Lacroix et al. 2016 for direct comparisons). In both WS and ASD, grammatical impairment has been shown to happen with aspects of grammar defined later in typical development, like raising and passives (Tager-Flusberg 1981, Perovic and Wexler 2007), grammatical morphology (Kjelgaard and Tager-Flusberg 2001, Roberts et al. 2004), relative clauses (Riches et al. 2010), and subject-control structures (Perovic and Janke 2013). Importantly for our concerns here, pragmatics (that is, the ability to use language for communicating in a social context) is impaired in both conditions (see Tager-Flusberg 2000, Doyle et al. 2004, Laws and Bishop 2004, Stojanovik 2006, Philofsky et al. 2007, Järvinen-Pasley et al. 2008, Asada and Itakura 2012, Lacroix et al. 2016 among others), although, as we will show in the next section, it is more so in ASD. Specifically, both ASD and WS individuals have been rated as impaired in the quality of their conversational initiations with others, with WS participants underperforming people with ASD (Philofsky et al. 2007). Likewise, narration, a basic pragmatic skill, has also been shown to be impaired in both conditions. Accordingly, Diez-Itza et al. (2016) reported in their study that WS individuals often give the impression of having in-tact narrative skills because of their overuse of discourse markers and exclamations, but in-depth analyses further reveal deficiencies in sequencing narratives. Similar deficits in sequencing narratives have been reported in ASD (see Freeman and Dake 1996, Happé and Frith 1996).
Studies have shown a direct correlation between socio-cognitive skills and deficits and pragmatic language (dis)abilities in both WS and ASD (Happé 1993, Surian et al. 1996, Hale and Tager-Flusberg 2005, John et al. 2009), suggesting that deficits in the social domain impact negatively on language use in both conditions. Difficulties with pragmatics can be hypothesized to derive from specific deficits that both groups have, particularly, problems with inferring the mental states of others (Asada and Itakura 2012). Likewise, problems with narration, and specifically, the lack of ability to sequence events, might stem from difficulties in spatial cognition, at least in the case of WS (Phillips et al. 2004). At the same time, socio-cognitive deficits impacting language use certainly contribute to language delay in both conditions, as many aspects of language acquisition require inference of the speaker’s intentions (Preissler and Carey 2005). Other probable factors contributing to language delay are deficits in joint attention, which especially impact the acquisition of vocabulary and semantic meaning of words (Baron-Cohen et al. 1985, Charman et al. 1997, Klein-Tasman et al. 2007, Mervis and Becerra 2007, Mervis and John 2012). At the same time, as noted, there are aspects of language that appear similarly preserved in both conditions in spite of socio-cognitive impairment. For instance, in their study on grammatical binding, Perovic and colleagues (2013) came to the conclusion that the impairment of grammatical knowledge on binding in both conditions is independent of difficulties in social interactions and pragmatics (Perovic et al. 2013).
Socio-cognitive differences between ASD and WS
Although, as discussed above, similarities between ASD and WS can indeed be found and while the social profiles of both conditions are in no way homogeneous, research also consistently points to differences in the socio communicative profiles of people with ASD and WS. Although the latter have been reported to have less socio communicative deficits than the former (e.g. Bellugi et al. 2000, Lincoln et al. 2007, Klein-Tasman et al. 2009, Lacroix et al. 2016), increased social behavior exhibited by people with WS (e.g. placing unreasonable demands on friendships, overfriendliness, social vulnerabilities, difficulties disengaging, increased vulnerability with strangers, etc.) can be considered to be just as disordered as the opposite features exhibited by people with ASD.
In general, people with WS and ASD have been shown to have opposite preferences in terms of their orientation to social vs. nonsocial information, as reported by eye tracking studies such as Riby and Hancock (2008, 2009). More specifically, in social situations, individuals with ASD tend to direct their attention inward towards themselves and prioritize information related to them as individuals, while those with WS direct their attention to others. As pointed out by Kuang (2016), in a social setting, neurotypical people rely on both systems of attention (attention to self and attention to others) in order to interact properly. This increased attention towards others may in turn help individuals with WS to preserve more emotional empathy than those with ASD can, in spite of the fact that, as discussed previously, both exhibit difficulties with imagining mental states of others (Tager-Flusberg and Sullivan 2000). As a consequence, individuals with WS are eager to engage socially and are highly motivated to approach familiar and unfamiliar people (Bellugi et al. 2000). In contrast, people with ASD attend much less to socially salient features and are generally reluctant to engage with others, regardless of whether they are familiar or not (Sigman et al. 2006, Riby and Hancock 2009). Specifically, ASD and WS show distinct differences in the area of face recognition skills; people with WS are hyper-atentive to faces and reportedly perform better than mental age matched controls on standardized tests of face recognition skills, while those with ASD attend much less to faces and perform distinctly worse (Bellugi et al 1994, Klin et al. 1999, Schultz 2005, Tager-Flusberg et al. 2006, Rose et al. 2007). It should be noted here that there are conflicting findings as to whether or not participants with WS have an advantage over participants with ASD in the area of emotion recognition. As discussed in Section 2 above, Järvinen and colleagues (2015) found that WS and ASD participants had similar degrees of difficulty identifying emotions from facial expressions. By contrast, Lacroix and colleagues (2009), in their study involving 12 participants with WS and 12 participants with ASD, found that on a task requiring participants to identify an emotion from photos of facial expressions, people with WS performed significantly worse than both the TD and ASD groups.
Interestingly, when participants are asked to analyze emotion in music, individuals with WS outperform those with ASD (Bhatara et al. 2010), in spite of both conditions exhibiting similar affinity and interest towards music in general (Heaton et al. 1998, Bonnel et al. 2003, Heaton 2003, Bhatara et al. 2010). In truth, enhanced musical abilities of people with WS do not concern the structural aspects of music, but instead are related to musicality and expressivity, commonly expressed through a heightened emotional responsiveness to music (Thakur et al. 2018).
Both conditions also show contrast in the management of anxiety in social settings. In both WS and ASD, levels of anxiety correlate with their degree of social impairment, although those with ASD are thought to have higher levels of anxiety in general (Rodgers et al. 2012, see also Section 2 above). Higher levels of restricted and repetitive behaviors correlated to higher levels of anxiety in ASD but not WS (Rodgers et al. 2012), which suggests that these behaviors may serve different functions in both conditions. Barak and Feng (2016) posited that these differences in anxiety levels correlate to social cognition, that is, it may be that the social drive of WS acts as a buffer against social anxiety, while in ASD social impairments might make them more vulnerable to anxiety (see also Frigerio et al. 2006). White et al. 2010).
Regarding language, and particularly, language use in social settings, differences between conditions can be observed as well. As noted above, generally individuals with ASD show marked problems with the communicative use of language, while those with WS show elaborate attention to detail and expressive phrases that are full of emotion and affect (Udwin and Yule 1990, Bellugi et al. 1994, Bellugi et al. 2000, Reilly et al 2004, Brock et al. 2007, Gothelf et al. 2008, Fishman et al. 2011). Still, there are indications that the processes involved in acquiring and using these characteristics are irregular, as can be seen from research exploring atypical activation of semantic networks after pointing (Lukács et al. 2004). By contrast, in ASD, vocabulary progresses steadily with age, but it contains a disproportionately high number of nouns and a much lower number of mental state terms when compared to typically developing children (Fein et al. 1996, Gastgeb et al. 2006, Kelley et al. 2006, Swensen et al. 2007, Tek et al. 2008).
Other studies (e.g. Frith and Snowling 1983, Tager-Flusberg 2003, 2004, Harris et al. 2006, Walenski et al. 2006) also document difficulties with comprehension of meaning from context in ASD; even individuals with high-functioning ASD show irregularities in an otherwise typical IQ profile, with significantly lower scores in comprehension tasks, such as comprehending idioms (Siegel et al. 1996, Goldstein et al. 2002). Fishman and colleagues (2011) also highlight the fact that ASD individuals have been shown to rely on visual imagery instead of linguistic cues to comprehend sentences (Kana et al. 2006), while people with WS tend to rely on sentence-level context cues. In their study, Fishman and colleagues compared 16 participants with WS, 12 participants with ASD, and 18 TD individuals between the ages of 17 and 46 years old. Participants were asked to judge the acceptability of a sentence (i.e. whether or not it made sense). The study measured the N400 effect, and ERP component inversely correlated to the semantic ‘fit’ or a word or phrase, which enables the measurement of the use of context to infer meaning, among other things. The study concluded that the WS group’s accuracy was not significantly different from that of the TD group, but that the ASD group’s accuracy was significantly lower. These findings suggest that participants with WS rely more heavily on context cues in linguistic processing than individuals with ASD, suggesting that semantic processing follows a different trajectory in both groups. More generally, the study by Fishman and colleagues provides an important insight into how different perceptual inputs (in this case, in the language domain) can eventually lead to different communicative behaviors, due to differences in processing information at the neural level. These differences point to a divergent organization of the brain in both ASD and WS, which is at the basis of their contrasting social and language phenotypes, as we will discuss in the two next sections of the paper.
Pragmatic problems, and differences between conditions, have been documented in other studies. For instance, in their comparative study of the pragmatic language profiles of children with ASD and WS, Philofsky and colleagues (2007) found that in certain areas (coherence, stereotyped language, nonverbal communication, and social relations scales) individuals with WS performed better than participants with ASD, although in other areas (inappropriate initiation, use of context, and interests scales) the impairment was similar. Also, WS children were rated by their parents as being slightly better than ASD individuals at communicative tasks like appropriately sequencing and referencing events for a listener, but they still had difficulties (Philofsky et al. 2007). As expected, these differences in language use between ASD and WS can be attributed to their divergent socio-cognitive profiles: after all, language is learned and used in social situations, and attention to the facial area and emotional state of the speaker, as well as interest in the interlocutor play a crucial role at these levels. Hence, Fishman and colleagues (2011) suggest that the different patterns of attention exhibited by people with ASD and participants with WS may lead to different perceptual inputs, which in turn lead to different communicative behaviors. Likewise, the decreased sensitivity to speech prosody documented in ASD (e.g. Korpilahti et al. 2007) could explain their impaired ability to infer meaning from context, whereas the auditory hypersensitivity documented in WS (e.g. Klein et al. 1990, Blomberg et al. 2006) could account for their enhanced ability at this level (see Fishman et al. 2011 for discussion).
Socio-cognitive similarities in ASD and WS: focusing on the brain (but not only)
In studies of both ASD and WS, a wealth of research has focused on specific brain networks thought to be implicated in behavioral and cognitive abnormalities of both conditions. In the next two sections we provide a brief overlook of research focused on the social realm, with the aim of achieving a more biologically-grounded account of the similarities and differences in the socio-cognitive phenotypes of ASD and WS.
Regarding the associations between irregularities in certain brain networks and some core social deficits found in both ASD and WS, four networks are worth considering. The first is the default mode network, which includes the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), the precuneus, the inferior parietal lobes (IPL), and medial temporal regions. This network is involved in basic cognitive processes important for social interaction, such as mentalizing, distinguishing between distinct individuals (Hassabis et al. 2014), autobiographical recollection, imagination, and self-referential processes such as memory retrieval and recollection (Spreng and Andrews-Hanna 2015). Parts of the default mode network, particularly those located in the inferior frontal and lateral temporal regions, have been shown to be activated during many social tasks (Binder and Desai 2011, Binder et al. 2009, Seghier 2013). The functional connectivity of the default mode network has been found to be irregular in ASD (Assaf et al. 2010, Lynch et al. 2013) as well as WS (Sampaio et al. 2016). Specifically, in their voxel-level study of resting state functional connectivity in ASD, Cheng and colleagues (2015) found that the medial temporal gyrus exhibits reduced cortical connectivity and increased connectivity to the medial thalamus in ASD participants, and posited that this may be related to face processing deficits and ToM impairments (Cheng et al. 2015). Cheng and colleagues (2015) also found in people with ASD a key system in the precuneus/superior parietal lobe with reduced functional connectivity, which is implicated in spatial functions, including those related to self and the environment. These elements are substrates of ToM, so it stands to reason that reduced connectivity in these regions may help to explain key elements in the social phenotype of ASD. In studies of WS, Sampaio and colleagues (2016) also found decreased functional connectivity in the precuneus, as well as the posterior cingulate of the left hemisphere, which is also implicated in the default mode network.
Even more relevant is the second network, namely, the social brain network, which includes the superior temporal sulcus (STS), the anterior cingulate cortex (ACC), the medial prefrontal cortex (mPFC), the inferior and superior frontal gyrus (IFG, SFG), the anterior insula, and the amygdala. This network is thought to influence a variety of skills and functions crucial to interpersonal interaction, such as facial expression imitation (involving the IFG), perception of facial expressions and eye gaze tasks (involving the posterior STS), theory of mind and perspective taking (involving the SFG), and emotion processing (involving the amygdala); for a thorough review of the various functions of the social brain network, see Misra 2014). Research has uncovered atypical connections and irregularities in this circuitry in both ASD (Gotts et al. 2012, Kennedy and Adolphs 2012) and WS (Barak and Feng 2016).
Also of interest is the circuitry involved in self-representation, which includes the mPFC, the PCC/precuneus, the temporo-parietal junction (TPJ), the anterior insula, the middle cingulate cortex (mCC), the ventral premotor cortex (PMv), and the somatosensory cortex. This circuit contributes to the ability to recognize oneself and form a concept of self as different from others, which is a fundamental part of social interaction (Uddin et al. 2008). The fact that this circuit is found to be atypical in ASD (Lombardo et al. 2010) and WS (Haas et al. 2014) may account for the decreased interest in others and increased focus on self which is typically found in individuals with ASD, as well as for the increased focus on others/decreased focus on self typically observed in participants with WS.
Finally, reward circuitry is an important brain network to consider as well. This brain circuit includes the ventral tegmental areaa (VTA), the striatum, the orbitofrontal cortex (OFC), the ventromedial prefrontal cortex (vmPFC) and the ACC. The reward circuit is crucial to social behavior since it controls learning, reinforcement, and value representation (Pujara and Koenigs 2014), but also the drive for social interaction or connection, deeming certain social interactions pleasurable or aversive (Pellissier et al. 2018). This network has been found to be irregular in ASD and WS (Dichter et al. 2012). Specifically, in an fMRI study, Kohls and colleagues found that participants with ASD showed significant hypoactivation in the reward circuit areas of the brain (in particular, the ACC, as well as the amygdala) in tasks involving a reward. Likewise, studies involving participants with WS (e.g. Haas et al. 2009) indicate a poorly modulated reward system in response to social cues, especially when assessing negative ones such as sad or angry faces.
Special attention regarding the similarities between both conditions has been paid to two particular brain structures mentioned above: the amygdala and the frontal lobes. Although differences are attenuated as participants get older (Martens et al. 2009), the amygdala is disproportionately large in both ASD and WS (Reiss et al. 2004, Schumann et al. 2004, Martens et al. 2009, Mosconi et al. 2009, Haas et al. 2014, Murphy et al. 2012, Järvinen et al. 2013, Gibbard et al. 2018). A key component of the limbic system, the amygdala is a set of brain structures that support emotion and motivation, among other functions (see Rolls 2015 for review), and is forefront in much of the research about the social (dys)function in both conditions (Stefanacci and Amaral 2000, Meyer-Lindenberg et al. 2005, Haas et al. 2010, Paul et al. 2010, Jawaid et al. 2012, Zalla and Sperduti 2013, Barak and Feng 2016). Mosconi et al. (2009) is one example of a robust study on amygdala volumes in ASD. This is a longitudinal study, in which amygdala volumes were measured in 50 participants with ASD and in 33 neurotypical controls, initially when they were 18–35 months old, and again at 42–59 months. The volume of the amygdala was measured with MRI and compared with the participants’ ability for joint attention, which was evaluated through tasks in which children directed another person’s attention through eye gaze or followed someone else’s eye gaze to attend to various objects or stimuli. The results showed that at both 2 and 4 years of age, the amygdala of the participants with ASD was enlarged in comparison to the TD group. The authors also found a significant association between amygdala volumes and joint attention abilities, suggesting that deficits in joint attention in this condition may result in part from this abnormality in the amygdala. In WS, similar results have been obtained. For instance, Haas and colleagues (2014) investigated the volume of the amygdala in 39 participants with WS compared to 40 TD controls. Using a surface based analytical modeling approach, they collected high resolution MRI data and found evidence of increased radial expansion on the surface of the amygdala in WS (specifically, on the bilateral posterior cortical nucleus, lateral nucleus, and central nucleus). Still, it is important to note that, on the subject of amygdala size in WS and ASD, contrasting findings abound. Hence, Campbell et al. (2009) observed that adolescents with WS have lower amygdalar volumes compared to neurotypical controls, whereas Martens et al. (2009) found higher amygdalar volumes compared to the TD control groups. Studies on adults also conflict: Reiss et al. (2004) found higher volumes of the amygdala while Chiang et al. (2007) found lower volumes compared to controls. Likewise, Aylward et al. (1999) or Pierce and colleagues found the amygdala to have a reduced volume in participants with ASD compared to controls, although one possible explanation for this discrepancy could be related, as noted above, to the age of the participants, as studies finding larger amygdala volumes (e.g. Mosconi et al. 2009, Schumann et al. 2004) were conducted with children, while studies finding reduced volume were conducted with adolescents and adults.
Apart from size differences, the amygdala also shows atypical functional connections to several brain regions, specifically with the ACC, the PFC, and the OFC (all of which are implicated in cognitive processing, attention, and inhibition) (Martens et al. 2008, Dedovic et al. 2009, Haas et al. 2014, Gibbard et al. 2018), and importantly, with various components of the social brain, particularly, the frontal lobes (Meyer-Lindenberg et al. 2005, Paul et al. 2010, Jawaid et al. 2012). Interestingly too, a common source of the anxiety reported in both ASD and WS has been found to be irregular hyper- and hypo- activation of the amygdala in response to both social and non-social stimuli in both conditions (see Reiss et al. 2004, Martens et al. 2009, Järvinen et al. 2013 for WS; see Baron-Cohen and Wheelwright 1999, Critchley et al. 2000, Dalton et al. 2005, Corbett et al. 2009, Kliemann et al. 2012, for ASD). Specifically, Barak and Feng (2016) highlight that the amygdala is the source of the non-social anxiety and phobias which are typical of WS, pointing to deficits in the prefrontal-amygdala white matter pathways as the cause (see also Avery et al. 2012). Research on a salience network including the amygdala, the ventral striatum, the dorsomedial thalamus, the hypothalamus, and the substantia nigra (SN)/VTA has shown these functional connections to be atypical in ASD (Uddin et al. 2013) as well as in WS (Haas and Reiss 2012). This network has been linked to attention switching, as well as detection and attention to sensory and emotional stimuli. Regarding the frontal areas, it should be noted that individuals with WS exhibit similar approach behavior to people with frontal lobe damage, suggesting that this abnormal approach behavior could be due to a lack of inhibitory control in the frontal lobe (Porter et al. 2007). Specifically, abnormal functional connectivity between the OFC and the amygdala has been linked to the uninhibited social nature in WS, since the frontal lobes have been shown to regulate and inhibit inappropriate social behavior (Meyer-Lindenberg et al. 2005, Mobbs et al. 2007, Porter et al. 2007, Little et al. 2013, Barak and Feng 2016). Similar lack of inhibitory control has also been shown in people with ASD, but in terms of abnormal personal space boundaries (Christ et al. 2007). Moreover, language deficits and language delay have been associated to a frontal lobe dysfunction and irregular functional connectivity to the amygdala, seemingly impacting on aspects like inferencing or joint attention (Lincoln et al. 2002, Cornish et al. 2007, Martens et al. 2008, Barak and Feng 2016).
Research has also implicated the mirror neuron system (MNS) in social dysfunction in ASD and WS (e.g. Järvinen et al. 2013). This network includes the frontal gyrus, the STS, and the IPL (Van Overwalle and Baetens 2009). Apart from its role in imitation, decoding, and implementation of actions (see e.g. Rizzolatti and Craighero 2004), the MNS is also related to the social realm in terms of empathy (e.g. Gallese 2001, Iacoboni 2009). Reduced cortical surface area, but preserved cortical thickness, in structures implicated in the MNS have been found in WS (Ng et al. 2016). Studies of the MNS in ASD report cortical thinning in selected areas, which positively correlate to degree of social dysfunction (Hadjikhani et al. 2006, Wallace et al. 2012). These findings indicate that the common social deficits found in both WS and ASD may stem from an atypical MNS, while the distinct social drive in WS is most likely derived from systems independent of this network (Ng et al. 2016).
Finally, the HPA axis (a major neuroendocrine system resulting from the interaction between the hypothalamus, the pituitary gland, and the adrenal glands) comes into play here, because of its involvement in stress response—particularly, the response to cortisol in the amygdala, the PFC, and the hippocampus– all of them areas implicated in situations of fear and social stress (see Martens et al. 2008, Dedovic et al. 2009, Lense and Dykens 2013, Bitsika et al. 2015). Both ASD and WS individuals have been shown to exhibit interrupted HPA axis function (Spratt et al. 2012, Jacobson 2014, Benítez-Burraco et al. 2016, Niego and Benítez-Burraco 2019, among many others). This might explain in part the prevalence of anxiety in the two disorders, although as mentioned above, the anxiety generally seems to happen in separate arenas for each group: social in ASD and non-social in WS (see also Dykens 2003, Graham et al. 2005, Rodgers et al. 2012, Lense and Dykens 2013).
Socio-cognitive differences in ASD and WS: focusing on the brain (but not only)
Just as the observed similarities in their respective social phenotypes happen to follow from similar dysfunctions in similar brain areas and circuits, the differences between ASD and WS in the realm of social behavior and social cognition stem in part from the impairment of different devices. One focus of attention has been the amygdala. As noted above, ASD and WS share similar irregularities in the amygdala. Nonetheless, some of the differences between these two conditions in the social domain can be hypothesized to lie in certain sub circuits of the amygdala, its differential response to stimuli, and/or its different connections to brain regions upstream or downstream from it. For instance, during eye gazing, a positive activation of the amygdala has been reported in participants with WS while aversive activation was reported in people with ASD (Barak and Feng 2016). An interesting point about this activation is that, according to Barak and Feng (2016), in both cases the amygdala is hyperactivated. However, this hyperactivation takes on different forms in the two conditions. In ASD, it seems that this hyper activation is negatively valenced, creating an aversive response in those with ASD. In contrast, those with WS experience an appetitive response from this hyperactivation, making them more apt to continue the eye gaze (Riby et al. 2009, Barak and Feng 2016). In terms of processing faces, there seems to be a difference at the level of responsiveness of the amygdala; it is hyper responsive to unfamiliar faces in ASD and hypo responsive to the same stimulus in WS (Lough et al. 2015). This might explain their distinctive response to faces and eye gaze. Accordingly, as mentioned above, the aversive response triggered by hyperactivation of the amygdala would cause an aversive response to faces, resulting in a reduced sustained attention to the facial region, and hence, problems with facial recognition (Dalton et al. 2005, Kliemann et al. 2012, Strauss et al. 2012). The opposite may happen in WS: the appetitive response triggered by amygdala hyperactivation may be a motivating factor to spend more time assessing facial features (Riby et al. 2009, Barak and Feng 2016). It has also been shown that individuals with WS have a heightened amygdala reaction to images depicting non-social fear, but a somewhat muted amygdala response to fearful social images and faces. In contrast, participants with ASD seem to present a negative over arousal of the amygdala when looking at faces (not necessarily fearful ones) which seems to follow the pattern of their social behavior (Meyer-Lindenberg et al. 2005, Haas et al. 2009, Mimura et al. 2010, Muñoz et al. 2010, Barak and Feng 2016). Eventually, one possible (complementary) explanation of all these differences is that the neurons within the amygdala responding to social stimuli come from different classes in each condition, e.g. glutamatergic or GABAergic (Barak and Feng 2016). Still, it should be noted here that the results are far from straightforward and contrasting results have also been reported (e.g. Thornton-Wells et al. 2011).
Other brain areas have been implicated in the differences between ASD and WS in the socio-cognitive domain. These include the fusiform gyrus (Haxby et al. 2000), the pSTS (Allison et al. 2000, Nummenmaa and Calder 2009), the amygdala (Adolphs and Spezio 2006), and parietal-frontal areas such as the TPJ and the mPFC (Gallese and Goldman 1998, Decety and Jackson 2004, Amodio and Frith 2006, Lieberman 2007, Sui et al. 2013). Specifically, recent research indicates that the mPFC and the pSTS both have crucial roles in both sides of the attention spectrum, i.e. attention to self vs. attention to others, responding to each function with an activation response or an inhibition response: whereas the pSTS area is key in ‘attention to others’ functions, the mPFC seems to support the ‘attention to self’ function (Sui et al. 2013, Kuang 2016). Not surprisingly, atypical connectivity and/or structures have been found in both the pSTS and the mPFC in both ASD and WS (Pelphrey et al. 2004, Amaral et al. 2008, Järvinen et al. 2013). Significant differences have also been found in the fusiform face area, an area in the fusiform gyrus involved in face processing. One important study addressing this issue is Golarai and colleagues’, who used fMRI imaging to measure the volume of the fusiform gyrus, particularly the fusiform face area (FFA), the region of the visual cortex involved in facial recognition. The study was conducted with 16 participants with WS and 15 TD individuals between the ages of 19–49. Participants were shown a total of 769 images in three different categories: faces, places, and textures. This study found that the FFA was significantly larger (approximately twice the size) in participants with WS compared to TD controls. Subsequent studies (e.g. O’Hearn et al. 2011, Haas and Reiss 2012) have similar findings. In contrast, findings on ASD suggest a different architecture in the FFA. One such study is Imke and colleagues’ (2008), who focused on areas within the FG that encompass the possible range of the FFA. This was a study on post-mortem brains from 7 individuals with ASD and 10 controls, looking for cytoarchitectonic differences in cell layer volumes, neuron density, and mean perikaryal volumes. Researchers concluded that brains of participants with ASD showed a significant reduction of mean neuron density, volume, and total number of neurons in areas of the FG that were in the range of the FFA, compared to controls. Other studies (e.g. Nickl-Jockschat et al. 2015), concur with these findings.
Lastly, differences in the structure and the connection patterns of the frontal lobes (particularly, with the amygdala) may contribute to distinctive features of the WS social phenotype, like sustained gaze towards faces, increased approachability perception for unfamiliar faces, difficulty in disengaging attention from faces, and difficulties with perceiving emotion from facial expressions (Bellugi et al. 1999, Porter et al. 2007). In contrast, it seems that altered connectivity with the frontal cortex in ASD is instrumental in decreased habituation of the amygdala response to emotional facial expressions, which correlates to their gaze aversion (Zalla and Sperduti 2013, Swartz et al. 2013).
Finally, it is worth mentioning the role of oxytocin, a hormone known to regulate social behavior and interaction (Wójciak et al. 2012). Specifically, oxytocin inhibits the Hypothalamic–Pituitary–Adrenal (HPA) axis’ stress triggered activity (Neumann 2002). Individuals with WS show an increased basal level of oxytocin, and levels of the hormone have been positively correlated with social engagement behaviors which are typical of this condition, such as tendency to approach strangers and emotionality (Dai et al. 2012). In contrast, lower levels of oxytocin have been reported in people with ASD (Modahl et al. 1998), with higher plasma concentrations of oxytocin correlating with enhanced verbal abilities (Zhang et al. 2016) and the retention of social information, like affective speech (Hollander et al. 2007). Administering oxytocin to individuals with ASD has been shown to help with attention to and retention of social cues, as well as promote eye contact (Hollander et al. 2007, Andari et al. 2010, Domes et al. 2013).
Discussion
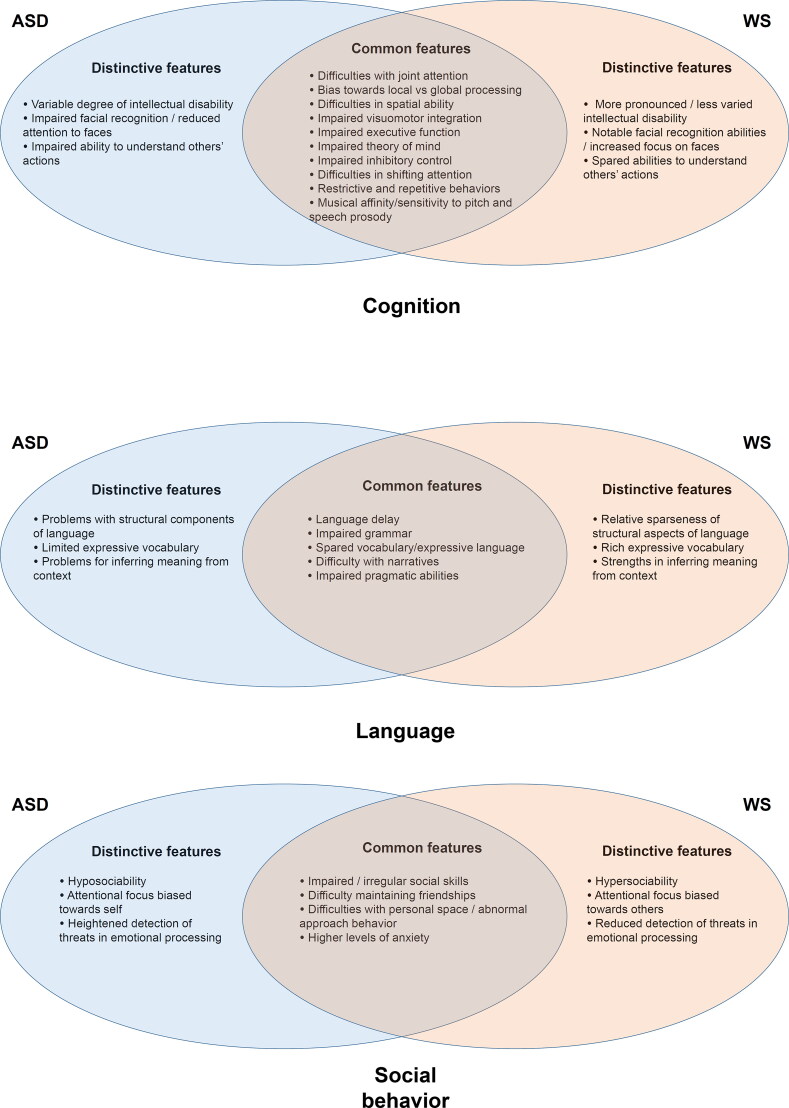
As we have shown in the previous sections, ASD and WS certainly exhibit important differences in the domains of social cognition and behavior, but also striking resemblances (summarized in Figure 1). Common features include difficulties with joint attention, impaired theory of mind, impaired inhibitory control, difficulties in shifting attention, restrictive and repetitive behaviors, language delay, impaired pragmatic abilities, impaired social skills, difficulty maintaining friendships, difficulties with personal space and abnormal approach behaviors, and high levels of anxiety. At the same time, participants with ASD exhibit a more variable degree of intellectual disability, a reduced attention to faces, a more marked inability to understand others’ actions, more problems with structural components of language, a limited expressive vocabulary, more acute problems for inferring meaning from context, hyposociability, an attentional focus biased towards self, and a heightened detection of threats in emotional processing. These deficits contrast with the profile of people with WS, who show a less varied intellectual disability, notable facial recognition abilities, spared abilities to understand others’ actions, a relative sparseness of structural aspects of language, a richer expressive vocabulary, notable abilities for inferring meaning from context, hypersociability, an attentional focus biased towards others and reduced detection of threats in emotional processing.
Figure 1.
Shared and distinctive features of ASD (left) and WS (right) in the domains of cognition (up), language (center) and social behavior (bottom) (see sections 2 and 3 for details).
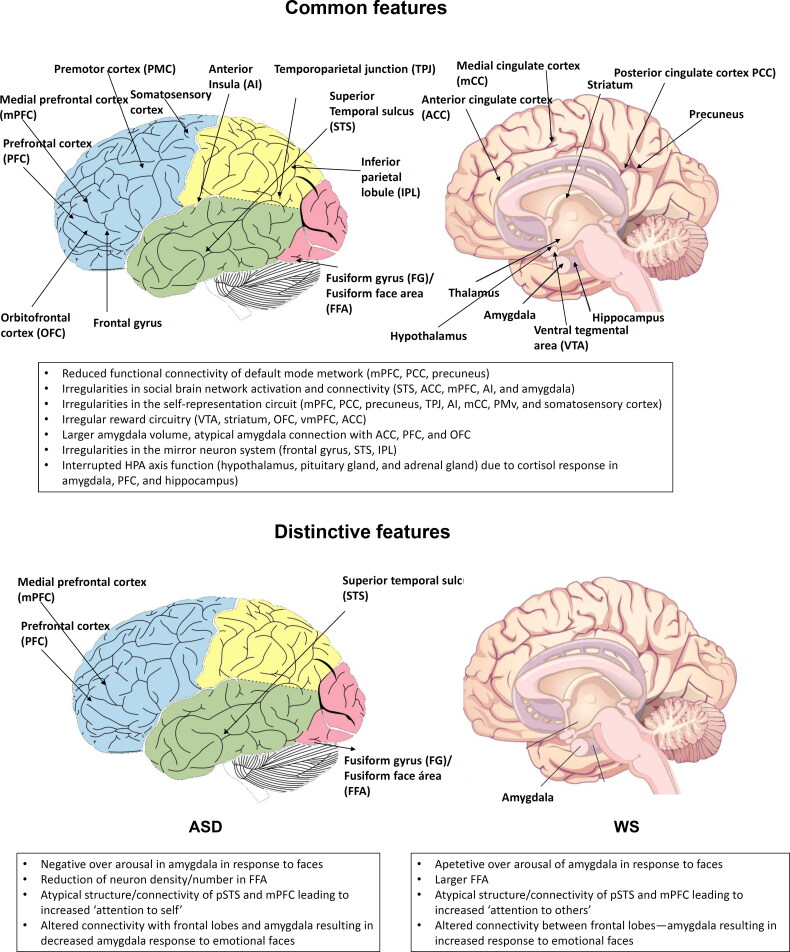
From a neurobiological perspective, it can be concluded that although some differences exist between conditions, similar anomalies in the same brain structures and their functional connectivity can be observed as well (summarized in Figure 2), these similar anomalies can translate, however, to different behaviors. To put it differently, research repeatedly shows that what may look like a similar impairment at the neurobiological level can result in a different trait at the phenotypic level. As noted in the Introduction, the same can be said of the genetic factors involved, as a significant overlap seems to exist between the genetic determinants of ASD and WS, and particularly, between the genes that are differentially-expressed compared to neurotypical controls, with most of these genes being found either upregulated or downregulated in both conditions. All in all, the evidence discussed in the paper is congruent with the view that the same brain areas and circuits, and ultimately, the same genes controlling their development and wiring can be safely expected to be involved in ASD and WS, with phenotypical differences across conditions resulting from subtle changes in gene regulation and ultimately, brain function.
Figure 2.
Shared (up) and distinctive (bottom) neurobiological features of ASD (left) and WS (right) in the sociocognitive domain (see sections 4 and 5 for details). The schematic view of the whole brain (left) is from Lumen, under Creative Commons Attribution License v4.0. The sagittal view of the brain (right) is from Psychology 2e, OpenStax, under Creative Commons Attribution License v4.0.
Overall, this complex scenario nicely fits the ‘diametric brain hypothesis’ (Crespi and Badcock 2008), according to which human evolution resulted in both non-social brain adaptations and social brain adaptations, which tend to exhibit tradeoffs. Accordingly, WS would involve a(n) (maladaptively) over-developed social-brain phenotype in conjunction with, to some degree, a(n) (maladaptive) under-development of non-social brain phenotype, whereas ASD would entail the opposite. In our previous research, we have extended this view to the recently-emerged account of human evolution, namely, the Self-Domestication hypothesis (SDH) of human evolution. According to this view, humans evolved many of the distinctive traits found in domesticated mammals as a result of our selection for reduced aggression (see Hare 2017 for discussion). In our work, we have shown that whereas features of human self-domestication are attenuated in ASD (Benítez-Burraco et al. 2016), they are found exaggerated in WS (Niego and Benítez-Burraco 2019). Because self-domestication seemingly resulted from changes in the activity of the HPA axis impacting on our brain and behavior, altered features of self-domestication in these two conditions are expected to account for their differences (and similarities) in the domains of social cognition and social behavior, as reviewed in this paper.
In sum, if we want to understand the deficits (and strong points) exhibited by people with ASD or WS, we need to move from simplistic views of these conditions as mirror conditions, and adopt instead a systems-biology approach, aimed at exploring the intricate connections between all the involved levels. In particular, an evo-devo approach seems compulsory, aimed at linking the abnormal phenotypes found in the human species with our cognitive and behavioral evolution.
Disclosure statement
The authors declare that they have no conflict of interest.
Authors’ contribution
ABB conceived the paper. AN and ABB conducted the literature search, analyzed the data, and wrote the paper. Both authors approved the final version of the manuscript
References
- Adolphs, R. and Spezio, M.. 2006. Role of the amygdala in processing visual social stimuli. Progress in Brain Research, 156, 363–378. [DOI] [PubMed] [Google Scholar]
- Allison, T., Puce, A. and McCarthy, G.. 2000. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences, 4, 267–278. [DOI] [PubMed] [Google Scholar]
- Amaral, D. G., Schumann, C. M. and Nordahl, C. W.. 2008. Neuroanatomy of autism. Trends in Neurosciences, 31, 137–145. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 2013. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author. [Google Scholar]
- Amodio, D. M. and Frith, C. D.. 2006. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7, 268–277. [DOI] [PubMed] [Google Scholar]
- Andari, E., Duhamel, J. R., Zalla, T., Herbrecht, E., Leboyer, M. and Sirigu, A.. 2010. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America, 107, 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada, K. and Itakura, S.. 2012. Social phenotypes of autism spectrum disorders and Williams syndrome: Similarities and differences. Frontiers in Psychology, 3, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf, M., Jagannathan, K., Calhoun, V. D., Miller, L., Stevens, M. C., Sahl, R., O'Boyle, J. G., Schultz, R. T. and Pearlson, G. D.. 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage, 53, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, S. N., Thornton-Wells, T. A., Anderson, A. W. and Blackford, J. U.. 2012. White matter integrity deficits in prefrontal-amygdala pathways in Williams syndrome. NeuroImage, 59, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward, E. H., Minshew, N. J., Goldstein, G., Honeycutt, N. A., Augustine, A. M., Yates, K. O., Barta, P. E. and Pearlson, G. D.. 1999. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology, 53, 2145–2150. [DOI] [PubMed] [Google Scholar]
- Barak, B. and Feng, G.. 2016. Neurobiology of social behavior abnormalities in autism and William’s syndrome. Nature Neuroscience, 19, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen, S. and Wheelwright, S.. 1999. 'Obsessions' in children with autism or Asperger syndrome. Content analysis in terms of core domains of cognition. British Journal of Psychiatry, 175, 484–490. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Leslie, A. M. and Frith, U.. 1985. Does the autistic child have a ‘‘theory of mind’’? Cognition, 21, 37–46. [DOI] [PubMed] [Google Scholar]
- Bellugi, U., Adolphs, R., Cassady, C. and Chiles, M.. 1999. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport, 10, 1653–1657. [DOI] [PubMed] [Google Scholar]
- Bellugi, U., Lichtenberger, L., Jones, W., Lai, Z. and St George, M. I.. 2000. The neurocognitive profile of Williams Syndrome: A complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience, 12, 7–29. [DOI] [PubMed] [Google Scholar]
- Bellugi, U., Wang, P. and Jernigan, T.. 1994. Williams syndrome: An unusual neuropsychological profile. In: Broman S., Grafman J., eds. 1994. Atypical cognitive deficits in developmental disorders: Implications for brain function. Hillsdale, NJ: Erlbaum Press, pp. 23–56. [Google Scholar]
- Benítez-Burraco, A., Lattanzi, W. and Murphy, E.. 2016. Language impairments in ASD resulting from a failed domestication of the human brain. Frontiers in Neuroscience, 10, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, N. I. and Ingersoll, B.. 2015. A further investigation of goal-directed intention understanding in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 4412, 3204–3214. [DOI] [PubMed] [Google Scholar]
- Bhatara, A., Quintin, E. M., Levy, B., Bellugi, U., Fombonne, E. and Levitin, D. J.. 2010. Perception of emotion in musical performance in adolescents with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research, 3, 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. and Desai, R. H.. 2011. The neurobiology of semantic memory. Trends in Cognitive Sciences, 15, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R., Desai, R. H., Graves, W. W. and Conant, L.. 2009. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsika, V., Sharpley, C. F., Andronicos, N. M. and Agnew, L. L.. 2015. Hypothalamus-pituitary-adrenal axis daily fluctuation, anxiety and age interact to predict cortisol concentrations in boys with an autism spectrum disorder. Physiology and Behavior, 138, 200–207. [DOI] [PubMed] [Google Scholar]
- Blomberg, S., Rosander, M. and Andersson, G.. 2006. Fears, hyperacusis and musicality in Williams’s syndrome. Research in Developmental Disabilities, 27, 668–680. [DOI] [PubMed] [Google Scholar]
- Bölte, S., Girdler, S. and Marschik, P. B.. 2019. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cellular and Molecular Life Sciences, 76, 1275–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel, A., Mottron, L., Peretz, I., Trudel, M., Gallun, E. and Bonnel, A. M.. 2003. Enhanced pitch sensitivity in individuals with autism: A signal detection analysis. Journal of Cognitive Neuroscience, 15, 226–235. [DOI] [PubMed] [Google Scholar]
- Brock, J., Jarrold, C., Farran, E.K., Laws, G. and Riby, D.M.. 2007. Do children with Williams syndrome really have good vocabulary knowledge? Methods for comparing cognitive and linguistic abilities in developmental disorders. Clinical Linguistics and Phonetics, 21, 673–688. [DOI] [PubMed] [Google Scholar]
- Campbell, L. E., Daly, E., Toal, F., Stevens, A., Azuma, R., Karmiloff-Smith, A., Murphy, D. G. and Murphy, K. C.. 2009. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Research, 1258, 96–107. [DOI] [PubMed] [Google Scholar]
- Charman, T., Swettenham, J., Baron-Cohen, S., Cox, A., Baird, G. and Drew, A.. 1997. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology, 33, 781–789. [DOI] [PubMed] [Google Scholar]
- Cheng, W., Rolls, E. T., Gu, H., Zhang, J. and Feng, J.. 2015. Autism: Reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain: A Journal of Neurology, 138, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M. C., Reiss, A. L., Lee, A. D., Bellugi, U., Galaburda, A. M., Korenberg, J. R., Mills, D. L., Toga, A. W. and Thompson, P. M.. 2007. 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. NeuroImage, 36, 1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ, S. E., Holt, D. D., White, D. A. and Green, L.. 2007. Inhibitory control in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 37, 1155–1165. [DOI] [PubMed] [Google Scholar]
- Corbett, B. A., Schupp, C. W., Levine, S. and Mendoza, S.. 2009. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research: Official Journal of the International Society for Autism Research, 2, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish, K., Scerif, G. and Karmiloff-Smith, A.. 2007. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 43, 672–685. [DOI] [PubMed] [Google Scholar]
- Crespi, B. and Badcock, C.. 2008. Psychosis and autism as diametrical disorders of the social brain. The Behavioral and Brain Sciences, 31, 241–320. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D., Daly, E. M., Bullmore, E. T., Williams, S. C., Van Amelsvoort, T., Robertson, D. M., Rowe, A., Phillips, M., McAlonan, G., Howlin, P. and Murphy, D. G.. 2000. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain, 123, 2203–2212. [DOI] [PubMed] [Google Scholar]
- Dai, L., Carter, C. S., Ying, J., Bellugi, U., Pournajafi-Nazarloo, H. and Korenberg, J. R.. 2012. Oxytocin and vasopressin are dysregulated in Williams syndrome, a genetic disorder affecting social behavior. PLoS One, 7, e38513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., Goldsmith, H. H., Alexander, A. L. and Davidson, R. J.. 2005. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8, 519– 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety, J. and Jackson, P. L.. 2004. The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 3, 71–100. [DOI] [PubMed] [Google Scholar]
- Dedovic, K., Duchesne, A., Andrews, J., Engert, V. and Pruessner, J. C.. 2009. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage, 47, 864–871. [DOI] [PubMed] [Google Scholar]
- Dewey, D., Cantell, M. and Crawford, S.G.. 2007. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society: JINS, 13, 246–256. [DOI] [PubMed] [Google Scholar]
- Dichter, G. S., Damiano, C. A. and Allen, J. A.. 2012. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. Journal of Neurodevelopmental Disorders, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Itza, E., Martínez, V. and Antón, A.. 2016. Narrative competence in Spanish-speaking adults with Williams syndrome. Psicothema, 283, 291–297. [DOI] [PubMed] [Google Scholar]
- Domes, G., Heinrichs, M., Kumbier, E., Grossmann, A., Hauenstein, K. and Herpertz, S. C.. 2013. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry, 74, 164–171. [DOI] [PubMed] [Google Scholar]
- Doyle, T.F., Bellugi, U., Korenberg, J.R. and Graham, J.. 2004. “Everybody in the world is my friend”: Hypersociability in young children with Williams Syndrome. American Journal of Medical Genetics Part A, 124A, 263–273. [DOI] [PubMed] [Google Scholar]
- Dykens, E. M. and Lense, M.. 2011. Intellectual disabilities and autism spectrum disorder: A cautionary note. In: Amaral D., Dawson G., Geschwind D., eds. Autism spectrum disorders. New York: Oxford University Press, pp. 261–269. [Google Scholar]
- Dykens, E. M. 2003. Anxiety, fears, and phobias in persons with Williams syndrome. Developmental Neuropsychology, 23, 291–316. [DOI] [PubMed] [Google Scholar]
- Elliott, D., Welsh, T. N., Lyons, J., Hansen, S. and Wu, M.. 2006. The visual regulation of goal-directed reaching movements in adults with Williams syndrome, Down syndrome, and other developmental delays. Motor Control, 10, 34–54. [DOI] [PubMed] [Google Scholar]
- Fein, D., Dunn, M.A., Allen, D. M., Aram, R., Hall, N. and Morris, R.. 1996. Neuropsychological and language findings In: Rapin I, ed. Preschool children with inadequate communication: Developmental language disorder, autism, low IQ. London: MacKeith Press, 123–154. [Google Scholar]
- Fisher, M. H., Lense, M. D. and Dykens, E. M. 2016. Longitudinal trajectories of intellectual and adaptive functioning in adolescents and adults with Williams syndrome. J Intellect Disabil Res. 60, 920–932. doi: 10.1111/jir.12303. [DOI] [PubMed] [Google Scholar]
- Fishman, I., Yam, A., Bellugi, U., Lincoln, A. and Mills, D.. 2011. Contrasting patterns of language-associated brain activity in autism and Williams syndrome. Social Cognitive and Affective Neuroscience, 6, 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith, U. and Snowling, M.. 1983. Reading for meaning and reading for sound in autistic and dyslexic children. British Journal of Developmental Psychology, 1, 329–342. [Google Scholar]
- Freeman, L. and Dake, S.. 1996. Teach me language: A language manual for children with autism, Asperger’s syndrome and related developmental disorders. British Columbia, Canada: Langley. [Google Scholar]
- Frigerio, E., Burt, D. M., Gagliardi, C., Cioffi, G., Martelli, S., Perrett, D. I. and Borgatti, R.. 2006. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia, 44, 254–259. [DOI] [PubMed] [Google Scholar]
- Gagliardi, C., Martelli, S., Burt, M. D. and Borgatti, R.. 2007. Evolution of neurologic features in Williams syndrome. Pediatric Neurology, 36, 301–306. [DOI] [PubMed] [Google Scholar]
- Gallese, V. 2001. The' shared manifold' hypothesis. From mirror neurons to empathy. Journal of Consciousness Studies, 8, 5–7. [Google Scholar]
- Gallese, V. and Goldman, A.. 1998. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2, 493–501. [DOI] [PubMed] [Google Scholar]
- Gastgeb, H. Z., Strauss, M. S. and Minshew, N. J.. 2006. Do individuals with autism process categories differently? The effect of typicality and development. Child Development, 77, 1717–1729. [DOI] [PubMed] [Google Scholar]
- Geschwind, G. H. and State, M. W.. 2015. Gene hunting in autism spectrum disorder: On the path to precision medicine. The Lancet Neurology, 14, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbard, C. R., Ren, J., Skuse, D. H., Clayden, J. D. and Clark, C. A.. 2018. Structural connectivity of the amygdala in young adults with autism spectrum disorder. Human Brain Mapping, 39, 1270–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg, C. and Rasmussen, P.. 1994. Brief report: Four case histories and a literature review of Williams syndrome and autistic behavior. Journal of Autism and Developmental Disorders, 24, 381–393. [DOI] [PubMed] [Google Scholar]
- Goldstein, G., Minshew, N.J., Allen, D.N. and Seaton, B.E.. 2002. High-functioning autism and schizophrenia: A comparison of an early and late onset neurodevelopmental disorder. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 17, 461–475. [PubMed] [Google Scholar]
- Gothelf, D., Searcy, Y. M., Reilly, J., Lai, P. T., Lanre-Amos, T., Mills, D., Korenberg, J. R., Galaburda, A., Bellugi, U. and Reiss, A. L.. 2008. Association between cerebral shape and social use of language in Williams syndrome. American Journal of Medical Genetics Part A, 146A, 2753–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts, S. J., Simmons, W. K., Milbury, L. A., Wallace, G. L., Cox, R. W. and Martin, A.. 2012. Fractionation of social brain circuits in autism spectrum disorders. Brain: A Journal of Neurology, 135, 2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, J. M., Rosner, B., Dykens, E. and Visootsak, J.. 2005. Behavioral features of CHARGE syndrome Hall-Hittner syndrome comparison with Down syndrome, Prader-Willi syndrome, and Williams syndrome. American Journal of Medical Genetics Part A, 133A, 240–247. [DOI] [PubMed] [Google Scholar]
- Gyawali, S. and Patra, B. N.. 2019. Autism spectrum disorder: Trends in research exploring etiopathogenesis. Psychiatry and Clinical Neurosciences, 73, 466–475. [DOI] [PubMed] [Google Scholar]
- Haas, B., Barnea-Goraly, M., Sheau, K., Yamagata, B., Ullas, S. and Reiss, A.. 2014. Altered microstructure within social-cognitive brain networks during childhood in Williams Syndrome. Cerebral Cortex, 24, 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B. W., Sheau, K., Kelley, R. G., Thompson, P. M. and Reiss, A. L.. 2014. Regionally specific increased volume of the amygdala in Williams syndrome: Evidence from surface-based modeling. Human Brain Mapping, 35, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B.W., Hoeft, F., Searcy, Y. M., Mills, D., Bellugi, U. and Reiss, A.. 2010. Individual differences in social behavior predict amygdala response to fearful facial expressions in Williams syndrome. Neuropsychologia, 48, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B. W., Mills, D., Yam, A., Hoeft, F., Bellugi, U. and Reiss, A.. 2009. Genetic influences on sociability: Heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B. W. and Reiss, A. L.. 2012. Social brain development in Williams syndrome: The current status and directions for future research. Frontiers in Psychology, 3, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani, N., Joseph, R. M., Snyder, J. and Tager-Flusberg, H.. 2006. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex, 16, 1276–1282. [DOI] [PubMed] [Google Scholar]
- Hale, C. M. and Tager-Flusberg, H.. 2005. Social communication in children with autism: The relationship between theory of mind and discourse development. Autism: The International Journal of Research and Practice, 9, 157–178. [DOI] [PubMed] [Google Scholar]
- Happé, F. and Frith, U.. 1996. The neuropsychology of autism. Brain, 119, 1377–1400. [DOI] [PubMed] [Google Scholar]
- Happé, F.G.E. 1993. Communicative competence and theory of mind in autism: A test of relevance theory. Cognition, 48, 101–119. [DOI] [PubMed] [Google Scholar]
- Hare, B. 2017. Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annual Review of Psychology, 68, 155–124. [DOI] [PubMed] [Google Scholar]
- Harris, G. J., Chabris, C. F., Clark, J., Urban, T., Aharon, I., Steele, S., McGrath, L., Condouris, K. and Tager-Flusberg, H.. 2006. Brain activation during semantic processing in autism spectrum disorders via function al magnetic imaging. Brain and Cognition, 61, 54–68. [DOI] [PubMed] [Google Scholar]
- Hassabis, D., Spreng, R. N., Rusu, A. A., Robbins, C. A., Mar, R. A. and Schacter, D. L.. 2014. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cerebral Cortex, 24, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby, J. V., Hoffman, E. A. and Gobbini, M. I.. 2000. The distributed human neural system for face perception. Trends in Cognitive Sciences, 4, 223–233. [DOI] [PubMed] [Google Scholar]
- Heaton, P., Hermelin, B. and Pring, L.. 1998. Autism and pitch processing: A precursor for savant musical ability. Music Perception, 15, 291–305. [Google Scholar]
- Heaton, P. 2003. Pitch memory, labelling and disembedding in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44, 543–551. [DOI] [PubMed] [Google Scholar]
- Hollander, E., Bartz, J., Chaplin, W., Phillips, A., Sumner, J., Soorya, L., Anagnostou, E. and Wasserman, S.. 2007. Oxytocin increases retention of social cognition in autism. Biological Psychiatry, 61, 498–503. [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. 2009. Imitation, empathy, and mirror neurons. Annual Review of Psychology, 60, 653–670. [DOI] [PubMed] [Google Scholar]
- Ingersoll, B., Walton, K., Carlsen, D. and Hamlin, T.. 2013. Social intervention for adolescents with autism and significant intellectual disability: Initial efficacy of reciprocal imitation training. American Journal on Intellectual and Developmental Disabilities, 118, 247–261. [DOI] [PubMed] [Google Scholar]
- Jacobson, L. 2014. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Comprehensive Physiology, 42, 715–738. [DOI] [PubMed] [Google Scholar]
- Jansiewicz, E. M., Goldberg, M. C., Newschaffer, C. J., Denckla, M. B., Landa, R. and Mostofsky, S. H.. 2006. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders, 36, 613–621. [DOI] [PubMed] [Google Scholar]
- Järvinen, A., Korenberg, J. R. and Bellugi, U.. 2013. The social phenotype of Williams syndrome. Current Opinion in Neurobiology, 23, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen, A., Ng, R. and Bellugi, U.. 2015. Autonomic response to approachability characteristics, approach behavior, and social functioning in Williams syndrome. Neuropsychologia, 78, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley, A., Wallace, G. L., Ramus, F., Happé, F. and Heaton, P.. 2008. Enhanced perceptual processing of speech in autism. Developmental Science, 11, 109–121. [DOI] [PubMed] [Google Scholar]
- Jawaid, A., Riby, D. M., Owens, J., White, S. W., Tarar, T. and Schulz, P. E.. 2012. 'Too withdrawn' or 'too friendly': considering social vulnerability in two neuro-developmental disorders. Journal of Intellectual Disability Research: JIDR, 56, 335–350. [DOI] [PubMed] [Google Scholar]
- John, A. E., Rowe, M. L. and Mervis, C. B.. 2009. Referential communication skills of children with Williams syndrome: Understanding when messages are not adequate. American Journal on Intellectual and Developmental Disabilities, 114, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, W., Bellugi, U., Lai, Z., Chiles, M., Reilly, J., Lincoln, A. and Adolphs, R.. 2000. II. Hypersociability in Williams Syndrome. Journal of Cognitive Neuroscience, 12 Suppl 1, 30–46. [DOI] [PubMed] [Google Scholar]
- Kana, R., Keller, T., Cherkassky, V., Minshew, N. and Just, M.. 2006. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain: A Journal of Neurology, 129, 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, E., Paul, J., Fein, D. and Naigles, L.. 2006. Residual Language Deficits in Optimal Outcome Children with a History of Autism. Journal of Autism and Developmental Disorders, 36, 807–828. [DOI] [PubMed] [Google Scholar]
- Kennedy, D. P. and Adolphs, R.. 2012. The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences, 16, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard, M. M. and Tager-Flusberg, H.. 2001. An Investigation of Language Impairment in Autism: Implications for Genetic Subgroups. Language and Cognitive Processes, 16, 287–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A. J., Armstrong, B. L., Greer, M. K. and Brown, F. R. 3rd.. 1990. Hyperacusis and otitis media in individuals with Williams syndrome. The Journal of Speech and Hearing Disorders, 55, 339–344. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman, B. P., Mervis, C. B., Lord, C. and Phillips, K. D.. 2007. Socio-communicative deficits in young children with Williams syndrome: Performance on the autism diagnostic observation schedule. Child Neuropsychology : a Journal on Normal and Abnormal Development in Childhood and Adolescence, 13, 444–467. [DOI] [PubMed] [Google Scholar]
- Klein-Tasman, B. P., Phillips, K. D., Lord, C., Mervis, C. B. and Gallo, F. J.. 2009. Overlap with the autism spectrum in young children with Williams syndrome. Journal of Developmental and Behavioral Pediatrics, 304, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann, D., Dziobek, I., Hatri, A., Baudewig, J. and Heekeren, H. R.. 2012. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32, 9469–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin, A., Sparrow, S. S., de Bildt, A., Cicchetti, D. V., Cohen, D. J. and Volkmar, F. R.. 1999. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders, 29, 499–508. [DOI] [PubMed] [Google Scholar]
- Korenberg, J. R., Chen, X. N., Hirota, H., Lai, Z., Bellugi, U., Burian, D., Roe, B. and Matsuoka, R.. 2000. Genome structure and cognitive map of Williams syndrome. Journal of Cognitive Neuroscience, 12, 89–107. [DOI] [PubMed] [Google Scholar]
- Korpilahti, P., Jansson-Verkasalo, E., Mattila, M. L., Kuusikko, S., Suominen, K., Rytky, S., Pauls, D. L. and Moilanen, I.. 2007. Processing of affective speech prosody is impaired in Asperger syndrome. Journal of Autism and Developmental Disorders, 37, 1539–1549. [DOI] [PubMed] [Google Scholar]
- Kuang, S. 2016. Two Polarities of Attention in Social Contexts: From Attending-to-Others to Attending-to-Self. Frontiers in Psychology, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix, A., Guidetti, M., Rogé, B. and Reilly, J.. 2009. Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Research in Developmental Disabilities, 30, 976–985. [DOI] [PubMed] [Google Scholar]
- Lacroix, A., Famelart, N. and Guidetti, M.. 2016. Language and emotional abilities in children with Williams syndrome and children with autism spectrum disorder: Similarities and differences. Pediatric Health, Medicine and Therapeutics, 7, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws, G. and Bishop, D.. 2004. Pragmatic language impairment and social deficits in Williams syndrome: A comparison with Down's syndrome and specific language impairment. International Journal of Language and Communication Disorders, 39, 45–64. [DOI] [PubMed] [Google Scholar]
- Lense, M. D. and Dykens, E. M.. 2013. Cortisol reactivity and performance abilities in social situations in adults with Williams syndrome. American Journal on Intellectual and Developmental Disabilities, 118, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, H. R. 2007. Cognitive methods for assessing mental energy. Nutritional Neuroscience, 10, 229–242. [DOI] [PubMed] [Google Scholar]
- Lincoln, A., Lai, Z. and Jones, W.. 2002. Shifting attention and joint attention dissociation in Williams síndrome: Implications for the cerebellum and social deficits in autism. Neurocase, 8, 226–232. [DOI] [PubMed] [Google Scholar]
- Lincoln, A. J., Searcy, Y. M., Jones, W. and Lord, C.. 2007. Social interaction behaviors discriminate young children with autism and Williams syndrome. Journal of the American Academy of Child and Adolescent Psychiatry, 46, 323–331. [DOI] [PubMed] [Google Scholar]
- Little, K., Riby, D. M., Janes, E., Clark, F., Fleck, R. and Rodgers, J.. 2013. Heterogeneity of social approach behaviour in Williams syndrome: The role of response inhibition. Research in Developmental Disabilities, 8, 959–967. [DOI] [PubMed] [Google Scholar]
- Liu, X., Li, Z., Fan, C., Zhang, D. and Chen, J.. 2017. Genetics implicate common mechanisms in autism and schizophrenia: Synaptic activity and immunity. Journal of Medicinal Genetics, 548, 511–520. [DOI] [PubMed] [Google Scholar]
- Lombardo, M., Chakrabarti, B., Bullmore, E., Sadek, S., Pasco, G., Wheelwright, S., Suckling, J., Aims Consortium, M. R. C. and Baron-Cohen, S, MRC AIMS Consortium . 2010. Atypical neural self-representation in autism. Brain : A Journal of Neurology, 133, 611–624. [DOI] [PubMed] [Google Scholar]
- Lough, E., Hanley, M., Rodgers, J., South, M., Kirk, H., Kennedy, D. P. and Riby, D. M.. 2015. Violations of personal space in young people with autism spectrum disorders and Williams syndrome: Insights from the social responsiveness scale. Journal of Autism and Developmental Disorders, 45, 4101–4108. [DOI] [PubMed] [Google Scholar]
- Lukács, A., Csaba, P. and Racsmány, M.. 2004. Language in hungarian children with Williams syndrome. Language Acquisition and Language Disorders, 36, 187–220. [Google Scholar]
- Lynch, C. J., Uddin, L. Q., Supekar, K., Khouzam, A., Phillips, J. and Menon, V.. 2013. Default mode network in childhood autism: Posteromedial cortex heterogeneity and relationship with social deficits. Biological Psychiatry, 74, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, M. A., Wilson, S. J. and Reutens, D. C.. 2008. Research review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. Journal of Child Psychology and Psychiatry 49, 576–608. [DOI] [PubMed] [Google Scholar]
- Martens, M. A., Wilson, S. J., Dudgeon, P. and Reutens, D. C.. 2009. Approachability and the amygdala: Insights from Williams syndrome. Neuropsychologia, 47, 2446–2453. [DOI] [PubMed] [Google Scholar]
- Mervis, C. B. and Becerra, A. M.. 2007. Language and communicative development in Williams syndrome. Mental Retardation and Developmental Disabilities Research Reviews, 13, 3–15. [DOI] [PubMed] [Google Scholar]
- Mervis, C. B., Klein-Tasman, B. P. and Mastin, M. E.. 2001. Adaptive behavior of 4- through 8-year-old children with Williams syndrome. American Journal of Mental Retardation, 1061, 82–93. [DOI] [PubMed] [Google Scholar]
- Mervis, C. B. and John, A. E.. 2012. Precursors to language and early language development in Williams syndrome. In: Farran EK, Karmiloff-Smith A., eds. Neurodevelopmental disorders across the lifespan: A neuroconstructivist approach. Oxford, UK: Oxford University Press, 187–204. [Google Scholar]
- Meyer-Lindenberg, A., Hariri, A. R., Munoz, K. E., Mervis, C. B., Mattay, V. S., Morris, C. A. and Berman, K. F.. 2005. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nature Neuroscience, 8, 991–993. [DOI] [PubMed] [Google Scholar]
- Mimura, M., Hoeft, F., Kato, M., Kobayashi, N., Sheau, K., Piggot, J., Mills, D., Galaburda, A., Korenberg, J. R., Bellugi, U. and Reiss, A. L.. 2010. A preliminary study of orbitofrontal activation and hypersociability in Williams Syndrome. Journal of Neurodevelopmental Disorders, 2, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra, V. 2014. The social brain network and autism. Annals of Neurosciences, 21, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs, D., Eckert, M. A., Mills, D., Korenberg, J., Bellugi, U., Galaburda, A. M. and Reiss, A. L.. 2007. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biological Psychiatry, 62, 256–261. [DOI] [PubMed] [Google Scholar]
- Modahl, C., Green, L., Fein, D., Morris, M., Waterhouse, L., Feinstein, C. and Levin, H.. 1998. Plasma oxytocin levels in autistic children. Biological Psychiatry, 43, 270–277. [DOI] [PubMed] [Google Scholar]
- Morris, C. A., , Mervis, C. B., , Hobart, H. H., , Gregg, R. G., , Bertrand, J., , Ensing, G. J., , Sommer, A., , Moore, C. A., , Hopkin, R. J., , Spallone, P. A., , Keating, M. T., , Osborne, L., , Kimberley, K. W. and , Stock, A. D. 2003. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: Genotype-phenotype analysis of five families with deletions in the Williams syndrome region. American Journal of Medical Genetics, 123A, 45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- Mosconi, M. W., Cody-Hazlett, H., Poe, M. D., Gerig, G., Gimpel-Smith, R. and Piven, J.. 2009. Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Archives of General Psychiatry, 66, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, K. E., Meyer-Lindenberg, A., Hariri, A. R., Mervis, C. B., Mattay, V. S., Morris, C. A. and Berman, K. F.. 2010. Abnormalities in neural processing of emotional stimuli in Williams syndrome vary according to social vs. non-social content. NeuroImage, 50, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, E. R., Foss-Feig, J., Kenworthy, L., Gaillard, W. D. and Vaidya, C. J.. 2012. Atypical functional connectivity of the amygdala in childhood autism spectrum disorders during spontaneous attention to eye-gaze. Autism Research and Treatment, 2012, 1–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, I. D. 2002. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo-pituitary-adrenal axis. Progress in Brain Research, 39, 147–162. [DOI] [PubMed] [Google Scholar]
- Newschaffer, C. J., Croen, L. A., Daniels, J., Giarelli, E., Grether, J. K., Levy, S. E., Mandell, D. S., Miller, L. A., Pinto-Martin, J., Reaven, J., Reynolds, A. M., Rice, C. E., Schendel, D. and Windham, G. C.. 2007. The epidemiology of autism spectrum disorders. Annual Review of Public Health, 28, 235–258. [DOI] [PubMed] [Google Scholar]
- Ng, R., Brown, T. T., Erhart, M., Järvinen, A. M., Korenberg, J. R., Bellugi, U. and Halgren, E.. 2016. Morphological differences in the mirror neuron system in Williams syndrome. Social Neuroscience, 11, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat, T., Rottschy, C., Thommes, J., Schneider, F., Laird, A. R., Fox, P. T. and Eickhoff, S. B.. 2015. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Structure and Function, 220, 2355–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niego, A. and Benítez-Burraco, A.. 2019. Williams syndrome, human self-domestication, and language evolution. Frontiers in Psychology, 10, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niego, A. and Benítez-Burraco, A.. 2020. Autism and Williams syndrome: Dissimilar socio-cognitive profiles with similar patterns of abnormal gene expression in the blood. bioRxiv.doi: 10.1101/2020.03.15.992479 [DOI] [PubMed] [Google Scholar]
- Nummenmaa, L. and Calder, A. J.. 2009. Neural mechanisms of social attention 2009. Trends in Cognitive Sciences, 13, 135–143. [DOI] [PubMed] [Google Scholar]
- O’Hearn, K., Roth, J. K., Courtney, S. M., Luna, B., Street, W., Terwillinger, R. and Landau, B.. 2011. Object recognition in Williams syndrome: Uneven ventral stream activation. Developmental Science, 14, 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman, K., Muchnik, S. K. and Sestan, N.. 2020. The evolution of the human brain and disease susceptibility. Current Opinion in Genetics and Development, 65, 91–97. [DOI] [PubMed] [Google Scholar]
- Paul, L. K., Corsello, C., Tranel, D. and Adolphs, R.. 2010. Does bilateral damage to the human amygdala produce autistic symptoms? Journal of Neurodevelopmental Disorders, 2, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier, L. P., Gandía, J., Laboute, T., Becker, J. and Le Merrer, J.. 2018. μ opioid receptor, social behaviour and autism spectrum disorder: Reward matters. British Journal of Pharmacology, 175, 2750–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey, L., Adolphs, R. and Morris, J.. 2004. Neuroanatomical substrates of social cognition dysfunction in autism. Mental Retardation and Developmental Disabilities Research Reviews, 10, 259–271. [DOI] [PubMed] [Google Scholar]
- Perovic, A., Modyanova, N. and Wexler, K.. 2013. Comparison of Grammar in Neurodevelopmental Disorders: The Case of Binding in Williams Syndrome and Autism With and Without Language Impairment. Language Acquisition, 20, 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perovic, A. and Janke, V.. 2013. Issues in the acquisition of binding, control and raising in high-functioning children with autism. UCL Working Papers in Linguistics, 25, 131–143. [Google Scholar]
- Perovic, A. and Wexler, K.. 2007. Complex grammar in Williams syndrome. Clinical Linguistics and Phonetics, 21, 729–745. [DOI] [PubMed] [Google Scholar]
- Phillips, C. E., Jarrold, C., Baddeley, A. D., Grant, J. and Karmiloff-Smith, A.. 2004. Comprehension of spatial language terms in Williams syndrome: Evidence for an interaction between domains of strength and weakness. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 40, 85–101. [DOI] [PubMed] [Google Scholar]
- Philofsky, A., Fidler, D. J. and Hepburn, S.. 2007. Pragmatic language profiles of school-age children with autism spectrum disorders and Williams syndrome. American Journal of Speech-Language Pathology, 16, 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober, B. R. 2010. Williams-Beuren syndrome. New England Journal of Medicine, 362, 239–252. [DOI] [PubMed] [Google Scholar]
- Porter, M.A., Coltheart, M. and Langdon, R.. 2007. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia, 45, 2839–2849. [DOI] [PubMed] [Google Scholar]
- Preissler, M. A. and Carey, S.. 2005. The role of inferences about referential intent in word learning: Evidence from autism. Cognition, 97, B13–B23. [DOI] [PubMed] [Google Scholar]
- Pujara, M. and Koenigs, M.. 2014. Mechanisms of reward circuit dysfunction in psychiatric illness: Prefrontal-striatal interactions. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 20, 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, J., Losh, M., Bellugi, U. and Wulfeck, B.. 2004. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain and Language, 88, 229–247. [DOI] [PubMed] [Google Scholar]
- Reiss, A. L., Eckert, M. A., Rose, F. E., Karchemskiy, A., Kesler, S., Chang, M., Reynolds, M. F., Kwon, H. and Galaburda, A.. 2004. An experiment of nature: Brain anatomy parallels cognition and behavior in Williams syndrome. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 5009–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, S., Riby, D.M., Park, J., Fraser, E. and Campbell, L.E.. 2010. Neuropsychological functioning and executive control in WS. Neuropsychologia, 48, 1216–1226. [DOI] [PubMed] [Google Scholar]
- Riby, D. M., , Doherty-Sneddon, G. and , Bruce, V. 2009. The eyes or the mouth? Feature salience and unfamiliar face processing in Williams syndrome and autism. Quarterly Journal of Experimental Psychology, 62, 189–203. doi: 10.1080/17470210701855629. [DOI] [PubMed] [Google Scholar]
- Riby, D. M. and Hancock, P. J.. 2008. Viewing it differently: Social scene perception in Williams syndrome and autism. Neuropsychologia, 46, 2855–2860. [DOI] [PubMed] [Google Scholar]
- Riby, D. M. and Hancock, P. J.. 2009. Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. Journal of Autism and Developmental Disorders, 39, 421–431. [DOI] [PubMed] [Google Scholar]
- Riby, D. M., Jones, N., Brown, P. H., Robinson, L. J., Langton, S. R. H., Bruce, V. and Riby, L. M.. 2011. Attention to faces in Williams syndrome. Journal of Autism and Developmental Disorders, 41, 1228–1239. [DOI] [PubMed] [Google Scholar]
- Riches, N., Loucas, T., Charman, T., Simonoff, E. and Bair, G.. 2010. Sentence repetition in adolescents with specific language impairments and autism: An investigation of complex syntax. International Journal of Language and Communication Disorders, 45, 47–60. [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. and Craighero, L.. 2004. The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. [DOI] [PubMed] [Google Scholar]
- Roberts, J. A., Rice, M. L. and Tager–Flusberg, H.. 2004. Tense marking in children with autism. Applied Psycholinguistics, 25, 429–448. [Google Scholar]
- Robichaux, M.A., Chenaux, G., Ho, H.Y., Soskis, M.J., Dravis, C., Kwan, K.Y., Šestan, N., Greenberg, M.E., Henkemeyer, M. and Cowan, C.W.. 2014. EphB receptor forward signaling regulates area-specific reciprocal thalamic and cortical axon pathfinding. Proceedings of the National Academy of Sciences, 111, 2188–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, J., Riby, D. M., Janes, E., Connolly, B. and McConachie, H.. 2012. Anxiety and repetitive behaviours in autism spectrum disorders and williams syndrome: A cross-syndrome comparison. Journal of Autism and Developmental Disorders, 42, 175–180. [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. 2015. Limbic systems for emotion and for memory, but no single limbic system. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 62, 119–157. [DOI] [PubMed] [Google Scholar]
- Rose, F. E., Lincoln, A. J., Lai, Z., Ene, M., Searcy, Y. M. and Bellugi, U.. 2007. Orientation and affective expression effects on face recognition in Williams syndrome and autism. Journal of Autism and Developmental Disorders, 37, 513–522. [DOI] [PubMed] [Google Scholar]
- Royston, R., Howlin, P., Waite, J. and Oliver, C.. 2017. Anxiety disorders in Williams Syndrome contrasted with intellectual disability and the general population: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 47, 3765–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio, A., Moreira, P. S., Osório, A., Magalhães, R., Vasconcelos, C., Férnandez, M., Carracedo, A., Alegria, J., Gonçalves, Ó. F. and Soares, J. M.. 2016. Altered functional connectivity of the default mode network in Williams syndrome: A multimodal approach. Developmental Science, 19, 686–695. [DOI] [PubMed] [Google Scholar]
- Schultz, R.T. 2005. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 23, 125–141. [DOI] [PubMed] [Google Scholar]
- Schumann, C. M., Hamstra, J., Goodlin-Jones, B. L., Lotspeich, L. J., Kwon, H., Buonocore, M. H., Lammers, C. R., Reiss, A. L. and Amaral, D. G.. 2004. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier, M. L. 2013. The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist, 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, D.J., Minshew, N.J. and Goldstein, G.. 1996. Wechsler IQ profiles in diagnosis of high-functioning autism. Journal of Autism and Developmental Disorders, 26, 389–406. [DOI] [PubMed] [Google Scholar]
- Sigman, M., Spence, S. J. and Wang, A. T.. 2006. Autism from developmental and neuropsychological perspectives. Annual Review of Clinical Psychology, 2, 327–355. [DOI] [PubMed] [Google Scholar]
- Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T. and Baird, G.. 2008. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Sparaci, L., Formica, D., Lasorsa, F. R., Mazzone, L., Valeri, G. and Vicari, S.. 2015. Untrivial Pursuit: Measuring Motor Procedures Learning in Children with Autism. Autism Research: Official Journal of the International Society for Autism Research, 8, 398–411. [DOI] [PubMed] [Google Scholar]
- Spratt, E. G., Nicholas, J. S., Brady, K. T., Carpenter, L. A., Hatcher, C. R., Meekins, K. A., Furlanetto, R. W. and Charles, J. M.. 2012. Enhanced cortisol response to stress in children in autism. Journal of Autism and Developmental Disorders, 42, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R. N. and Andrews-Hanna, J.. 2015. The default network and social cognition. Brain Mapping: An Encyclopedic Reference, 3, 165–169. [Google Scholar]
- Stefanacci, L. and Amaral, D. G.. 2000. Topographic organization ofcortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. The Journal of Comparative Neurology, 421, 52–79. 10. [DOI] [PubMed] [Google Scholar]
- Stojanovik, V. 2006. Social interaction deficits and conversational inadequacy in Williams syndrome. Journal of Neurolinguistics, 19, 157–173. [Google Scholar]
- Strauss, M. S., Newell, L. C., Best, C. A., Hannigen, S. F., Gastgeb, H. Z. and Giovannelli, J. L.. 2012. The development of facial gender discrimination in typically developing individuals and individuals with autism. Journal of Autism and Developmental Disorders, 42, 1847–1855.doi: 10.1007/s10803-011-1428-1 [DOI] [PubMed] [Google Scholar]
- Sui, J., Rotshtein, P. and Humphreys, G. W.. 2013. Coupling social attention to the self forms a network for personal significance. Proceedings of the National Academy of Sciences of the United States of America, 110, 7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, K., Winner, E. and Tager-Flusberg, H.. 2003. Can adolescents with Williams syndrome tell the difference between lies and jokes?. Developmental Neuropsychology, 231–2, 85–103. [DOI] [PubMed] [Google Scholar]
- Surian, L., Baron-Cohen, S. and Van der Lely, H.. 1996. Are children with autism deaf to Gricean maxims? Cognitive Neuropsychiatry, 1, 55–71. [DOI] [PubMed] [Google Scholar]
- Swartz, J. R., Wiggins, J. L., Carrasco, M., Lord, C. and Monk, C. S.. 2013. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen, L. D., Kelley, E., Fein, D. and Naigles, L. R.. 2007. Processes of language acquisition in children with autism: Evidence from preferential looking. Child Development, 78, 542–557. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg, H., Skwerer, D. P. and Joseph, R. M.. 2006. Model syndromes for investigating social cognitive and affective neuroscience: A comparison of Autism and Williams syndrome. Social Cognitive and Affective Neuroscience, 1, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg, H. and Sullivan, K.. 2000. A componential view of theory of mind: Evidence from Williams syndrome. Cognition, 76, 59–90. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg, H. 1981. On the nature of linguistic functioning in early infantile autism. Journal of Autism and Developmental Disorders, 11, 45–56. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg, H. 2000. Understanding the language and communicative impairments in autism. International Review of Research in Mental Retardation, 23, 185–205. [Google Scholar]
- Tager-Flusberg, H. 2003. Language impairments in children with complex neurodevelopmental disorders: The case of autism. In: Levy Y., Schaeffer J.C., eds. Language competence across populations: Toward a definition of specific language impairment. Mahwah, NJ: Lawrence Erlbaum Associates, pp. 297–321. [Google Scholar]
- Tager-Flusberg, H. 2004. Language and communicative deficits and their effects on learning and behavior. In: Prior M., ed. Asperger syndrome: Behavioral and educational aspects. New York: Guilford Press, pp. 85–103. [Google Scholar]
- Tebbenkamp, A. T., , Willsey, A. J., , State, M. W. and , Šestan, N. 2014. The developmental transcriptome of the human brain. Current Opinion in Neurology, 27, 149–156. doi: 10.1097/WCO.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J. and Maurer, R.G.. 1998. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences of the United States of America, 95, 13982–13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek, S., Jaffery, G., Fein, D. and Naigles, L.. 2008. Do children with autism spectrum disorders show a shape bias in word learning? Autism Research: Official Journal of the International Society for Autism Research, 1, 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, D., Martens, M. A., Smith, D. S. and Roth, E.. 2018. Williams syndrome and music: A systematic integrative review. Frontiers in Psychology, 9, 2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Wells, T. A., Avery, S. N. and Blackford, J. U.. 2011. Using novel control groups to dissect the amygdala's role in Williams syndrome. Developmental Cognitive Neuroscience, 1, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro, R., Konyukh, M., Delorme, R., Leblond, C., Chaste, P., Fauchereau, F., Coleman, M., Leboyer, M., Gillberg, C. and Bourgeron, T.. 2010. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends in Genetics : Tig, 26, 363–372. [DOI] [PubMed] [Google Scholar]
- Trauner, D. A., Bellugi, U. and Chase, C.. 1989. Neurologic features of Williams and Down syndromes. Pediatric Neurology, 5, 166–168. [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q., Supekar, K., Lynch, C. J., Khouzam, A., Phillips, J., Feinstein, C., Ryali, S. and Menon, V.. 2013. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry, 70, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, L. Q., Davies, M. S., Scott, A. A., Zaidel, E., Bookheimer, S. Y., Iacoboni, M. and Dapretto, M.. 2008. Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PLoS One, 3, e3526. doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udwin, O. and Yule, W.. 1990. Expressive language of children with Williams syndrome. American Journal of Medical Genetics Supplement, 6 Suppl, 108–114. [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. and Baetens, K.. 2009. Understanding others' actions and goals by mirror and mentalizing systems: A meta-analysis. NeuroImage, 48, 564–584. [DOI] [PubMed] [Google Scholar]
- Vivanti, G., Hocking, D. R., Fanning, P. and Dissanayake, C.. 2016. Social affiliation motives modulate spontaneous learning in Williams syndrome but not in autism. Molecular Autism, 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski, M., Tager-Flusberg, H. and Ullman, M.T.. 2006. Language in autism. In: Moldin S., Rubenstein J., eds. Understanding autism: From basic neuroscience to treatment. New York: Taylor and Francis, pp. 175–203. [Google Scholar]
- Wallace, G. L., Shaw, P., Lee, N. R., Clasen, L. S., Raznahan, A., Lenroot, R. K., Martin, A. and Giedd, J. N.. 2012. Distinct cortical correlates of autistic versus antisocial personality traits in a longitudinal sample of typically developing youth. Journal of Neuroscience, 32, 4856–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S. W., Albano, A. M., Johnson, C. R., Kasari, C., Ollendick, T., Klin, A., Oswald, D. and Scahill, L.. 2010. Development of a cognitive behavioural intervention program to treat anxiety and social deficits in teens with high functioning autism. Clinical Child and Family Psychology Review, 13, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójciak, P., Remlinger-Molenda, A. and Rybakowski, J.. 2012. The role of oxytocin and vasopressin in central nervous system activity and mental disorders. Psychiatr Polska, 466, 1043–1052. [PubMed] [Google Scholar]
- Yang, X., Ru, W., Wang, B., Gao, X., Yang, L., Li, S., Xi, S. and Gong, P.. 2016. Investigating the genetic basis of attention to facial expressions: The role of the norepinephrine transporter gene. Psychiatric Genetics, 26, 266–271. [DOI] [PubMed] [Google Scholar]
- Zalla, T. and Sperduti, M.. 2013. The amygdala and the relevance detection theory of autism: An evolutionary perspective. Frontiers in Human Neuroscience, 7, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrei, M., Burton, C. L., Engchuan, W., Young, E. J., Higginbotham, E. J., MacDonald, J. R., Trost, B., Chan, A. J. S., Walker, S., Lamoureux, S., Heung, T., Mojarad, B. A., Kellam, B., Paton, T., Faheem, M., Miron, K., Lu, C., Wang, T., Samler, K., Wang, X., Costain, G., Hoang, N., Pellecchia, G., Wei, J., Patel, R. V., Thiruvahindrapuram, B., Roifman, M., Merico, D., Goodale, T., Drmic, I., Speevak, M., Howe, J. L., Yuen, R. K. C., Buchanan, J. A., Vorstman, J. A. S., Marshall, C. R., Wintle, R. F., Rosenberg, D. R., Hanna, G. L., Woodbury-Smith, M., Cytrynbaum, C., Zwaigenbaum, L., Elsabbagh, M., Flanagan, J., Fernandez, B. A., Carter, M. T., Szatmari, P., Roberts, W., Lerch, J., Liu, X., Nicolson, R., Georgiades, S., Weksberg, R., Arnold, P. D., Bassett, A. S., Crosbie, J., Schachar, R., Stavropoulos, D. J., Anagnostou, E. and Scherer, S. W.. 2019. A large data resource of genomic copy number variation across neurodevelopmental disorders. NPJ Genomic Medicine, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. F., Dai, Y. C., Wu, J., Jia, M. X., Zhang, J. S., Shou, X. J., Han, S. P., Zhang, R. and Han, J. S.. 2016. Plasma Oxytocin and Arginine-Vasopressin Levels in Children with. Neuroscience Bulletin, 32, 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., Kaiser, T., Monteiro, P., Zhang, X., Van der Goes, M. S., Wang, D., Barak, B., Zeng, M., Li, C., Lu, C., Wells, M., Amaya, A., Nguyen, S., Lewis, M., Sanjana, N., Zhou, Y., Zhang, M., Zhang, F., Fu, Z. and Feng, G.. 2016. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron, 89, 147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]