Abstract
Arylamine N-acetyltransferases (NATs) are found in many eukaryotic organisms, including humans, and have previously been identified in the prokaryote Salmonella typhimurium. NATs from many sources acetylate the antitubercular drug isoniazid and so inactivate it. nat genes were cloned from Mycobacterium smegmatis and Mycobacterium tuberculosis, and expressed in Escherichia coli and M. smegmatis. The induced M. smegmatis NAT catalyzes the acetylation of isoniazid. A monospecific antiserum raised against pure NAT from S. typhimurium recognizes NAT from M. smegmatis and cross-reacts with recombinant NAT from M. tuberculosis. Overexpression of mycobacterial nat genes in E. coli results in predominantly insoluble recombinant protein; however, with M. smegmatis as the host using the vector pACE-1, NAT proteins from M. tuberculosis and M. smegmatis are soluble. M. smegmatis transformants induced to express the M. tuberculosis nat gene in culture demonstrated a threefold higher resistance to isoniazid. We propose that NAT in mycobacteria could have a role in acetylating, and hence inactivating, isoniazid.
Arylamine N-acetyltransferases (NATs) are cytosolic enzymes which acetylate arylamines and hydrazines by transfer of the acetyl group from acetyl coenzyme A to the free amino group forming an acetylamide (33). The same enzymes are also able to catalyze the transfer of an acetyl group to the oxygen of an arylhydroxylamine (10, 32). NAT is widespread among eukaryotes (28, 33), and the existence of NAT in prokaryotes was, until recently, thought to be confined to Salmonella typhimurium (32). S. typhimurium NAT has the ability to N-acetylate arylamines and the hydrazine isoniazid (24, 32). In humans there are now known to be two isoenzymes, NAT1 and NAT2 (2). The human enzyme NAT2, whose substrates include sulfonamide-based antibacterial compounds (20), was first identified as the enzyme which inactivates the front-line antitubercular drug isoniazid (9). The sulfonamides sulfamethoxazole and p-aminosalicylate are acetylated predominantly by the human isoenzyme NAT1 (4, 13), as is p-aminobenzoic acid (18, 30). Both NAT1 (22, 34) and NAT2 (for a review, see reference 5) show polymorphism in human populations. Identification of human NAT2 polymorphisms provides an explanation for the different effective therapeutic doses of isoniazid in fast and slow acetylators (8, 9).
We report here the cloning of the nat genes from the eubacteria Mycobacterium smegmatis and Mycobacterium tuberculosis and show that the M. smegmatis NAT is active with a range of substrates, including isoniazid. We demonstrate, using antibodies raised against recombinant S. typhimurium NAT, that NAT is present in wild-type M. smegmatis. It was reasoned that if isoniazid was acetylated by NAT prior to activation, an elevated resistance to isoniazid would be observed if more NAT were present in M. smegmatis. We therefore expressed the M. tuberculosis nat gene in M. smegmatis using the shuttle vector pACE-1 (21) and observed the effect of induction of NAT protein on the growth of mycobacteria cultured in isoniazid.
Identification of nat genes in M. smegmatis and M. tuberculosis.
A radiolabelled DNA probe representing a 264-bp HindIII fragment of S. typhimurium nat (32), corresponding to a region which spans two highly conserved areas in NAT, was used to isolate clones containing nat from M. smegmatis and M. tuberculosis (strain H37Rv). Gridded libraries containing at least two copies of the M. smegmatis and M. tuberculosis genomes were screened, and double positives were selected. All sequence analyses were performed by an automated (ABI 377) sequencer (The Advanced Biotechnology Centre, ICSM, London) with Fidelase, an enzyme suitable for regions rich in GC. Amino acid sequences representing putative open reading frames of M. smegmatis and M. tuberculosis NAT are illustrated in Fig. 1 together with a PILEUP comparison of known NAT amino acid sequences. The sequence of M. tuberculosis NAT corresponds exactly to the sequence deposited previously in the database (3), assigned as a hypothetical protein. NAT from the genome of Escherichia coli is also shown for comparison and was obtained by database searching, again assigned as a hypothetical protein (1). These sequences are illustrated in order to emphasize the common features in all NAT proteins; the conserved PFENL and RGGDC sequences (where D is either W or Y), containing the active-site cysteine and the arginine residues proposed to participate in the reaction mechanism (6, 32), are indicated in Fig. 1. The M. smegmatis nat gene open reading frame is >60% identical to M. tuberculosis nat, and these genes have GC contents of 69 and 65%, respectively, compatible with the genes being of mycobacterial origin (15). The mycobacterial NATs each show amino acid sequence identities of ∼35 and 30% with the NATs from S. typhimurium and human NAT2, respectively (Fig. 1).
FIG. 1.
Amino acid alignment of the predicted M. smegmatis and M. tuberculosis NAT against NAT homologues of eukaryotic and prokaryotic origins. Amino acids are represented by the single-letter code. Numbers refer to amino acid positions of the aligned sequences, which are ranked in order of decreasing similarity in comparison with human NAT1. Codes on the left identify the relevant NAT sequence. Human(1) is human NAT1, accession no. P18440; Human(2) is human NAT2, accession no. P11245; Hamster(1) is hamster NAT1, accession no. P50292; Mouse(1) is mouse NAT1, accession no. P50294; Hamster(2) is hamster NAT2, accession no. P50293; Mouse(2) is mouse NAT2, accession no. P50295; E. coli is E. coli NAT, accession no. P77567; S. typhimurium is S. typhimurium NAT, accession no. Q00267; M. tuberculosis is M. tuberculosis NAT, accession no. P96848, which codes for a hypothetical protein (3) and has the same sequence as the M. tuberculosis NAT described in this report; and M. smegmatis is M. smegmatis NAT, accession no. AJ006588. Amino acids showing identity in all NATs are shown in white on a black background, while residues showing conservation and similarity in more than four species of NAT are indicated in white on a grey background. A dot represents a gap introduced to maximize homology. The proposed active site cysteine is indicated (§) (32), as are the arginine residues thought to participate in the reaction (†) (6, 32). Regions of complete identity are underscored.
Expression of mycobacterial nat genes in E. coli.
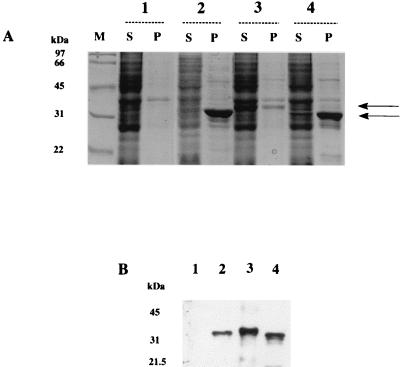
The predicted open reading frames of S. typhimurium, M. smegmatis, and M. tuberculosis nat genes were cloned into the expression vector pET28b (Novagen). E. coli cells (DE23-pLysS) were transformed with the constructs for recombinant protein production, which also encode a 2.1-kDa thrombin-cleavable N-terminal histidine tag. Recombinant protein production was carried out as described previously (24). E. coli cells were disrupted by sonication on ice, eight times each for 30 s with 15-s intervals between sonications, and centrifuged at 100,000 × g for 60 min at 4°C. Supernatant was removed, and the pellet was resuspended in an equal volume of resuspension buffer (50 mM Tris-HCl [pH 8.0], 2 mM EDTA, 4 mM dithiothreitol, and 1 mM Pefabloc [protease inhibitor]). It was demonstrated that the nat sequence from M. smegmatis induces the synthesis of a protein of ∼32 kDa, close to the predicted size of 32.3 kDa, corresponding to the open reading frame coding for the NAT protein (275 amino acids) plus the hexahistidine fusion tag (2.1 kDa). The recombinant NAT protein from M. smegmatis was found associated predominantly with the insoluble pellet (Fig. 2A, lane 4). The molecular mass of the M. tuberculosis NAT protein (275 amino acids) plus the hexahistidine tag is predicted to be 33.5 kDa. The NAT recombinant protein of M. tuberculosis has a molecular mass of around 32 kDa and is found exclusively in the insoluble pellet when produced in E. coli (Fig. 2A, lane 2). The molecular mass of the S. typhimurium NAT protein (283 amino acids) with the hexahistidine tag is predicted to be 34.3 kDa and is found predominantly in the soluble, supernatant fraction (Fig. 2A, lane 3).
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant NAT proteins in E. coli. (A) Gels were stained with Coomassie blue. E. coli cells were transformed with pET28b alone (lanes 1) or with pET28b constructs containing the coding region of nat from M. tuberculosis (lanes 2), S. typhimurium (lanes 3), or M. smegmatis (lanes 4). Lysates were used to generate supernatants (S) and pellets resuspended in the original volume of lysate (P). Individual lanes were loaded with 15 μl of each fraction. The upper and lower arrows indicate the migration of recombinant S. typhimurium and mycobacterial NATs, respectively. Lane M, molecular mass markers. (B) Western blot analysis using an antiserum (1:100,000) against recombinant S. typhimurium NAT developed by using an enhanced chemiluminescence detection system (27). Lane 1, supernatant from E. coli transformed with pET28b alone; lane 2, pellet from E. coli transformed with M. tuberculosis NAT; lane 3, supernatant from E. coli transformed with S. typhimurium NAT; lane 4, supernatant from E. coli transformed with M. smegmatis NAT.
An antiserum raised against purified recombinant S. typhimurium NAT (24) was used to confirm the identity of the induced protein bands observed in Fig. 2A. The immunization schedule and detection were carried out as previously described (27). The antiserum was used at a dilution of 1:100,000 and did not cross-react with the control E. coli supernatant that had been transformed with vector alone under the conditions used (Fig. 2B, lane 1). The antiserum does identify a low level of endogenous E. coli NAT when used at a dilution of 1:50,000 (data not shown). The antiserum, at 1:100,000, cross-reacted with a strong band in the supernatant of E. coli transformed with nat from S. typhimurium (Fig. 2B, lane 3) and in the supernatant of E. coli which had been transformed with the putative nat from M. smegmatis (Fig. 2B, lane 4). No band corresponding to M. tuberculosis NAT was found in the supernatant fraction (data not shown). However, a protein in the pellet fraction of E. coli cells expressing the M. tuberculosis nat gene was shown to cross-react with the antiserum to S. typhimurium NAT. The degree of recognition of the NAT from M. tuberculosis in the pellet (Fig. 2B, lane 2) is less than that expected based on the protein staining intensity (Fig. 2A, lane 2P), suggesting that the antiserum to the S. typhimurium NAT cross-reacts less with the M. tuberculosis protein than with the M. smegmatis protein (Fig. 2A, lane 4S, and Fig. 2B, lane 4).
The detection of NAT activity using the arylamines anisidine, 4-aminoveratrole, and p-aminobenzoic acid was carried out by a colorimetric assay (24), and activity with the hydrazine isoniazid was detected by the method of Olson et al. (19). Bacterial lysates and supernatants were diluted up to 50-fold with 20 mM Tris-HCl (pH 7.5) and 2 mM dithiothreitol before being used. Controls were carried out with identically diluted samples of E. coli cell lysate fractions that had been transformed with pET28b alone and demonstrated no activity under the conditions used. Likewise, insoluble pellets from E. coli cells expressing the M. tuberculosis nat gene or the M. smegmatis nat gene had no NAT enzymic activity. It was concluded that the NAT protein in these pellets was in inclusion bodies. Recombinant NAT from M. smegmatis in the supernatant fraction of E. coli is active in acetylating a series of substrates, including isoniazid, anisidine, and 4-aminoveratrole, which are also substrates of the S. typhimurium enzyme. The arylamine p-aminobenzoic acid is poorly acetylated by NAT from both M. smegmatis and S. typhimurium (24). Isoniazid is a better substrate (Km, 25 μM; Vmax/Km 2,520 × 10−6 liter · min−1 · mg of protein−1) than are the arylamines anisidine (Km, 300 μM; Vmax/Km, 16 × 10−6 liter · min−1 · mg of protein−1) and 4-aminoveratrole (Km, 650 μM; Vmax/Km, 25 × 10−6 liter · min−1 · mg of protein−1) for the NAT enzyme from M. smegmatis. These results suggest the enzyme has a substrate specificity similar to that of the S. typhimurium enzyme (24, 32).
Expression of mycobacterial nat genes in M. smegmatis.
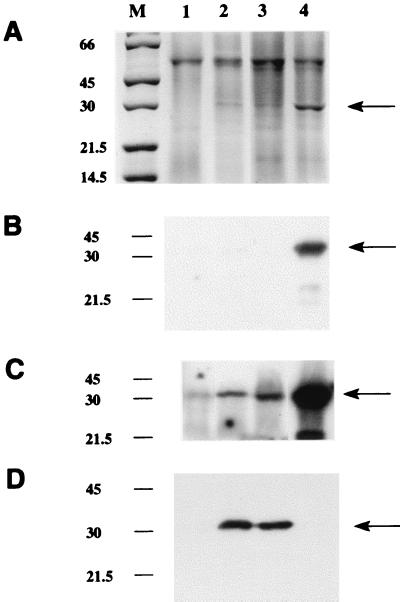
M. smegmatis and M. tuberculosis nat genes were also expressed by using the mycobacterial expression vector pACE-1. Competent M. smegmatis cells (Mc2155 [26]) were electroporated with 1 μg of plasmid DNA, either alone or containing the nat insert, at 700 Ω, 2.5 kV, and 25 μF (26). M. smegmatis cells were grown on the selective medium 7H9 supplemented with albumin-dextrose-catalase (Difco) or 7H11 agar supplemented with oleic acid-albumin-dextrose-catalase (Difco) containing hygromycin (50 μg/ml). Minimal medium was used for gene expression using the pACE-1 constructs (21). These constructs yield recombinant protein when acetamide (2 mg/ml) is supplied as the sole carbon source in the growth medium. M. smegmatis cell pellets were sonicated on ice 10 times, each for 1 min, with 30-s intervals between sonications. The resulting cell lysates were centrifuged at 100,000 × g for 60 min at 4°C. Supernatants were kept, and the pellets were resuspended to the original volume in resuspension buffer. With this expression vector, recombinant mycobacterial NAT protein was detected predominantly in the supernatant fraction (Fig. 3A). Cells expressing the M. smegmatis nat (Fig. 3A, lane 4) routinely had more recombinant protein than the cells expressing the M. tuberculosis nat gene (Fig. 3A, lanes 2 and 3). The sizes of the induced protein bands were around 30 kDa, which corresponds to the expected sizes for NAT proteins without a hexahistidine tag. The antiserum raised against the pure S. typhimurium NAT identified a band in the supernatants of M. smegmatis induced to express the M. smegmatis nat gene (Fig. 3B and C, lane 4) and, with longer exposure of the autoradiograph, the presence of M. tuberculosis NAT (Fig. 3C, lanes 2 and 3). There was also an indication of endogenous NAT (Fig. 3C, lane 1). The sizes of the endogenous and induced proteins (which have no hexahistidine tags) illustrated in Fig. 3 are indistinguishable, as expected. An antiserum raised against recombinant M. tuberculosis NAT, prepared by immunizing rabbits with insoluble recombinant protein excised from a polyacrylamide gel slice, was used at a dilution of 1:100,000 and is highly specific for M. tuberculosis NAT (Fig. 3D, lanes 2 and 3). It does not recognize M. smegmatis NAT under the conditions shown in Fig. 3D.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant NAT proteins in M. smegmatis. Gels were loaded with 15 μl of supernatants from lysates of M. smegmatis transformed with pACE-1 alone (lane 1), with M. tuberculosis nat (lanes 2 and 3), or with M. smegmatis nat (lane 4) (M, molecular mass markers [in kilodaltons]). (A) Staining with Coomassie blue. The arrow indicates the additional band in the supernatants transformed with a nat gene. (B to D) Western blots developed by using an enhanced chemiluminescence detection system as described previously (27), where exposure time for detection of the second antibody is either 1 min (B and D) or 20 min (C). In each panel, lane 1 has been loaded with 10 μg of total protein and lanes 2 to 4 have been loaded with 600 ng of total protein. An antiserum raised against recombinant M. tuberculosis NAT protein synthesized in E. coli is shown in panel D. The antiserum used in panels B and C is the antiserum against pure S. typhimurium NAT (24). Both antisera were used at a dilution of 1:100,000.
Overproduction of mycobacterial nat genes in M. smegmatis results in increased resistance to isoniazid.
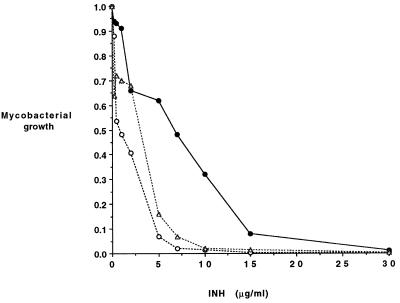
We have investigated whether the expression of the nat gene from M. tuberculosis alters the sensitivity of M. smegmatis to isoniazid in vivo. M. smegmatis transformants of pACE-1 either alone or containing the nat gene from M. tuberculosis were grown in minimal medium containing either acetamide to induce synthesis of protein or an equivalent carbon concentration of glucose which does not induce protein synthesis from the pACE-1 vector. Cultures were grown for up to 48 h in the presence of different amounts of isoniazid, and cell growth was determined at 600 nm with a plate reader (Titertek Multiskan). It was observed that when the expression of the nat gene was induced, there was an increase in the concentration of isoniazid in the growth medium that could be tolerated (Fig. 4). There was no change in the response to isoniazid in M. smegmatis cultures containing the nat gene construct when the growth conditions did not induce synthesis of recombinant NAT protein. In contrast, M. smegmatis cells, which contained only vector, were equally sensitive to isoniazid, irrespective of whether acetamide or glucose was the carbon source.
FIG. 4.
Effect of expression of M. tuberculosis nat on the growth of M. smegmatis in the presence of isoniazid (INH). Results for cultures of M. smegmatis either transformed with pACE-1 alone (open circles) or with pACE-1 containing M. tuberculosis nat (solid circles) grown in minimal medium containing acetamide and cultures of pACE-1 containing M. tuberculosis nat grown in minimal medium containing glucose (triangles) are shown.
Discussion.
The existence of highly conserved arylamine N-acetyltransferase sequences in bacteria other than S. typhimurium suggests that the enzyme is conserved in evolution. The role of NAT in endogenous metabolism is unclear, although it has been suggested that in eukaryotes, NAT (in particular, the human NAT1 isoenzyme) plays a role in folate catabolism (16, 31). The very poor activity of the NAT from M. smegmatis or S. typhimurium with p-aminobenzoic acid can be rationalized on the basis that a supply of p-aminobenzoic acid is essential for folate synthesis in prokaryotes. The activity profile is more like that of the human isoenzyme NAT2 which is responsible for the inactivation, by acetylation, of isoniazid (18). NATs are present in several bacterial species, and a sequence similar to NAT has been identified in the completed genome sequence of E. coli (1). It has been demonstrated in the present study that the activity in E. coli with all substrates tested is less than 1% of the activity of the other recombinant NATs overproduced in E. coli. In addition to the overall homology, alignment of NAT sequences (Fig. 1) necessitates the introduction, in the bacterial sequences, of a gap of around 20 amino acids on the C-terminal region flanking the N- and C-terminal regions of the molecule (12). It has been suggested that the N-terminal region is predominantly alpha helix, while the C-terminal portion is predominantly beta sheet. The gap corresponds to the region linking these two secondary structure domains of the molecule. The bacterial NAT enzymes appear to be particularly stable to proteolysis, in contrast to the mammalian NAT enzymes (25), which may be due to the lack of a randomly structured loop in the bacterial NATS.
The expression of NAT in mycobacteria has important implications. Mycobacteria are exquisitely sensitive to isoniazid (14), although there are differences among the mycobacteria in their levels of sensitivity to the drug. M. tuberculosis will not grow in 0.2 μg of isoniazid per ml (21), and M. smegmatis will not grow in 5 μg of isoniazid per ml (7). When we induced the synthesis of more NAT in M. smegmatis, growth of the mycobacteria was not arrested until the concentration of isoniazid was 15 μg/ml. Isoniazid is inactivated in humans through acetylation (8, 9). The results presented here demonstrate that NAT is expressed endogenously in M. smegmatis and that the NAT proteins from mycobacteria can acetylate isoniazid and thus appear able to inactivate isoniazid in vivo. There is a body of evidence to demonstrate that the antimycobacterial activity of isoniazid in mycobacteria relies on the drug first becoming activated (11, 17, 23). It is already known that the acetylation of isoniazid inactivates the drug. The results presented here support the concept that the acetylation of isoniazid in mycobacteria acts in competition with the activation through oxidation. It is therefore important to investigate nat expression in other mycobacteria, including particularly M. tuberculosis, in which development of isoniazid resistance cannot be accounted for completely by currently identified loci (29).
Acknowledgments
We are extremely grateful to the Wellcome Trust for financial support.
We thank S. Martin for the preparation of the gridded libraries and D. Young, K. Duncan, and J. Sinclair for very helpful discussions. We also thank James Sandy for excellent technical assistance.
REFERENCES
- 1.Blattner F, Plunkett G, Bloch C, Perna N, Burland V, Riley M, Collado-Vides J, Glasner J, Rode C, Mayhew G, Gregor J, Davis N, Kirkpatrick H, Goeden M, Rose D, Mau B, Shao Y. The complete genome sequence of Escherichia coli K12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 2.Blum M, Grant D M, McBride W, Heim M, Meyer U A. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990;9:193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- 3.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Barrel B. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 4.Cribb A E, Nakamura H, Grant D M, Miller M A, Spielberg S P. Role of polymorphic and monomorphic human arylamine N-acetyltransferases in determining sulfamethoxazole metabolism. Biochem Pharmacol. 1993;45:1277–1282. doi: 10.1016/0006-2952(93)90280-a. [DOI] [PubMed] [Google Scholar]
- 5.Deguchi T. Physiology and molecular biology of arylamine N-acetyltransferases. Biomed Res. 1992;13:231–242. [Google Scholar]
- 6.Delomenie C, Goodfellow G H, Krishnamoorthy R, Grant D M, Dupret J M. Study of the role of the highly conserved residues Arg(9) and Arg(64) in the catalytic function of human N-acetyltransferases NAT1 and NAT2 by site-directed mutagenesis. Biochem J. 1997;323:207–215. doi: 10.1042/bj3230207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhandayuthapani S, Zhang Y, Mudd M H, Deretic V. Oxidadtive stress response and its role in sensitivity to isoniazid in mycobacteria: characterization and inducibility of ahpC by peroxides in Mycobacterium smegmatis and lack of expression in M. aurum and M. tuberculosis. J Bacteriol. 1996;178:3641–3649. doi: 10.1128/jb.178.12.3641-3649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellard G A, Gammon P T. Pharmacokenetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976;4:83–113. doi: 10.1007/BF01086149. [DOI] [PubMed] [Google Scholar]
- 9.Evans D A P, Manley K A, McKuisick V A. Genetic control of isoniazid acetylation in man. Br Med J. 1960;2:485–491. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hein D W, Doll M A, Gray K, Rustan T D, Ferguson R J. Metabolic activation of N-hydroxy-2-aminofluorene and N-hydroxy-2-acetylaminofluorene by monomorphic N-acetyltransferase (NAT1) and polymorphic N-acetyltransferase (NAT2) in colon cytosols of Syrian hamsters congenic at the NAT2 locus. Cancer Res. 1993;53:509–514. [PubMed] [Google Scholar]
- 11.Heym B, Zhang Y, Poulet S, Young D, Cole S T. Characterization of the katG gene encoding catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993;175:4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard T, Tramontano A, Team I W. Update on protein structure prediction: results of the 1995 IRBM Workshop. Protein Folding Design. 1996;1:R55–R63. doi: 10.1016/S1359-0278(96)00028-4. [DOI] [PubMed] [Google Scholar]
- 13.Jehne J W. Partial purification and properties of the isoniazid transacetylase in human liver: its relationship to the acetylation of p-aminosalicylic acid. J Clin Investig. 1965;44:1992–2002. doi: 10.1172/JCI105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruger-Thiemer E. Isonicotinic acid hypothesis of the anti-tuberculosis action of isoniazid. Am Rev Tuberc. 1958;78:364–367. doi: 10.1164/artpd.1958.77.2.364. [DOI] [PubMed] [Google Scholar]
- 15.Lévy-Frébault V V, Portaels F. Proposed minimum standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int J Syst Bacteriol. 1992;42:315–323. doi: 10.1099/00207713-42-2-315. [DOI] [PubMed] [Google Scholar]
- 16.Minchin R F. Acetylation of para-aminobenzoylglutamate, a folate catabolite, by recombinant human NAT and U937 cells. Biochem J. 1995;307:1–3. doi: 10.1042/bj3070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser M, Kapur V, Williams D, Kreiswirth B, van Soolingen D, Van Embden J. Characterisation of the catalase-peroxidase gene (katG) and inhA locus in isoniazid resistant and susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis. 1996;173:196–202. doi: 10.1093/infdis/173.1.196. [DOI] [PubMed] [Google Scholar]
- 18.Ohsako S, Deguchi T. Cloning and expression of cDNAs for polymorphic and monomorphic arylamine N-acetyltransferases of human liver. J Biol Chem. 1990;265:4630–4634. [PubMed] [Google Scholar]
- 19.Olson W, Dayton P, Israili Z, Pruitt A. Spectrophotofluorimetric assay for isoniazid and acetylisoniazid in plasma adapted to paediatric studies. Clin Chem. 1977;23/24:745–748. [PubMed] [Google Scholar]
- 20.Palamanda J, Hickman D, Ward A, Sim E, Romkes-Sparks M, Unadkat J. Dapsone acetylation by human liver N-acetyltransferase and interaction with anti-opportunistic infection drugs. Drug Metab Dispos. 1995;23:473–477. [PubMed] [Google Scholar]
- 21.Parish T, Mahenthiralingam E, Draper P, Davis E O, Colston M J. Regulation of the inducible amidase gene of Mycobacterium smegmatis. Microbiology. 1997;143:2267–2276. doi: 10.1099/00221287-143-7-2267. [DOI] [PubMed] [Google Scholar]
- 22.Risch A, Smelt V, Lane D, Stanely L, van der Slot W, Ward A, Sim E. Arylamine N-acetyltransferase in erythrocytes of cystic fibrosis patients. Pharmacol Toxicol. 1996;78:235–240. doi: 10.1111/j.1600-0773.1996.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Rozwarski D A, Grant G A, Barton D H R, Jacobs W R, Sacchettini J C. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair J, Delgoda R, Noble M, Jarmin S, Goh N, Sim E. Purification, characterisation and crystallisation of an N-hydroxyarylamine O-acetyltransferase from Salmonella typhimurium. Prot Expr Purif. 1998;12:371–380. doi: 10.1006/prep.1997.0856. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair J, Sim E. A fragment consisting of the first 204 amino-terminal amino acids of human arylamine N-acetyl transferase one (NAT1) and the first transacetylation step of catalysis. Biochem Pharmacol. 1997;53:11–16. doi: 10.1016/s0006-2952(96)00592-8. [DOI] [PubMed] [Google Scholar]
- 26.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R. Isolation and characterisation of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 27.Stanley L, Mills I, Sim E. Localisation of polymorphic N-acetyltransferase (NAT2) in tissues of inbred mice. Pharmacogenetics. 1997;7:121–130. doi: 10.1097/00008571-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Vatsis K P, Weber W W, Bell D A, Dupret J M, Evans D A, Grant D M, Hein D W, Lin H J, Meyer U A, Relling M V, et al. Nomenclature for N-acetyltransferases. Pharmacogenetics. 1995;5:1–17. doi: 10.1097/00008571-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Victor T C, Warren R, Butt J L, Jordaan A M, Felix J V, Venter A, Sirgel F A, Schaaf H S, Donald P R, Richardson M, Cynamon M H, VanHelden P D. Genome and MIC stability in Mycobacterium tuberculosis and indications for continuation of use of isoniazid in multidrug-resistant tuberculosis. J Med Microbiol. 1997;46:847–857. doi: 10.1099/00222615-46-10-847. [DOI] [PubMed] [Google Scholar]
- 30.Ward A, Hickman D, Gordon J W, Sim E. Arylamine N-acetyltransferase in human red blood cells. Biochem Pharmacol. 1992;44:1099–1104. doi: 10.1016/0006-2952(92)90373-q. [DOI] [PubMed] [Google Scholar]
- 31.Ward A, Summers M, Sim E. Purification of recombinant human NAT1 expressed in E. coli. Biochem Pharm. 1995;49:1759–1767. doi: 10.1016/0006-2952(95)00087-g. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M, Sofuni T, Nohmi T. Involvement of Cys69 residue in the catalytic mechanism of N-hydroxyarylamine O-acetyltransferase of Salmonella typhimurium. Sequence similarity at the amino acid level suggests a common catalytic mechanism of acetyltransferase for S. typhimurium and higher organisms. J Biol Chem. 1992;267:8429–8436. [PubMed] [Google Scholar]
- 33.Weber W W, Hein D W. Arylamine N-acetyltransferases. Pharmacol Rev. 1985;37:25–79. [PubMed] [Google Scholar]
- 34.Weber W W, Vatsis K P. Individual variability in p-aminobenzoic acid acetylation by human N-acetyltransferase (NAT1) of peripheral blood. Pharmacogenetics. 1993;3:209–212. doi: 10.1097/00008571-199308000-00006. [DOI] [PubMed] [Google Scholar]