Abstract
Patients with non-supernumerary ring chromosome 7 syndrome have an increased incidence of hemangiomas, café-au-lait spots and melanocytic nevi. The mechanism for the increased incidence of these benign neoplasms is unknown. We present the case of a 22-year old man with ring chromosome 7 and multiple melanocytic nevi. Two nevi, one on the right ear and the other on the right knee, were biopsied and diagnosed as desmoplastic Spitz nevi. Upon targeted next-generation DNA sequencing, both harbored BRAF fusions. Copy number alterations and fluorescence in situ hybridization (FISH) for BRAF suggested that the fusions arose on the ring chromosome 7. Hence, one reason for increased numbers of nevi in patients with non-supernumerary ring chromosome 7 syndrome may be increased likelihood of BRAF fusions, due to the instability of the ring chromosome.
Keywords: BRAF fusion, BRAF gene, ring chromosome 7, ring chromosome seven, melanocytic nevi, melanocytic nevus, Spitz nevus, Spitz tumor, spitzoid, BRAF fusion
Introduction
Non-supernumerary ring chromosome 7 syndrome is defined genetically by one abnormal chromosome 7 that forms a ring and has a characteristic phenotype. The ring is caused by double-stranded DNA breaks of the short and long arms of the chromosome, which become subsequently joined, resulting in the formation of the ring. As a consequence the subtelomeric regions of both chromosomal arms may become lost (Velagaleti et al., 2002). The ring chromosome 7 may be detected by traditional G-banded karyotyping or fluorescence in situ hybridization (FISH) and may be performed on peripheral lymphocytes, normal skin fibroblasts, bone marrow or nevi (Salas-Labadía et al., 2014; Velagaleti et al., 2002). The phenotypic traits may include neurodevelopmental delay, psychomotor delay, microcephaly, dysmorphic facial features, genital anomalies, skeletal anomalies and cutaneous lesions, including capillary malformations (so-called “nevus flammeus” or port wine stain), capillary hemangiomas of the head and neck, café-au-lait spots, hypopigmented macules and numerous melanocytic nevi (Salas-Labadía et al., 2014; Vollenweider Roten, Masouyé, et al., 1993). These melanocytic nevi have been described as darkly pigmented papules or macules that may occur on varied anatomical sites (abdomen, thorax, upper and lower extremities). In 5 of 11 ring chromosome 7 patients with melanocytic nevi, the nevi were reported to be congenital (Table 1). Their histomorphology has not been well studied, and their genomic alterations remain to be elucidated.
TABLE 1.
Literature review of melanocytic lesions in patients with ring chromosome 7 syndrome
| Article | Patient demographics | Cutaneous phenotype | Skin lesions, histology | Non-cutaneous phenotype | Cytogenetics |
|---|---|---|---|---|---|
| Salas-Labadia et al. Cytogenomic and phenotypic analysis in low-level monosomy 7 mosaicism with non-supernumerary ring chromosome 7. Am J Med Genet A. 2014 (PMID: 24677512) | 7 months – 6 years M | Congenital nevi, nevus flammeus, café-au-lait spots, hypo/hyper pigmented spots following the lines of Blaschko (face, dorsum and limbs) | NA | Dysmorphic features, developmental delay, hypogenitalism, lumbar dextroscoliosis, cerebellar and ophtalmological abnormalities. | 46, XY, r(7)(p22.3q36.1) 0.8 Mb deletion in 7p22.3, and a 7.5 Mb deletion in 7q36.1 arr 7p22.3(113,336-954,145)x1,7q36.1q36.3(151306863-158812247)x1, (Human Genome Build, hg18)) |
| Kaur et al. Ring chromosome 7 in an Indian woman. J Intellect Dev Disabil. 2008 (PMID 18300171) | 25F | Dark pigmented nevi (abdomen, thorax and thigh). Café-au-lait spots. | NA | Facial dysmorphism, upward slanting palpebral fissures, broad nose, large lips, short neck, widely spaced nipples, puffy hands and feet, short stature. Speech delay, microcephaly. | 46,XX, r(7) |
| Mehraein et al. Somatic mosaicism of chromosome 7 in a highly proliferating melanocytic congenital naevus in a ring chromosome 7 patient. Am J Med Genet A 2004 (PMID 15523614) | 14M | Nevus flammeus of the forehead and back, multiple small flat congenital melanocytic nevi (one of which had rapidly grown, measured 2.5 in greatest diameter) | Surgical excision of the largest nevus: compound melanocytic congenital nevus | Failure to thrive, microcephaly, seizures. Dysmorphic features: short neck, deep set ears, short palpebral fissures, arched palate, klinodaktyly. | 46,XY,r(7)(p22q36) Loss, gain or duplication of the ring chromosome in 15.4% of lymphocytes. In the nevus, gain of chromosome 6 in 36.8% of cells. |
| Rodriguez et al. Ring chromosome 7 and sacral agenesis. (PMID 10982483) | 19 month F | Nevus flammeus (forehead), multiple nevi (head, labia majora, abdomen, lower limbs), café-au-lait spots (head and back of lower limbs). | NA | Microcephaly, hypotelorism, sacral agenesis. | 46, XX, r(7) |
| Ceballos-Quintal et al. Severe congenital hypoacusia in a a patient with mosaic ring chromosome 7. Rev Biomed 1999 (PMID: not indexed) | 8M | Capillary hemangiomas (forehead, occiput, neck). Café-au-lait spots (clavicular region, thorax, abdomen, arms and hands). Dark pigmented nevi (hand and back). Hypopigmented spots (legs). | NA | Microcephaly, psychomotor delay, failure to thrive, upslanting palpebral fissures, broad nasal base, short neck, syndactyly. Hypoacusia. | 46, XY, r(7) (p22.2q36.3) |
| Wahlstrom et al. Boy with a ring 7 chromosome: a case report with special reference to dermatological findings. Acta Paediatric. 1996 (PMID 8922097) | 8M | Café-au-lait spots and multiple heavily pigmented nevi, most of which were clinically considered “dysplastic” | Histopathology showed “pigmented nevi with pronounced dysplasia”. No signs of melanoma in situ. Melanocytes were “heavily pigmented”. One lesion showed large cells that “confluence to syncytia-like giant cells”. | Short stature, facial dysmorphism. Normal psycho-motor development. | 46, XY, r(7)(p22.3q36.3) |
| Vollenweider et al. Cutaneous findings in ring chromosome 7 syndrome. Dermatology, 1993. (PMID 8428052) | 9 cases. 3 days-17 years. 7/9: M 2/9: F | 7/9 had vascular lesions (nevus flammeus, hemangiomas). 3 had café-au-lait spots. 5 showed large pigmented congenital nevi. | NA | Skeletal anomalies, short stature, microcephaly, ocular and genital anomalies. | NA |
| Vollenweider et al. Melanoma associated with ring chromosome 7. Dermatology. 1993. (PMID: 8428043) | 17F | Multiple pigmented congenital nevi. Disseminated nevi (largest 5.5 cm in diameter, clinical photograph available). Largest nevi on the thigh, knee and calf. At 17 years of age, presented with itching of a right retroauricular pigmented lesion. |
Seven excised nevi, shown to be compound melanocytic nevi. At 17 years of age, the retroauricular lesion was biopsied and showed melanoma (Breslow 1.8mm), composed of “pigmented epithelioid-type cells invading the epidermis and reticular dermis”. Satellite cutaneous metastases found. No recurrence at 24 months after follow-up nor nodal metastases. |
None. | 46, XX, r(7) |
| Biesecker et al. Severe anomalies associated with ring chromosome 7, Am J Med Genet, 1991. (PMID 1746606) | 3 day M | Capillary hemangiomas (forehead, occiput, back and neck), darkly pigmented congenital nevus (thigh) | NA | Polyasplenia, intrauterine growth retardation, wide palpebral fissures, short ears, heart murmur, unilateral hydrocele. | 46, XY, r(7)(p22q36) |
| Caramia et al. Ring chromosome 7: report of the fifth case. Eur J Pediatr. 1990 (PMID 2189730) | 13M | Multiple pigmented nevi. Hypopigmented spots. Several capillary hemangiomas. | NA | Growth failure, microcephaly, clinodactyly | 46, XY, r(7)(p22q36) |
| Koiffmann et al. Ring chromosome 7 in a man with multiple congenital anomalies and mental retardation. J Med Genetics. 1990 (PMID: 2395166) | 39M | Hemangiomas (forehead, neck). Pigmented nevi (back and abdomen). | NA | Microcephaly, short palpebral fissures, inguinal hernia, hypospadias. Growth and developmental delay. Short stature. | 46, XY, r(7) |
| DeLozier-Blanchet and Guenin. Cytogenetics of ring chromosome 7, Clin Genet 1984. PMID (6705244) AND DeLozier et al. A fourth case of ring chromosome 7, Clin Genet, 1982 (PMID 7172483) |
8F | Numerous pigmented congenital nevi on all parts of the body (0.3–1.0cm). Also, three large verrucous and pigmented nevi on the lower limbs (largest 5.5 cm in diameter), firm on palpation. Cutis marmorata (abdomen and thighs). Nevus flammeus (vertex) | Biopsy showed “typical congenital nevi” | Short stature dysmorphic features (round face, arched palate, wide-spaced nipples and short 4th metacarpals). | 46, XX, r(7) |
Materials and Methods
The study was approved by the human research ethics committee of the University of California, San Francisco (11–07922) and was conducted according to the Declaration of Helsinki.
Tumor DNA was analyzed using capture-based next-generation sequencing with the UCSF500 Cancer Gene Panel which examines the coding regions of 479 genes (Supplementary Table 1) and the select introns of 47 genes. Analysis was performed as previously described (Afshar et al., 2019).
Fluorescence in situ hybridization (FISH) using the ZytoLight SPEC BRAF dual color break apart probe (Zytovision, Bremerhaven, Germany) was carried out on 5 μm sections.
Results
The patient was a 22-year old man with a 46 XY, r(7) karyotype who had global developmental delay, relative microcephaly and several papules (presumed to be melanocytic nevi) present since early childhood. Hemangiomas and café-au-lait spots were not present. Two of his nevi (right ear and right knee) which had been present since early childhood and were stable in size and shape for 3 years, were biopsied and showed similar histopathologic findings. Melanocytes were arrayed in a predominantly dermal distribution with rare Kamino bodies in the slightly hyperplastic epidermis. In the papillary dermis, large epithelioid melanocytes had moderately abundant gray cytoplasm and large nuclei with prominent nucleoli. Some showed multinucleation and pigmentation was faint or partial. Nests and fascicles of melanocytes positioned between desmoplastic collagen bundles demonstrated “maturation” (diminished size of melanocytes and nests of melanocytes) with increased depth in the reticular dermis (Figure 1A and C). Both tumors were negative for PRAME and demonstrated mosaic expression of p16 by immunohistochemistry (Figure 1B and D).
Figure 1.
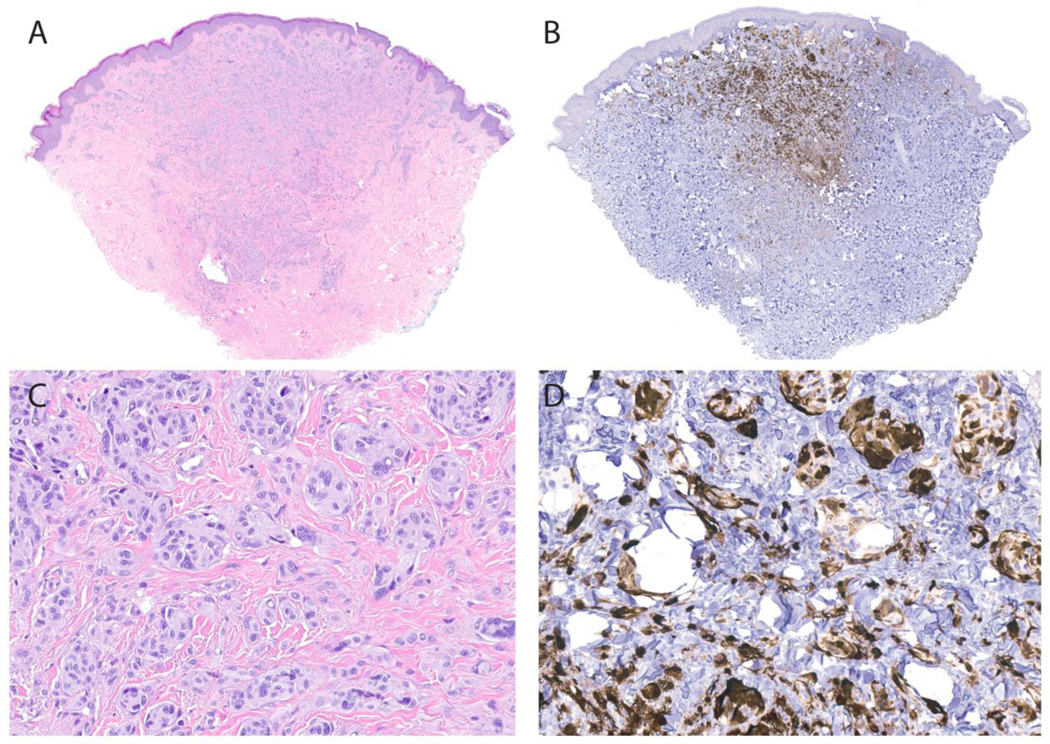
Histopathology of the Spitz nevus from the knee showing a predominantly dermal distribution of melanocytes, with nests and fascicles of melanocytes extending into the reticular dermis with a wedge-shaped profile. (1A,C: H&E) Large epithelioid melanocytes with abundant cytoplasm and prominent nucleoli are observed. The melanocytes demonstrated strong mosaic expression of p16 by immunohistochemistry with less expression in the deeper aspects of the nevus (a gradient pattern of expression) (1B,D).
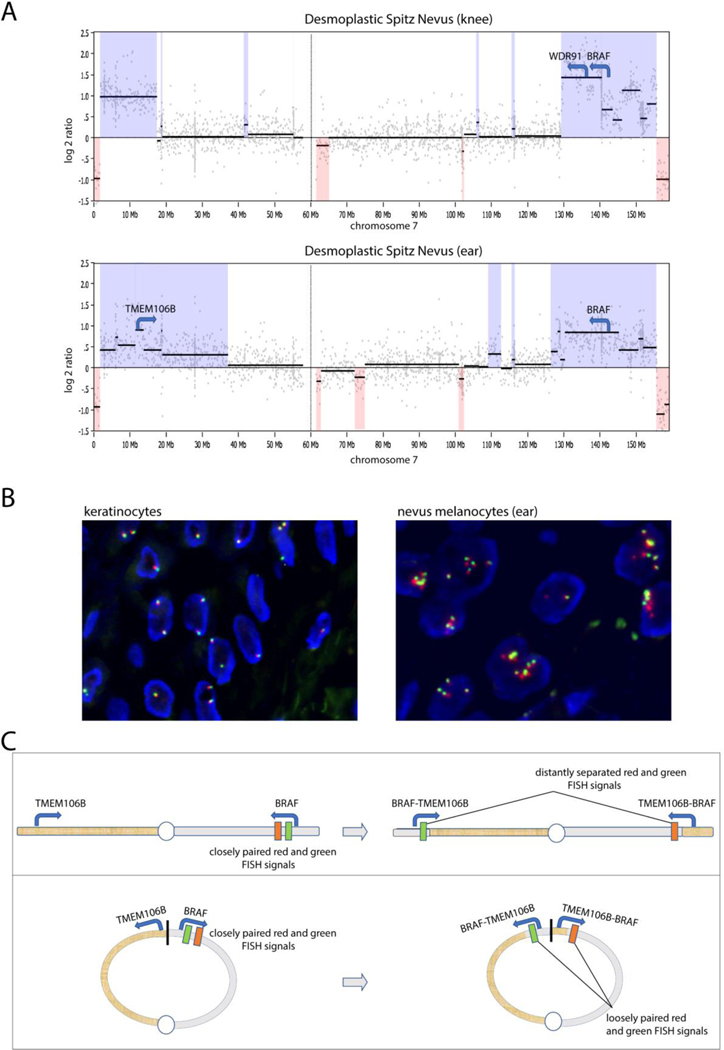
By targeted gene panel sequencing, the Spitz nevus on the ear harbored both a TMEM106B-BRAF fusion and its reciprocal BRAF-TMEMB106B fusion (Supplementary Data 1). The resulting TMEM106B-BRAF gene retained exons 8–18 of BRAF and was predicted to result in an in-frame transcript. The Spitz nevus on the knee harbored a WDR91-BRAF fusion that retained exons 9–18 of BRAF and was also predicted to result in an in-frame transcript. Both the TMEMB106B-BRAF and WDR91-BRAF fusions are predicted to encode fusion proteins with constitutive BRAF kinase activity as they include the BRAF kinase domain but not the autoinhibitory N-terminal domain of BRAF (Botton et al., 2013; Ross et al., 2016; Tran et al., 2005). Both tumors showed amplification of the genomic regions containing the BRAF fusion gene, with identical losses of the tips of both arms of chromosome 7 (likely reflecting DNA lost in the process of the ring formation) and amplification of the portions of chromosome 7 proximal to the losses at the tips of chromosome 7, suggesting that the amplifications occurred on the ring chromosome (Figure 2A). No additional genetic alterations including TERT promoter or BRAF mutations or CDKN2A mutations or deletions were identified. Except the aforementioned copy number changes on chromosome 7 there were no additional copy number changes in the rest of the genome in either tumor.
Figure 2.
Molecular characterization of the two Spitz nevi. (A) Copy number profiles derived by next-generation DNA sequencing. In both tumors, there is loss of the identical distal portions of chromosome 7, reflecting the genomic material lost from the ring chromosome 7. Both tumors demonstrate amplified regions consistent with amplification of the BRAF fusion. (B) FISH with break-apart probes for the BRAF region showed two closely paired red and green signals in keratinocytes and several loosely paired signals in neoplastic melanocytes from the Spitz nevus on the ear. (C) The illustration shows the BRAF and TMEM106B loci, which are far apart on the opposing arms of chromosome 7. The location targeted by the red and green FISH probes flanking the BRAF locus are shown. The top panel illustrates how a TMEM106B-BRAF fusion would lead to widely split signals. The lower panel shows closer proximity of the BRAF and TMEM106B loci in the setting of a ring chromosome 7, and how a TMEM106B-BRAF fusion would result in loosely split FISH signals as seen in the neoplastic melanocytes of our case.
Fluorescence in situ hybridization (FISH) with BRAF break apart probes was performed on both tumors. The probes flank the BRAF locus with red on the 3’ and green on the 5’ side. Normal keratinocytes showed 1–2 closely juxtaposed red and green signals, representing the intact BRAF loci. By contrast, in both tumors, neoplastic melanocytes had approximately 5 pairs of red and green signals, most of which appeared to be slightly more distant than those in keratinocytes (Figure 2B). Given the FISH findings, the TMEM106B-BRAF fusion must occur on the ring chromosome. Because TMEM106B and BRAF are far apart on chromosome 7, a complete spatial separation of BRAF probes would be expected if the fusion occurred on a normal chromosome 7 (Figure 2C). However, a TMEM106B-BRAF fusion on the ring chromosome 7 could occur through an inversion of the genomic region spanning the 5’ end of BRAF, the distal q-arm, the junction with the p-arm, the distal p-arm, and the 5’ end of TMEM106B and would result in a partial split of FISH probes and the reciprocal fusion as seen in our case. This finding indicates that the TMEM106B-BRAF fusion affected the BRAF locus on the ring chromosome and not on the normal chromosome 7. The WDR91-BRAF fusion can arise from tandem duplication, and the FISH findings are compatible with tandem duplication of the 5.6 Mb segment between WDR91 and BRAF occurring on either the ring or normal chromosome 7. However, the number of reads supporting the WDR91-BRAF fusion indicate that the fusion was amplified, and the copy number profile of chromosome 7 suggests that the amplification (and thus the BRAF fusion) occurred on the ring chromosome 7.
Discussion
Herein, we present a patient with non-supernumerary ring chromosome 7 syndrome and multiple melanocytic nevi. Two of the nevi were biopsied and both were histopathologically characterized as desmoplastic Spitz nevi and not common or conventional melanocytic nevi. Both harbored BRAF fusions occurring on the pre-existing ring chromosome 7. Because of their topology, ring chromosomes are likely to be unstable during cell divisions, making them prone to double-stranded DNA breaks and structural rearrangements (Velagaleti et al., 2002). Melanocytic nevi, specifically Spitz nevi, can be initiated by a structural rearrangement that generates constitutively active kinases (Wiesner et al., 2014; Yeh et al., 2015). Two of the kinases found to be rearranged in Spitz nevi are located on chromosome 7: BRAF and MET. Kinase fusions involving these genes in Spitz tumors often have a fusion partner that also is contained in chromosome 7 (Botton et al., 2019). Our findings suggest that kinase fusions resulting from intrachromosomal structural rearrangements on chromosome 7 may contribute to the increased number of melanocytic nevi observed in patients with non-supernumerary ring chromosome 7 syndrome.
Another observation that supports this hypothesis is that increased numbers of melanocytic nevi are not characteristic in the supernumerary form of the ring chromosome 7 syndrome; they are only observed in the non-supernumerary form (Velagaleti et al., 2002). Supernumerary ring chromosomes 7 contain comparatively little genetic material without the distal portion of the long arm on which BRAF and MET are located (Velagaleti et al., 2002).
BRAF fusions are associated with Spitz cytomorphology and are among the defining genetic alteration of Spitz nevus in the 2018 WHO classification of skin tumors (Elder et al., 2018). Amin et al. described that melanocytic tumors with BRAF fusions tend to show a sheet-like, sclerosing growth involving both the epidermis and dermis (Amin et al., 2017). Epidermal hyperplasia was frequently observed and cytomorphology was more likely to be epithelioid, with “severe nuclear atypia” (Amin et al., 2017). The nevi in our case demonstrated features of Spitz nevus, such as Kamino bodies, epidermal hyperplasia and epithelioid melanocytes with clefting around nests of melanocytes. There also was desmoplasia, which is seen in Spitz nevi with HRAS mutation but also may be common in Spitz tumors with BRAF fusion.
The histopathology of melanocytic nevi in ring chromosome 7 syndrome has not been extensively studied. We reviewed published cases of ring chromosome 7 syndrome (Table 1). Few case reports show figures of the pertinent histopathology. One interesting histopathologic description from the nevi of an 8-year old male (Wahlström et al., 1996) described “pigmented nevi with pronounced dysplasia”, in which the melanocytes were “heavily pigmented” and large cells were confluent as “syncytia-like giant cells”, possibly indicating a spitzoid or pigmented epithelioid melanocytoma-like morphology. There has been a report of a melanoma developing in a 17-year old girl with ring chromosome 7 syndrome and multiple congenital nevi (Vollenweider Roten, Delozier-Blanchet, et al., 1993). The melanoma was retroauricular and showed “pigmented epithelioid-type cells invading the epidermis and the reticular dermis”(Vollenweider Roten, Delozier-Blanchet, et al., 1993). Reviewing their provided figure, we do observe large cells with abundant cytoplasm, prominent nucleoli and large melanophages. This lesion could potentially fit within the spectrum of Spitz tumor. As for our patient, the absence of secondary genetic alterations associated with progression such as TERT promoter mutation and homozygous 9p21 (CKDN2A locus) deletions ruled out melanoma. We recommended clinical follow-up and/or re-biopsy of any recurrent lesions.
BRAF fusions are expressed under the promoter of the 5’ gene, TMEM106B and WDR91 in our case. It is conceivable that expression of one copy of these BRAF fusions does not lead to enough BRAF fusion protein to initiate tumor growth and that tumor formation was contingent on the secondary amplifications of the BRAF fusion genes. This would be akin to the situation in nevus spilus, which has a mutant HRAS in the background lentigo with gain of the mutant allele in the compound melanocytic proliferations within it (Sarin et al., 2013). The genomic instability of ring chromosomes could increase the likelihood of not only BRAF fusion but also subsequent amplification.
To conclude, the cutaneous phenotype of non-supernumerary ring chromosome 7 syndrome consists of capillary hemangiomas, café-au-lait spots and dark, pigmented nevi which may show large epithelioid cells on histomorphology. Herein, we molecularly characterize two desmoplastic Spitz nevi in a 22-year-old man with ring chromosome 7 syndrome. The presence of two distinct BRAF fusions occurring on the ring chromosome 7 suggest that nevi with BRAF fusion may be observed more frequently in patients afflicted with this genetic syndrome.
Supplementary Material
Significance.
The mechanism underpinning the increased number of melanocytic nevi observed in patients with non-supernumerary ring chromosome 7 syndrome is unknown. Our findings suggest that kinase fusions such as BRAF gene fusions resulting from intrachromosomal structural rearrangements on chromosome 7 due to the instability of the ring may be a major contributing factor. Nevi occurring in the ring chromosome 7 syndrome may best correspond to the clinical, pathological and genomic category of Spitz tumors.
Acknowledgements:
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number 1R35CA220481).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Afshar AR, Damato BE, Stewart JM, Zablotska LB, Roy R, Olshen AB, Joseph NM, & Bastian BC (2019). Next-Generation Sequencing of Uveal Melanoma for Detection of Genetic Alterations Predicting Metastasis. Translational Vision Science & Technology, 8(2). 10.1167/tvst.8.2.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin SM, Haugh AM, Lee CY, Zhang B, Bubley JA, Merkel EA, Verzì AE, & Gerami P (2017). A Comparison of Morphologic and Molecular Features of BRAF, ALK, and NTRK1 Fusion Spitzoid Neoplasms. The American Journal of Surgical Pathology, 41(4), 491. 10.1097/PAS.0000000000000761 [DOI] [PubMed] [Google Scholar]
- Botton T, Talevich E, Mishra VK, Zhang T, Shain AH, Berquet C, Gagnon A, Judson RL, Ballotti R, Ribas A, Herlyn M, Rocchi S, Brown KM, Hayward NK, Yeh I, & Bastian BC (2019). Genetic Heterogeneity of BRAF Fusion Kinases in Melanoma Affects Drug Responses. Cell Reports, 29(3), 573–588.e7. 10.1016/j.celrep.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton T, Yeh I, Nelson T, Vemula SS, Sparatta A, Garrido MC, Allegra M, Rocchi S, Bahadoran P, McCalmont TH, LeBoit PE, Burton EA, Bollag G, Ballotti R, & Bastian BC (2013). Recurrent BRAF kinase fusions in melanocytic tumors offer an opportunity for targeted therapy. Pigment Cell & Melanoma Research, 26(6), 845–851. 10.1111/pcmr.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DE, Massi D, Scolyer R, & Willemze R (2018). WHO Classification of Skin Tumours (4th Edition). IARC Press. [Google Scholar]
- Raghavan SS, Kapler ES, Dinges MM, Bastian BC, & Yeh I (n.d.). Eruptive Spitz nevus, a striking example of benign metastasis. Scientific Reports, in press. [DOI] [PMC free article] [PubMed]
- Ross JS, Wang K, Chmielecki J, Gay L, Johnson A, Chudnovsky J, Yelensky R, Lipson D, Ali SM, Elvin JA, Vergilio J-A, Roels S, Miller VA, Nakamura BN, Gray A, Wong MK, & Stephens PJ (2016). The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. International Journal of Cancer, 138(4), 881–890. 10.1002/ijc.29825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Labadía C, Cervantes-Barragán DE, Cruz-Alcívar R, Daber RD, Conlin LK, Leonard LD, Spinner NB, Durán-McKinster C, Dávila-Ortíz de Montellano DJ, Del Castillo-Ruiz V, & Pérez-Vera P (2014). Cytogenomic and phenotypic analysis in low-level monosomy 7 mosaicism with non-supernumerary ring chromosome 7. American Journal of Medical Genetics. Part A, 164A(7), 1765–1769. 10.1002/ajmg.a.36503 [DOI] [PubMed] [Google Scholar]
- Sarin KY, Sun BK, Bangs CD, Cherry A, Swetter SM, Kim J, & Khavari PA (2013). Activating HRAS Mutation in Agminated Spitz Nevi Arising in a Nevus Spilus. JAMA Dermatology, 149(9), 1077–1081. 10.1001/jamadermatol.2013.4745 [DOI] [PubMed] [Google Scholar]
- Tran NH, Wu X, & Frost JA (2005). B-Raf and Raf-1 Are Regulated by Distinct Autoregulatory Mechanisms. Journal of Biological Chemistry, 280(16), 16244–16253. 10.1074/jbc.M501185200 [DOI] [PubMed] [Google Scholar]
- Velagaleti GVN, Jalal SM, Kukolich MK, Lockhart LH, & Tonk VS (2002). De novo supernumerary ring chromosome 7: First report of a non-mosaic patient and review of the literature. Clinical Genetics, 61(3), 202–206. 10.1034/j.1399-0004.2002.610306.x [DOI] [PubMed] [Google Scholar]
- Vollenweider Roten S, Delozier-Blanchet CD, Masouyé I, & Saurat JH (1993). Melanoma associated with ring chromosome 7. Dermatology (Basel, Switzerland), 186(2), 138–143. 10.1159/000247325 [DOI] [PubMed] [Google Scholar]
- Vollenweider Roten S, Masouyé I, Delozier-Blanchet CD, & Saurat JH (1993). Cutaneous findings in ring chromosome 7 syndrome. Dermatology (Basel, Switzerland), 186(2), 84–87. 10.1159/000247313 [DOI] [PubMed] [Google Scholar]
- Wahlström J, Bjarnason R, Rosdahl I, & Albertsson-Wikland K (1996). Boy with a ring 7 chromosome: A case report with special reference to dermatological findings. Acta Paediatrica (Oslo, Norway: 1992), 85(10), 1256–1260. 10.1111/j.1651-2227.1996.tb18243.x [DOI] [PubMed] [Google Scholar]
- Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, Lipson D, Otto G, Brennan K, Murali R, Garrido M, Miller VA, Ross JS, Berger MF, Sparatta A, Palmedo G, Cerroni L, Busam KJ, Kutzner H, … Bastian BC (2014). Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nature Communications, 5, 3116. 10.1038/ncomms4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de la Fouchardiere A, Mully TW, North JP, Garrido MC, Gagnon A, Vemula SS, McCalmont TH, LeBoit PE, & Bastian BC (2015). Activating MET kinase rearrangements in melanoma and Spitz tumours. Nature Communications, 6, 7174. 10.1038/ncomms8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.