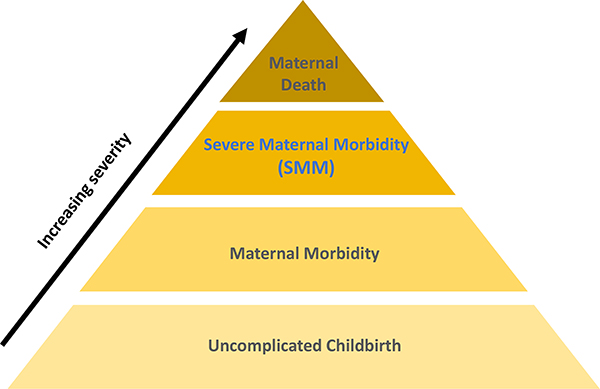
The United States ranks worse on maternal mortality than other high-resource countries (Tikkanen, Gunja, FitzGerald, & Zephyrin, 2020). It is one of few countries where maternal mortality has not improved in recent decades (GBD 2015 Maternal Mortality Collaborators, 2016). Racial/ethnic and geographic inequities in maternal mortality are substantial and persistent. Black and Native American individuals are particularly burdened, with at least two-to threefold higher mortality than the rest of the population (Admon et al., 2018). Severe maternal morbidity (SMM) is a sentinel or near-miss maternal health outcome proximate in severity to maternal mortality (Figure 1). SMM encompasses unexpected outcomes of labor and birth that put women most at risk of dying, such as eclampsia, hemorrhage, cardiovascular events, sepsis, and organ failure (American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, Kilpatrick, & Ecker, 2016). SMM is 50–100 times more common than maternal mortality, affecting 1–2% of people giving birth, and thus more feasible to study. Like maternal mortality, SMM has increased in recent decades (although the reasons for the increases are uncertain) (Leonard, Main, & Carmichael, 2019) and disproportionately affects women of color (Admon et al., 2018). A better understanding of how and why SMM occurs is key to improving maternal health and preventing maternal mortality. This includes recognizing both clinical and social drivers of maternal morbidity (e.g., racism), and their modifiability.
Figure 1.
Continuum of maternal morbidity, by severity. (Adapted from NYC Department of Health & Mental Hygiene. 2016. Severe Maternal Morbidity in NYC, 2008–12. New York, NY.)
The objective of this commentary is to call attention to challenges to identifying population-level strategies for preventing SMM and its inequities, and to propose solutions. We focus on challenges to conducting research related to SMM within the U.S. context, although the points raised have broader global applicability. The challenges discussed include 1) the conceptual frameworks used to understand SMM, 2) defining SMM, and 3) the availability of data to assess SMM. By addressing these issues, we aim to advance research and efforts to improve health across the life course for people who give birth.
Here, we use terms that are both gendered (e.g., maternal) and gender-neutral (e.g., individual) to be inclusive of the identities of all persons with capacity for pregnancy and birth, which span the gender spectrum (Moseson et al., 2020).
Conceptual and Theoretical Frameworks to Guide SMM Research
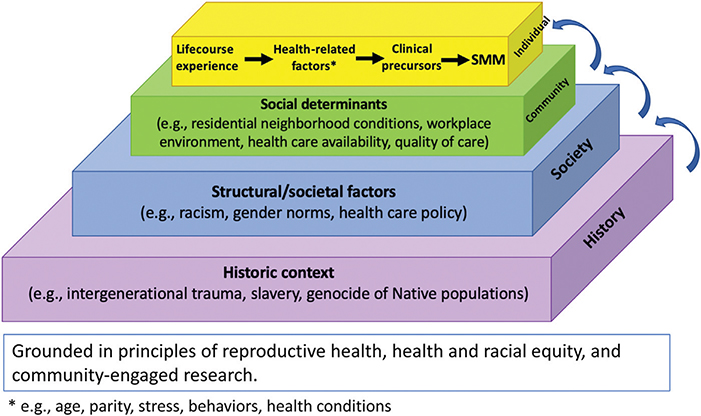
We conceptualize SMM and its inequities within a multidimensional causal chain of events framework that spans the “macrosocial” (i.e., structural and societal factors) to the “micro-clinical” (i.e., pathways from specific clinical precursors to specific SMM indicators) (Figure 2). Transformative, sustainable improvements require identifying effective interventions that span this entire continuum: preventing acute progression to life-threatening situations and interrupting higher-order social processes that threaten health. Transformative change will thus require centering on equity, which in turn requires acknowledging our historical legacy (Halfon et al., 2014; Kramer et al., 2019) rooted in the historical context of the enslavement of Black Americans, the genocide of Native and Indigenous Americans, and a system of structural racism (i.e., mutually reinforcing systems that foster racial discrimination and differential access to resources and opportunities) that has led to intergenerational trauma for minoritized groups in the United States (Bailey, Feldman, & Bassett, 2021).
Figure 2.
Conceptual framework for describing multilevel pathways to severe maternal morbidity (SMM) and its inequities.
Several conceptual and theoretical frameworks and approaches are particularly important to guiding research on SMM (Table 1). First, SMM research should be grounded within a broader context of reproductive health that acknowledges its multilevel, life course, and intergenerational nature (recommendation 1A). Reproductive health is influenced by experiences that span the entire life course and multiple generations, and that occur at multiple levels (e.g., individual, family, neighborhood, societal) (Halfon & Hochstein, 2002; Lu & Halfon, 2003; McLeroy, Bibeau, Steckler, & Glanz, 1988). For example, adverse childhood events, as well as intergenerational poverty, may affect adult reproductive outcomes (Mersky & Lee, 2019).
Table 1.
Recommendations for Improving Population-based Research on SMM
| 1. Conceptual and theoretical frameworks to guide SMM research |
| 1A. Reproductive health |
| SMM research should be grounded in the broader context of reproductive health (not just “pregnancy” health), acknowledging that reproductive health is |
| • Multilevel - Supported by the Social-Ecological Model, this framework recognizes the influence of multilevel domains of influence on reproductive health, including individual, neighborhood, health system, and societal factors (McLeroy et al., 1988). |
| • Life course health - Pregnancy outcomes like SMM are affected by life course experiences, and SMM may in turn affect subsequent life course health (Lu & Halfon, 2003). |
| • Intergenerational health - Historic context of one’s family and society affect the reproductive health of current and subsequent generations (e.g., intergenerational trauma, slavery, genocide of Native populations) (Halfon & Hochstein, 2002; McLeroy et al., 1988). |
| 1B. Health and racial equity |
| Given stark disparities by race and social disadvantage, SMM research should be centered on achieving equity, within a framework that is informed by multiple relevant movements and theories, including, for example, Critical Race Theory, EcoSocial Theory, Intersectionality, and Reproductive Justice (BlackWomen Scholars and the Research Working Group of the Black Mamas Matter Alliance, 2020; Roberts, 1998; Ford & Airhihenbuwa, 2010; Krieger, 2020; Ross, 2017) [see references for further explication]. |
| 1C. Community-engaged research |
| SMM research should be guided by principles of community-engaged research, which acknowledge that contributions from people with lived experience and highest burden, at every stage of the research process, are essential to its effectiveness (Ortiz et al., 2020; Wang et al., 2020b). |
| 2. Defining SMM |
| 2A. Timing of SMM |
| • SMM indicators should characterize severe complications that arise during pregnancy or postpartum, and not situations at risk of leading to severe complications. |
| 2C. Indicators |
| • Standardized approaches that link precursor clinical conditions with SMM events are needed to fully understand the causes of SMM, how best to approach it analytically, and how to prevent it. |
| 2C. Transparency |
| • Research should provide a clear definition of SMM, report all codes and criteria that are used, and use existing validated indices whenever possible. |
| • Coding experts should be included in the development and revision of SMM indices. |
| • If transfusion is included in the definition of SMM and volume of transfusion is not available, findings should be reported with and without including transfusion as an indicator. |
| • Research should state the timing of SMM events that are included, provide justification, and discuss potential concomitant limitations. |
| 2D. Continuum of care |
| • When possible, research should include SMM events that arise during pregnancy or childbirth, through at least 42 days postpartum, and the approach should be clearly described. |
| • Further research is needed that compares SMM that emerges during the prenatal, peripartum, and postpartum periods. |
| 3. Data improvements |
| • Improve the availability of data resources that |
| - Allow rigorous characterization of SMM |
| - Include critical individual-level sociodemographic variables (e.g., maternal race-ethnicity, age, parity, socioeconomic status) |
| and |
| - Enable characterization of social and structural determinants (see Figure 2 for examples). |
| • Some suggested strategies are as follows: |
| - Develop state-based datasets that link vital records with maternal and infant hospital discharge records, including indicators of where people live so that social and structural determinants can be studied. |
| - Improve the quality of maternal health information recorded in vital records. Using current versions of birth and fetal death certificates to study SMM is not recommended. |
| - Improve the quality of social determinant information in hospital discharge records. |
| - Fund the exploration of birthing people’s and communities’ perspectives and priorities regarding SMM. |
Abbreviation: SMM, severe maternal morbidity.
Second, research should be centered on principles of health and racial equity, which can be informed by several movements and theories (recommendation 1B). Research should be grounded in reproductive justice and an explicit acknowledgment that health-related inequities arise from social forces rather than innate biologic differences. Reproductive justice is a framework created by Black women that emphasizes the human right to maintain personal bodily autonomy, to have or not have children, and to parent one’s children in safe, supportive communities (Black Women Scholars and the Research Working Group of the Black Mamas Matter Alliance, 2020; Ross, 2017). As articulated by Critical Race Theory and Krieger’s EcoSocial model, disparities emanate from a historical context of bias and racism, rooted in societal power structures, that is manifested over time and across generations in multiple aspects of one’s social context and living environment (e.g., social policy, health care quality, and safety) (Krieger, 2020). The resulting exposures are embodied over the life course and ultimately lead to greater biologic vulnerability and adverse health outcomes (Roberts, 1998; Krieger, 2020). Furthermore, health equity must be acknowledged, understood, and improved along multiple intersectional dimensions, including sexual and gender identification, disability, socioeconomic status, and migrant and/or documentation status. Frameworks to guide the incorporation of these tenets into research exist and should be used (e.g., Public Health Critical Race praxis, intersectionality) (Ford & Airhihenbuwa, 2010).
Third, principles of community-engaged research, which acknowledge that contributions from people who represent and directly advocate for those who have experienced SMM, and especially from groups bearing the highest burden of SMM, are essential to effective, impactful research and intervention (Wang, Glazer, Sofaer, Balbierz, & Howell, 2020b) and are needed across all stages of the research process, from inception to dissemination (Ortiz et al., 2020) (recommendation 1C). Declaration and integration of these principles into study designs and dissemination, by inclusive research teams that center the voices and experiences of socially marginalized investigators and communities, will help ensure that we ask meaningful questions that yield meaningful answers and do not perpetuate racism and other forms of oppression (Boyd, Lindo, Weeks, & McLemore, 2020; Hardeman, Karbeah, & Kozhimannil, 2020; Julian et al., 2020; Vyas, Einstein, & Jones, 2020). Future research should center the perspectives of those affected by SMM and implement their priorities for research and intervention (Eniola, Nack, Niles, Morton, & Searing, 2020; Wang et al., 2020b).
In summary, SMM and its inequities should be conceptualized within a multidimensional causal chain of events framework that intentionally incorporates multiple relevant theoretical frameworks (see Table 1.) Although not every study or prevention strategy will address all of the complexities we have described, grounding in their essence will produce better research that is more likely to lead to sustainable, equitable improvement of maternal health.
Defining SMM
Another important obstacle to understanding SMM—overall and with respect to equity—is variability in how SMM is conceptually defined and operationalized or identified from data (England et al., 2020; Knight & Joseph, 2020). The American College of Obstetricians and Gynecologists defines SMM as unintended outcomes of the process of labor and delivery that have significant short-term or long-term consequences for maternal health and can be considered a near miss for maternal mortality (American College of Obstetricians and Gynecologists et al., 2016). SMM cases are typically identified from a composite of SMM indicators (i.e., complications or events that qualify as SMM). For example, the Centers for Disease Control and Prevention (CDC) SMM index includes a broad array of indicators that can be obtained from International Classification of Disease (ICD) codes in hospital discharge data (CDC, 2017). In contrast, the World Health Organization definition focuses on organ dysfunction criteria, which tend to require laboratory results (Say, Souza, & Pattinson, 2009). Greater consensus is needed regarding processes to ascertain cases (be it from case review or administrative data) and what conditions to include, as discussed elsewhere (Knight, 2020; Knight & Joseph, 2020). Here we point out some more general conceptual points that we believe are important to improving consistency and clarity in how SMM is defined.
First, we recommend that SMM indicators should characterize severe complications that arise during or following pregnancy, and not conditions at risk of severe complications (recommendation 2A). In this case, preexisting conditions such as sickle-cell anemia, HIV disease, or severe obesity would not be considered SMM, but sickle-cell crisis, HIV-related complications, or acute myocardial infarction might be. This view guided the development of an SMM index by the CDC, which is used commonly in U.S. studies of SMM but has not been applied in all studies of SMM (CDC, 2017; Chantry et al., 2020; Dzakpasu et al., 2020).
Second, we recommend development of standardized approaches that link precursor clinical conditions with SMM indicators (recommendation 2B). SMM-defining events may or may not directly indicate the immediate underlying clinical condition that preceded SMM. For example, major respiratory events typically identified as SMM, such as pulmonary edema or acute respiratory distress syndrome, can be caused by varied precursor clinical conditions, such as preeclampsia, infection, cardiac disease, or hemorrhage. In some cases, the connection between SMM and its proximal clinical precursor is obvious (e.g., eclampsia follows preeclampsia). Either way, determining the clinical precursor to SMM is a separate step from identifying the SMM event itself. This step requires further attention, as it will help us understand pathways leading to SMM and opportunities for prevention.
Third, we recommend transparency in how SMM is defined and reported (recommendation 2C). SMM measures vary in the indicators they include, which can lead to considerable variability in prevalence estimates (England et al., 2020; Snowden et al., 2021). The use of auxiliary factors to refine case identification, such as length of hospital stay or intensive care unit admission, also varies across studies (Snowden et al., 2021). One example is blood transfusion. Transfusion is included in the CDC index, but ICD codes do not indicate transfusion volume. This lack of specificity can result in misclassification when transfusion, which may or may not qualify as “severe,” is the only SMM indicator present (Main et al., 2016); indeed, approximately half of cases have transfusion as their only indicator in studies using the CDC index. CDC thus currently reports SMM with and without including transfusion as an indicator. Inconsistency in the actual codes selected to identify indicators within similar coding systems is another problem. For example, the CDC index and the Bateman index use some different ICD codes for the same indicators (Snowden et al., 2021). Even when using the same indices, variability in coding systems and their application may affect cross-study or cross-population comparison (Chantry et al., 2020). To facilitate comparisons across studies, we recommend the following: detailed description of how SMM is defined; inclusion of medical coding experts in the development of SMM indices; use of existing, validated indices; and reporting of findings with and without including transfusion as an indicator.
Fourth, we recommend SMM research and intervention address the continuum of care from the prenatal through postpartum periods (recommendation 2D). Clarity about SMM timing is important. SMM may emerge during pregnancy, at the time of childbirth, or postpartum. A recent review of SMM definitions reported that only about half of prior studies of SMM actually stated the range of timing of SMM-defining events (England et al., 2020). Most studies are limited to data from childbirth hospitalizations, which capture most but not all cases (Girsen et al., 2020). Further complicating this matter is inconsistency in defining the length of the postpartum period, typically varying from 42 days to 1 year (National Academies of Sciences, Engineering, and Medicine, 2020). The extent of underascertainment of SMM that occurs postpartum, such as what types of SMM events are most likely to be missed, and whether cases are more likely to be missed among certain subgroups, is unknown. We recommend that studies clarify timing for ascertaining SMM and include cases that emerge during childbirth, and afterward whenever possible; that differences in these two sets of cases should be considered when possible; that studies clearly state their data sources and timing for ascertaining SMM; and that researchers conduct additional studies of SMM that occurs after birth hospitalizations.
In sum, greater consensus and consistency in how SMM is defined is needed, both within the United States and beyond, in order for the field to truly move forward (Knight, 2020; Knight & Joseph, 2020). A recent study described a process to develop consensus-based criteria for SMM using hospital discharge data among several European countries, albeit focused on a few select indicators (Chantry et al., 2020). The study serves as an excellent learning template for others and reinforces how essential collaboration is to achieving progress.
Data Improvements
Another roadblock to progress in understanding SMM is data availability. In the United States, hospital discharge or claims data typically contain sufficient coding of procedures and conditions to identify SMM as it is currently defined. However, this type of dataset often lacks information on patient experience and important nonclinical factors that are likely part of the pathways leading to SMM, such as social-structural, sociodemographic, environmental, and behavioral factors. For example, the U.S. National Inpatient Sample (NIS) is a publicly available data source from a representative sample of U.S. hospitalizations that can provide nationally representative SMM rates. However, the NIS (and most hospital discharge data) does not include factors such as parity, education, geography, or gestational age at birth (all of which are available from U.S. vital records). Before 2012, the NIS had substantial missing data on race/ethnicity (>20%), but this limitation has been rectified. Of note, some definitions of SMM rely on data that are not available in discharge records, such as vital sign measurements and laboratory values (American College of Obstetricians, Gynecologists et al., 2016; Say et al., 2009; World Health Organization, 2011); however, the degree to which such additional information would increase validity of existing SMM indices like the CDC index is unclear, and the feasibility of its inclusion is also uncertain. Many large-scale datasets (including NIS) are also lacking longitudinally linked data, for example, to reflect postpartum health care encounters or to link multiple births over time to the same woman.
To understand and eliminate inequities, we need to be able to study social and structural determinants of health, many of which include features of where people live (Kramer et al., 2019). These features include aspects of the health care system (e.g., health care quality and availability), characteristics of the physical and built environment (e.g., socioeconomic resources, crime, green space, food availability, pollution), and policies (e.g., laws regarding access to reproductive health care). Hospital discharge data do not typically indicate where a patient lived (before, during, or after pregnancy). Since 2012, even state of residence is not available from the NIS. Thus, we currently cannot readily compare the prevalence of SMM across states, and our understanding of the contribution of social determinants to SMM is limited (Wang, Glazer, Howell, & Janevic, 2020a). County of birth can be obtained from U.S. vital records, and in some states, more refined indicators of where a birthing parent lives (e.g., ZIP code) may be available with permission, because the maternal address at the time of birth is part of the vital record. However, in the United States, vital records (birth certificates and fetal death certificates) alone do not provide sufficient information to understand SMM (Luke, Brown, Liu, Diop, & Stern, 2018; Snowden et al., 2021). They currently include checkboxes for a few maternal conditions that occur during the childbirth hospitalization and are indicative of SMM (e.g., transfusion). The sensitivity is unacceptably low; in a study of California births, the sensitivity was 0.08 for transfusion and 0.07 for sepsis, when comparing birth certificates with hospital discharge data (Snowden et al., 2021). Further, the availability of data that address patient experience of SMM, via quantitative or qualitative data, is limited; producing high-quality data regarding patient experiences and priorities requires community involvement and greater attention from funders. Thus, a major barrier to improving SMM research is the absence of the types of data needed to study some of its most important determinants.
Linkage of hospital discharge data (which enable identification of SMM cases) and vital records (which can provide data on sociodemographic variables and where a birthing parent lived) is one mechanism to improve data availability and quality needed to study, understand, and address SMM and its inequities. Such linkages are technically straightforward and highly successful via probabilistic linkage of variables, such as date, time, and hospital of birth (>98% success in California) (Herrchen, Gould, & Nesbitt, 1997). Other linkages would also be useful but often less feasible, such as with prenatal or postpartum outpatient claims records. The onus of responsibility to make such data available falls largely on government-funded public health agencies at state and national levels. Resources are needed to make such data available, both to create the datasets and to manage data access and security. These hurdles are difficult to overcome, in light of competing priorities and tight budgets. Another mechanism is that hospitals could develop systems to download electronic health record data straight into the vital record, to improve its reporting of maternal conditions that are currently included. This approach could be extended statewide. A third approach is to better incorporate nonclinical information (e.g., sociodemographics, community resources) into hospital discharge and electronic health records, following existing recommendations (Institute of Medicine, 2014).
In sum, some useful data do exist, and the technical challenges to creating accessible data sources for studying SMM are surmountable. We recommend that mechanisms for creating more comprehensive data resources to study SMM and social determinants be developed, and that such development be a national priority to improve maternal and child health (Table 1).
Conclusions
Reducing SMM is critical to improving maternal health. SMM is now a national outcome measure for Title V, it is part of new Healthy People 2030 Goals, and it is one of the few quality indicators that focuses on maternal health (versus neonatal, perinatal, or obstetric care)—all of which attest to the importance of understanding its causes. To make progress on understanding SMM, we need a solid conceptual orientation that spans the continuum of broad structural to specific clinical factors and centers equity; better consensus on its definition and measurement; a confluence of population-level data on SMM, sociodemographic variables, and place; and better understanding of patient and community experience of SMM. The time is right for progress, now that the urgency of the U.S. maternal health crisis is broadly understood. Media attention and public demand to improve maternal health and achieve racial equity is growing. Congress has proposed multiple pieces of legislation to improve maternal health, including omnibus bills addressing improved care for Black women, supported by the Black Maternal Health Caucus. The National Institutes of Health launched its IMPROVE initiative in 2020, the Patient-Centered Outcomes Research Institute’s reauthorization includes a focus on maternal health, and the CDC launched its “Hear Her” campaign. The Health Resources and Services Administration–supported Alliance for Innovation on Maternal Health initiative and the CDC National Network of Perinatal Quality Collaboratives support the development and implementation of toolkits to improve the quality of maternal care. Heightened intolerance of racism and concerns about the impact of the COVID-19 pandemic on maternal health and health equity will likely further galvanize action to improve maternal health. Documenting and calling attention to the U.S. maternal health crisis are critical steps on the path to improving maternal health and mitigating inequities, but they are not sufficient on their own. Achieving progress toward addressing this largely preventable crisis requires coordinated, multifaceted action. It is essential that we act using a multilevel framework informed by evidence, centered on equity and the voices of the people who are most affected, to ensure health across the life course for every person giving birth in the United States.
Acknowledgments
This work was supported by National Institutes of Health grant NR017020, funded by the National Institute for Nursing Research and the Office of Research on Women’s Health.
Biographies
Author Descriptions
Suzan Carmichael, PhD, is a perinatal epidemiologist and Professor in the Departments of Pediatrics and Obstetrics and Gynecology at the Stanford University School of Medicine.
Barbara Abrams, DrPH, RD, is a nutritional and perinatal epidemiologist and Professor in the Division of Epidemiology, School of Public Health, University of California, Berkeley.
Alison El Ayadi, ScD, MPH, is an epidemiologist and Professor in the Department of Obstetrics, Gynecology, and Reproductive Sciences and Department of Epidemiology and Biostatistics, University of California, San Francisco.
Henry Lee, MD, MS, is a neonatologist and Associate Professor in the Department of Pediatrics, Stanford University School of Medicine.
Can Liu, PhD, is an epidemiologist and researcher in the Department of Public Health Sciences at Stockholm University, Sweden.
Deirdre Lyell, MD, is a maternal-fetal medicine specialist and Professor in the Department of Obstetrics and Gynecology, Stanford University School of Medicine.
Audrey Lyndon, RN, PhD, FAAN, is Professor of Nursing and Medicine and Assistant Dean for Clinical Research in the Rory Meyers College of Nursing, New York University, New York, NY.
Elliott Main, MD, is a maternal-fetal medicine specialist and Professor in the Department of Obstetrics and Gynecology, Stanford University School of Medicine, and Director of the California Maternal Quality Care Collaborative.
Mahasin Mujahid, PhD, MS, FAHA, is a social epidemiologist and Associate Professor in the Division of Epidemiology, School of Public Health, University of California, Berkeley.
Lu Tian, PhD, is a biostatistician and Professor in the Department of Biomedical Data Science, Stanford University School of Medicine.
Jonathan Snowden, PhD, is a perinatal epidemiologist and Associate Professor in the School of Public Health, Oregon Health & Science University-Portland State University, Portland, Oregon; and Department of Obstetrics & Gynecology, Oregon Health & Science University.
References
- Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, & Dalton VK (2018). Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012–2015. Obstetrics & Gynecology, 132(5), 1158–1166. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, Kilpatrick SK, & Ecker JL (2016). Severe maternal morbidity: Screening and review. American Journal of Obstetrics and Gynecology, 215(3), B17–B22. [DOI] [PubMed] [Google Scholar]
- Bailey ZD, Feldman JM, & Bassett MT (2021). How structural racism works - Racist policies as a root cause of U.S. racial health inequities. New England Journal of Medicine, 384, 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black Women Scholars and the Research Working Group of the Black Mamas Matter Alliance. (2020). Black maternal health research re-envisioned: Best practives for the conduct of research with, for, and by Black mamas. Harvard Law & Policy Review, 14(2), 393–415. [Google Scholar]
- Boyd RW, Lindo EG, Weeks LD, & McLemore MR (2020). On racism: A new standard for publishing on racial health inequities. Health Affairs Blog. Available: https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/. Accessed: August 31, 2020. [Google Scholar]
- CDC. (2017). Severe maternal morbidity in the United States. Available: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html#anchor_SMM. Accessed: May 30, 2018.
- Chantry AA, Berrut S, Donati S, Gissler M, Goldacre R, Knight M,. Deneux-Tharaux C (2020). Monitoring severe acute maternal morbidity across Europe: A feasibility study. Paediatric and Perinatal Epidemiology, 34(4), 416–426. [DOI] [PubMed] [Google Scholar]
- Dzakpasu S, Deb-Rinker P, Arbour L, Darling EK, Kramer MS, Liu S,. Joseph KS (2020). Severe maternal morbidity surveillance: Monitoring pregnant women at high risk for prolonged hospitalisation and death. Paediatric and Perinatal Epidemiology, 34(4), 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England N, Madill J, Metcalfe A, Magee L, Cooper S, Salmon C, & Adhikari K (2020). Monitoring maternal near miss/severe maternal morbidity: A systematic review of global practices. PLoS One, 15(5), e0233697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eniola F, Nack A, Niles P, Morton CH, & Searing H (2020). Women’s experiences with severe maternal morbidity in New York City: A qualitative report. Available: https://www1.nyc.gov/assets/doh/downloads/pdf/csi/womens-experience-with-severe-maternal-morbidity-nyc-qualitative-report.pdf. Accessed: December 14, 2021.
- Ford CL, & Airhihenbuwa CO (2010). The public health critical race methodology: Praxis for antiracism research. Social Science Medicine, 71(8), 1390–1398. [DOI] [PubMed] [Google Scholar]
- Girsen AI, Sie L, Carmichael SL, Lee HC, Foeller ME, Druzin ML, & Gibbs RS (2020). Rate and causes of severe maternal morbidity at readmission: California births in 2008–2012. Journal of Perinatolofy, 40(1), 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Maternal Mortality Collaborators. (2016). Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N, & Hochstein M (2002). Life course health development: An integrated framework for developing health, policy, and research. Milbank Quarterly, 80(3), 433–479, iii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N, Long P, Chang DI, Hester J, Inkelas M, & Rodgers A (2014). Applying a 3.0 transformation framework to guide large-scale health system reform. Health Affairs (Millwood), 33(11), 2003–2011. [DOI] [PubMed] [Google Scholar]
- Hardeman RR, Karbeah J, & Kozhimannil KB (2020). Applying a critical race lens to relationship-centered care in pregnancy and childbirth: An antidote to structural racism. Birth, 47(1), 3–7. [DOI] [PubMed] [Google Scholar]
- Herrchen B, Gould JB, & Nesbitt TS (1997). Vital statistics linked birth/infant death and hospital discharge record linkage for epidemiological studies. Computers and Biomedical Research, 30(4), 290–305. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2014). Capturing social and behavioral domains in electronic health records, Phase 1. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Julian Z, Robles D, Whetstone S, Perritt JB, Jackson AV, Hardeman RR, & Scott KA (2020). Community-informed models of perinatal and reproductive health services provision: A justice-centered paradigm toward equity among Black birthing communities. Semin Perinatol, 44(5), 151267. [DOI] [PubMed] [Google Scholar]
- Knight M (2020). Defining severe maternal morbidity-When is it time to stop? Paediatric and Perinatal Epidemiology, 34(4), 384–385. [DOI] [PubMed] [Google Scholar]
- Knight M, & Joseph KS (2020). Severe maternal morbidity and maternal mortality: A need for consensus on concepts and prevention efforts. Paediatric Perinatal Epidemiology, 34(4), 377–378. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Strahan AE, Preslar J, Zaharatos J, St Pierre A, Grant JE, … Callaghan WM (2019). Changing the conversation: applying a health equity framework to maternal mortality reviews. American Journal of Obstetrics and Gynecology, 221(6). 609.e601–609.e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (2020). Measures of racism, sexism, heterosexism, and gender binarism for health equity research: from structural injustice to embodied harm-an ecosocial analysis. Annual Review of Public Health, 41, 37–62. [DOI] [PubMed] [Google Scholar]
- Leonard SA, Main EK, & Carmichael SL (2019). The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy and Childbirth, 19(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, & Halfon N (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal, 7(1), 13–30. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Liu CL, Diop H, & Stern JE (2018). Validation of severe maternal morbidity on the US certificate of live birth. Epidemiology, 29(4), e31–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, … Kilpatrick S (2016). Measuring severe maternal morbidity: Validation of potential measures. American Journal of Obstetrics and Gynecology, 214(5). 643 e641–643.e610. [DOI] [PubMed] [Google Scholar]
- McLeroy KR, Bibeau D, Steckler A, & Glanz K(1988). Anecological perspective on health promotion programs. Health and Education Quarterly, 15(4), 351–377. [DOI] [PubMed] [Google Scholar]
- Mersky JP, & Lee CP (2019). Adverse childhood experiences and poor birth outcomes in a diverse, low-income sample. BMC Pregnancy and Childbirth,19(1), 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseson H, Zazanis N, Goldberg E, Fix L, Durden M, Stoeffler A, … Obedin-Maliver J (2020). The imperative for transgender and gender nonbinary inclusion: Beyond women’s health. Obstetrics & Gynecology, 135(5), 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2020). Birth settings in America: Outcomes, quality, access, and choice. Washington, D.C.: The National Academies Press. [PubMed] [Google Scholar]
- Ortiz K, Nash J, Shea L, Oetzel J, Garoutte J, Sanchez-Youngman S, & Wallerstein N (2020). Partnerships, processes, and outcomes: A health equity-focused scoping meta-review of community-engaged scholarship. Annual Review of Public Health, 41, 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D (1998). Killing the Black body: Race, reproduction, and the meaning of liberty. New York, NY: Pantheon. [Google Scholar]
- Ross LJ (2017). Reproductive justice as intersectional feminist activism. Souls, 19(3), 286–314. [Google Scholar]
- Say L, Souza JP, & Pattinson RC (2009). Maternal near miss—towards a standard tool for monitoring quality of maternal health care. Best Practice & Research: Clinical Obstetrics & Gynaecology, 23(3), 287–296. [DOI] [PubMed] [Google Scholar]
- Snowden JM, Lyndon A, Kan P, El Ayadi A, Main E, & Carmichael SL (2021). Severe maternal morbidity: A comparison of definitions and data sources. American Journal of Epidemiology, 190(9), 1890–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen R, Gunja MZ, FitzGerald M, & Zephyrin L (2020). Maternal mortality and maternity care in the United States compared to 10 other developed countries. Available: https://www.commonwealthfund.org/publications/issue-briefs/2020/nov/maternal-mortality-maternity-care-us-compared-10-countries. Accessed: December 14, 2021.
- Vyas DA, Eisenstein LG, & Jones DS (2020). Hidden in plain sight – Reconsidering the use of race correction in clinical algorithms. New England Journal of Medicine, 383(9), 874–882. [DOI] [PubMed] [Google Scholar]
- Wang E, Glazer KB, Howell EA, & Janevic TM (2020a). Social determinants of pregnancy-related mortality and morbidity in the United States: A systematic review. Obstetrics & Gynecology, 135(4), 896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Glazer KB, Sofaer S, Balbierz A, & Howell EA (2020b). Racial and ethnic disparities in severe maternal morbidity: A qualitative study of women’s experiences of peripartum care. Womens Health Issues, 31(1), 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Department of Reproductive Health and Research. (2011). Evaluating the quality of care for severe pregnancy complications. The WHO near-miss approach for maternal health. Available: http://whqlibdoc.who.int/publications/2011/9789241502221_eng.pdf?ua=1. Accessed: December 14, 2021. [Google Scholar]