Abstract
Interest in Obsessive-Compulsive Disorder’s pathology has focused on brain network profiles of the dorsal Anterior Cingulate Cortex (dACC), given its role as a principal control region. Both motor control and working memory tasks induce dysfunctional dACC profiles in OCD, and here, we contrasted dACC network profiles in OCD and age-comparable controls during both tasks (from data collected in the same participants). The motor task required participants to tap their right forefinger in response to a flashing white probe; the memory task was a standard n-back (2-Back) that required participants to identify if a current stimulus was identical to the one presented two items before it in the sequence. Network interactions were modeled using Psychophysiological Interactions (PPI), a model of directional functional connectivity. Our inter-group analyses indicated a) that the motor control task evoked greater dACC modulation than the working memory task, and b) that the modulatory effect was significantly greater in the OCD group. We also investigated the relationship between OCD symptom dimensions (lifetime obsession and lifetime compulsion measured using the CY-BOCS) and dACC network profiles in OCD. This regression analysis revealed a dichotomy between Obsessive-Compulsive symptom dimensions and the degree of dACC modulation: it was primarily increased obsessions that predicted increased modulation during the motor control task, but primarily increased compulsions that predicted increased modulation during the working memory task. These results re-emphasize the salience of the dACC in OCD, and the primacy of tasks of motor control in evoking dACC pathology in the disorder.
Introduction:
Obsessive-Compulsive Disorder (OCD) is a neuropsychiatric disorder characterized by intrusive, anxiety provoking thoughts and urges (obsessions), and repetitive behaviors performed to reduce anxiety (compulsions) (Abramowitz et al., 2009). OCD is prevalent in youth, with one-year incidence rates in sub-clinical and clinical adolescent populations estimated at 8.4% and 0.7% respectively (Valleni-Basile et al., 1996). Approximately 45% of adolescents report OCD symptoms (Apter et al., 1996; Berg et al., 1988). Despite relatively high frequencies of occurrence, understanding patterns of task-induced network dysfunction in the brain’s pathways has proven challenging. OCD’s complex symptoms have been linked to an inability to disengage intrusive thoughts from eliciting behavior, implying a pathology that increases inhibitory control (Bari and Robbins, 2013; Chamberlain et al., 2005; Fineberg et al., 2014; Lipszyc and Schachar, 2010; van Velzen et al., 2014), a pattern highly suggestive of aberrant functioning of brain networks that sub serve control processing (Piras et al., 2013).
Salient aspects of OCD pathology suggest that the dorsal Anterior Cingulate Cortex (dACC) is of particular structural and functional interest (Diwadkar et al., 2015). This region plays a prominent role in cognition, attention, and motivation (Bush et al., 2002; To et al., 2017; van Veen and Carter, 2002). Neuroimaging studies indicate that it is typically engaged during response conflict (occurring between incompatible streams of information processing) (Botvinick et al., 2001) and performance monitoring (Gilbert et al., 2018; Shenhav et al., 2013; van Veen and Carter, 2002). The literature on the role of the dACC in conflict processing is substantial (Nakamura et al., 2005), but through its mediation of multiple brain networks, the dACC has also been implicated as a principal control region associated particularly with motor and memory control (Paus, 2001). The anatomy of the dACC interjects heavily with networks for motor control (Stevens et al., 2011), providing a) a structural bases for the direct modulation of motor networks (Asemi et al., 2015) and b) a link between sensorimotor and cognitive processing (Friedman et al., 2017). In addition, through its connections to the prefrontal cortex (Stevens et al., 2011), the dACC functions as a regulator of executive processes including decision making and working memory (Lenartowicz and McIntosh, 2005). This interface with regions implicated in the maintenance and manipulation of mnemonic representations suggests that the dACC is part of a general control system that coordinates processes during working memory (Chafee and Goldman-Rakic, 2000; Chein and Schneider, 2005; Koenigs et al., 2009; Mars and Grol, 2007; McCarthy et al., 1997). Indeed, executive control is central to performance during tasks such as working memory, as such tasks induce varying demands, require constant encoding and updating of stimuli, resulting in a substantial propensity for errors (Bakshi et al., 2011; Diwadkar et al., 2015; Diwadkar et al., 2011). Here, the established role of the dACC in error monitoring during conflict assumes significance (Bakshi et al., 2011; MacDonald et al., 2000; Schulz et al., 2011), as demanding task characteristics elevate the role of regions such as the dACC during tasks that elicit a high degree of control.
Previous studies in OCD have demonstrated that dACC network profiles significantly differ from those of healthy controls during motor (Friedman et al., 2017) and mnemonic tasks (Diwadkar et al., 2015), where in both task domains, OCD were characterized by exaggerated modulation by the dACC. This exaggerated modulation suggests that the obsessive dimension of the illness may alter dACC function so as to induce exaggerated control of motor and memory networks (Diwadkar et al., 2015; Li and Mody, 2016). Task complexity in and of itself does not explain these effects as employed motor paradigms are generally more tractably performed than working memory tasks (Diwadkar et al., 2017; Diwadkar et al., 2018).
Clearly both working memory and motor function can evoke dACC-related network pathology in OCD, though in principle, the dACC is purported to play slightly different roles in each domain. Despite this general understanding, the relative degree of dACC dysfunction in OCD evoked by tasks of motor control versus tasks of working memory remains unclear. Understanding whether one or the other task domain is more evocative of network dysfunction will be informative about the interjection between task demands and dACC dysfunction, something that in turn will be an effective means for discovering network pathology in OCD. Moreover, exploratory analyses of the relationship between OCD symptom dimensions and the degree of (any) dACC dysfunction will provide a measure of support for how aspects of the illness are linked with evoked dACC function in each domain.
Thus, our primary aim was to discover relative dysfunction associated with dACC network profiles across these two task active states. To achieve this, we modeled and analyzed dACC network profiles under two specific experimental paradigms acquired in the same participants and the same imaging session; motor control, induced using an established visuo-motor integration paradigm (Asemi et al., 2015; Diwadkar et al., 2015; Friedman et al., 2017; Morris et al., 2018) and working memory, induced using a standard verbal n-back task (Casey et al., 1995; Diwadkar et al., 2017; Diwadkar et al., 2015; Diwadkar et al., 2011).
Methods:
Subjects
Twenty-eight participants with a diagnosis of OCD and twenty-seven healthy controls (HC) participated in the fMRI study (Refer to Table 1 for demographic data). Participants and parents were interviewed with the Schedule for Obsessive-Compulsive and Other Behavioral Syndromes and Schedule for Schizophrenia and Affective Disorders for School-Aged Children-Present and Lifetime Version (Kaufman et al., 1997; Wolff and Wolff, 1991). The lifetime (maximum) and current severity of OCD were assessed with a modified version of the Children's Yale-Brown Obsessive-Compulsive Disorder Scale (CY-BOCS) (Goodman et al., 1989; Scahill et al., 1997). Lifetime and current Axis I diagnosis were independently confirmed by clinicians (DRR, GLH), using DSM-5 criteria. Exclusion criteria included lifetime history of schizophrenia, other psychotic disorder, bipolar disorder, substance abuse or dependence, anorexia nervosa, bulimia nervosa, epilepsy, head injury with sustained loss of consciousness, Huntington's disease, dyskinesia, autism, IQ ≤ 80, or ≥ 15 on the lifetime version of the Social Communication Questionnaire (Mulligan et al., 2009; Rutter et al., 2003). The study was approved by the Wayne State University School of Medicine and the UM Human Investigation Committee. Legal guardians and participants provided written informed consent prior to participating in the study.
Table 1.
depicts the demographic data of our OCD and HC participants.
| Group (n) | Sex (M/F) | Age (SD) | Age Range | CY-BOCS Lifetime Obsessions (SD) | CY-BOCS Lifetime Compulsions (SD) | Comorbidities | Handedness |
|---|---|---|---|---|---|---|---|
| Healthy Controls (27) | 10/17 | 16.32 (2.70) | 12.05–22.94 | - | - | - | R |
| OCD (28) | 10/18 | 16.53 (3.07) | 12.36–21.88 | 13.28 (4.40) | 13.25 (3.76) | None | R |
fMRI
Gradient echo EPI fMRI data acquisition was conducted at the Vaitkevicius Magnetic Resonance Center (WSU) on a 3T Siemens Verio system using a 12-channel volume head coil (TR: 2.6s, TE: 29ms, FOV: 256×256mm2, acquisition matrix: 128×128, 36 axial slices, voxel dimensions: 2 × 2 × 3mm). In addition, a 3D T1-weighted anatomical MRI image was acquired (TR: 2200ms, TI: 778ms, TE: 3ms, flip-angle=13°, FOV: 256×256mm2, 256 axial slices of thickness = 1.0mm, matrix=256×256). A neuroradiologist reviewed all scans to rule out clinically significant abnormalities.
Uni-Manual Motor Paradigm
The established uni-manual motor task (Asemi et al., 2015; Diwadkar et al., 2017; Friedman et al., 2017; Morris et al., 2018) required participants to tap their right forefinger to a flashing white probe presented at different frequencies (.5 Hz or 1 Hz) and with different stimulus onset asynchronies (SOA). Periodic epochs (fixed SOA) allowed participants to establish and maintain a motor set. The SOAs for Random epochs were generated by pseudo-randomly sampling values from Gaussian distributions (μ = 1.0/2.0 s; σ = 0.5/1.0 s). The SOA lower bound was set at 300 ms (to exceed the lower limit of response latency). This condition preempted formation of a motor set, eliciting reactive responding. The number of elicited responses was held constant across all Periodic and Random epochs. Participants alternated between tapping and rest epochs each lasting 30 s (four epochs for each tapping condition and two rest epochs). Epochs were interspersed with short (10 s) rest intervals. Tapping responses were collected from the surface of a response touchpad (Current Design Systems, Inc.).
Working Memory Task Paradigm
During an established verbal working memory paradigm (n-back task) (Casey et al., 1995; Diwadkar et al., 2017; Diwadkar et al., 2015; Diwadkar et al., 2011), participants were presented with a sequence of letters indicating if the current letter was identical to the one present “n” stimuli previously in the sequence. Subjects responded via a two-choice button unit. Working memory load was varied (n=0 or n=2). Each condition lasted 30 s (presentation time: 500 ms; SOA: 2500 ms; 10 letters per condition) interspersed with rest epochs (20 s).
fMRI Processing
fMRI images were preprocessed and analyzed using SPM 12 (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK) using standard processing methods. Spatial pre-processing included manually orienting images to the AC-PC line, correcting for head movement by aligning images to a reference image, and co-registering images to a high-resolution T1 image which was normalized to the Montreal Neurological Institute template brain. Images were subsequently resliced into 3.375 mm3 voxels (1.5 mm × 1.5 mm × 1.5 mm). A low pass filter (128 s) was used to remove low frequency components and a Gaussian filter was used to spatially smooth all images (8 mm full-width half maximum; FWHM). An autoregressive AR(1) model was used to account for serial correlation, and regressors modeled as box-car vectors (for each task-related condition) were convolved with a canonical hemodynamic reference waveform.
Brain network interactions were modeled using psycho-physiological interaction (PPI), a technique utilizing the general linear model to investigate functional connectivity by assessing modulatory effects of a seed region in a task active state (Friston et al., 1997; O'Reilly et al., 2012; Silverstein et al., 2016). PPI analyses were conducted for each task and subject using a seed (maximal response) in the dACC.
First level analyses modeled the degree of dACC modulation in each of the two task-active states. Time series from the dACC were extracted for all subjects using the effects of interest contrast of each task, and subsequently convolved with the corresponding task-active conditions. Thus, for working memory, analyses were conducted across all 2-back epochs, and for motor control, across all the periodic and random epochs. This approach allowed us to estimate veridical profiles associated with each of the working memory and motor control domains. The resulting interaction terms were positively weighted (O'Reilly et al., 2012). Thus, each participant contributed two first-level PPI maps (one per task) to subsequent 2nd level analyses. The 2nd level analysis utilized a two-factor design with Group (OCD vs. HC) as independent factor, and Task (Memory vs. Motor) as non-independent factor. This design structure flexibly allowed us to model effects associated with Group (OCD ≠ HC) and Task (Motor ≠ Memory). For all contrasts, significant clusters were identified using 104 Monte Carlo probability simulations of the data (p<.05 cluster level) which compute the probability of a random field of noise (after spatial correlations of voxels based on image smoothness are accounted for). These simulations generate a minimum cluster size threshold for significance after thresholding noise to a certain level (Morris et al., 2018). Therefore, our analysis used both individual voxel thresholding and a minimum cluster size thresholding. The statistical approach is based on an underlying and tenable assumption that activation occurs over contiguous voxels while noise does not aggregate in clusters (Ward, 2000).
Results:
Results are organized to present evidence of (1) dysfunctional dACC network profiles in OCD, (2) differences in network profiles between tasks, and (3) correlations between OCD symptom dimensions and the degree of dACC network dysfunction.
Group Related Effects on dACC Modulation
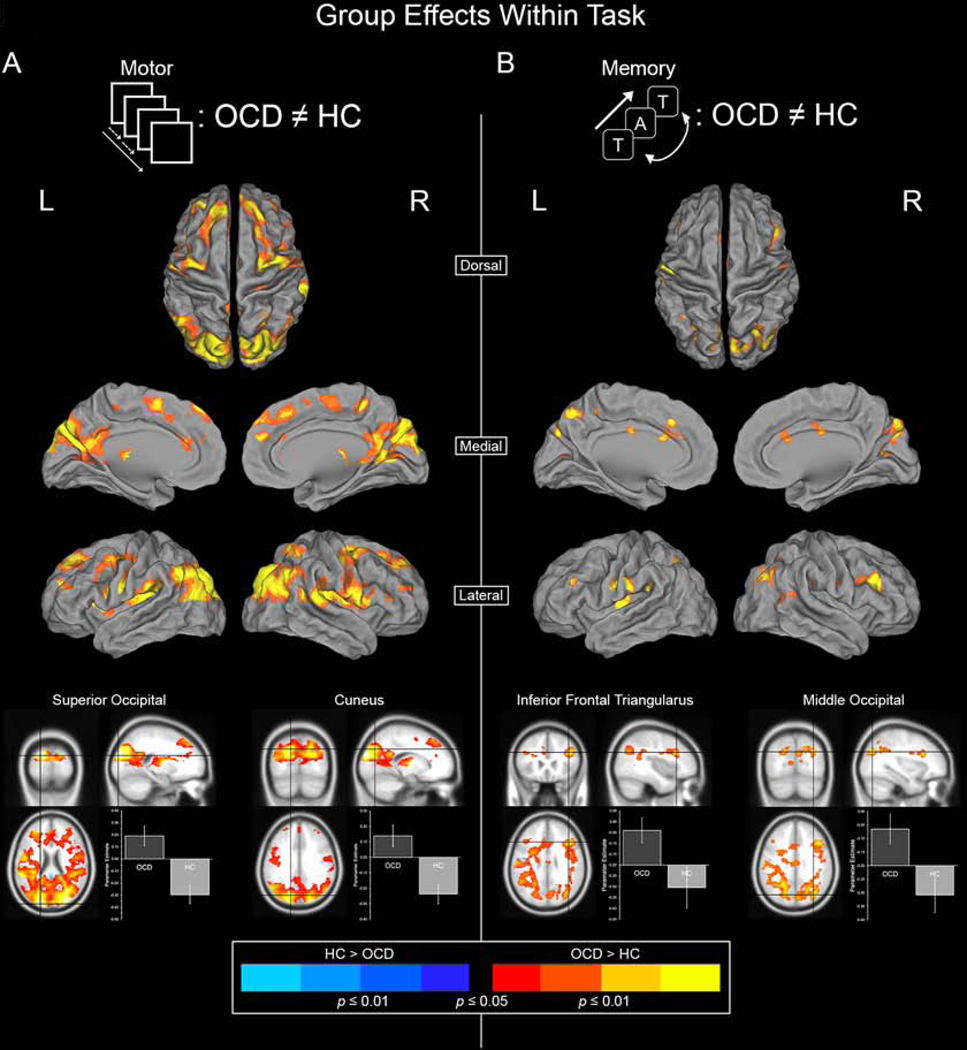
Figure 1 depicts clusters under the effect of Group (OCD ≠ HC) in each of the Motor and Memory task paradigms. Clusters depict regions characterized by differential modulation by the dACC.
Figure 1.
Significant clusters depict areas where dACC modulation is unequal between the OCD and HC groups. Warm colors represent areas where dACC modulation is greater in OCD whereas cool colors represent areas where dACC modulation is greater in HC. (A) Inter-group differences (OCD ≠ HC) under the Motor Control task are depicted. (B) Inter-group differences under the Working Memory task are depicted. Two highly significant peaks are sampled for each task to depict the specific effects (bottom).
OCD were characterized by significantly greater dACC modulation under both experimental paradigms, with minimal effects observed in the opposite direction (HC > OCD). This hyper-modulation was particularly salient for the motor task (Figure 1A). During the motor task, OCD were characterized by increased dACC modulation of multiple targets, including the Superior and Middle Occipital Lobules, Precuneus, Cuneus, Insula, Pre- and Post-Central Gyri, Frontal Gyrus, and the Superior Parietal Lobule. More circumscribed effects were observed for the memory paradigm (Figure 1B). Here, effects were observed in areas including Precuneus, Cuneus, Frontal Gyrus, Superior and Middle Occipital Lobules, and the Supramarginal Gyrus (Table 2 reports significance under the Effect of Group).
Table 2.
provides information on significant clusters under the effect of group (OCD ≠ HC) during the motor control and working memory tasks. These clusters are mapped in Figure 1.
| Contrast | Lobe | Automated Anatomical Labeling (AAL) | MNI Coordinates | Cluster Extent | T Score | P (peak) | ||
|---|---|---|---|---|---|---|---|---|
| Effect of Group | OCD > HC | Motor | Frontal | Frontal_Inf_Tri_L | −38 24 10 | 279 | 4.09 | <0.001 |
|
| ||||||||
| Frontal_Inf_Tri_R | 34 24 12 | 253 | 2.69 | 0.004 | ||||
|
| ||||||||
| Frontal_Mid_L | −27 44 23 | 723 | 3.30 | 0.001 | ||||
|
| ||||||||
| Frontal_Mid_R | 39 −7 56 | 633 | 3.06 | 0.001 | ||||
|
| ||||||||
| Frontal_Sup_L | −10 38 48 | 1061 | 2.88 | 0.002 | ||||
|
| ||||||||
| Frontal_Sup_R | 36 −8 58 | 1377 | 2.94 | 0.002 | ||||
|
| ||||||||
| Frontal_Sup_Medial_L | −6 38 52 | 1941 | 3.07 | 0.001 | ||||
|
| ||||||||
| Parietal | Parietal_Sup_L | −16 −74 40 | 207 | 3.26 | 0.001 | |||
|
| ||||||||
| Parietal_Sup_R | 21 −55 53 | 827 | 3.02 | 0.002 | ||||
|
| ||||||||
| Postcentral_L | −48 −4 17 | 1344 | 3.55 | <0.001 | ||||
|
| ||||||||
| Postcentral_R | 62 −4 16 | 796 | 2.87 | 0.002 | ||||
|
| ||||||||
| Precentral_L | −48 −1 17 | 2348 | 3.45 | <0.001 | ||||
|
| ||||||||
| Precentral_R | 39 −12 46 | 1908 | 3.14 | 0.001 | ||||
|
| ||||||||
| Precunues_L | −16 −73 32 | 4246 | 3.70 | <0.001 | ||||
|
| ||||||||
| Precunues_R | 20 −74 40 | 112 | 3.00 | 0.002 | ||||
|
| ||||||||
| SupraMarginal_L | −56 −40 23 | 710 | 3.66 | <0.001 | ||||
|
| ||||||||
| SupraMarginal_R | 45 −3 22 | 806 | 3.17 | 0.001 | ||||
|
| ||||||||
| Supp_Motor_Area_L | −10 −1 54 | 2079 | 3.00 | 0.002 | ||||
|
| ||||||||
| Occipital | Occipital_Mid_L | −22 −91 20 | 3477 | 4.53 | <0.001 | |||
|
| ||||||||
| Occipital_Mid_R | 30 −86 24 | 1911 | 4.89 | <0.001 | ||||
|
| ||||||||
| Occipital_Sup_L | −21 −91 23 | 2086 | 4.96 | <0.001 | ||||
|
| ||||||||
| Occipital_Sup_R | 27 −88 26 | 2065 | 4.86 | <0.001 | ||||
|
| ||||||||
| Lingual_R | 2 −67 6 | 435 | 2.77 | 0.003 | ||||
|
| ||||||||
| Cuneus_L | −16 −76 34 | 5091 | 4.19 | <0.001 | ||||
|
| ||||||||
| Temporal | Temporal_Sup_L | −54 −40 22 | 712 | 3.83 | <0.001 | |||
|
| ||||||||
| Temporal_Sup_R | 57 −36 14 | 1713 | 3.48 | <0.001 | ||||
|
| ||||||||
| Insula_L | −38 10 8 | 1427 | 3.99 | <0.001 | ||||
|
| ||||||||
| Insula_R | 38 14 8 | 959 | 3.67 | <0.001 | ||||
|
| ||||||||
| Thalamic | Thalamus_L | −12 −18 10 | 713 | 3.26 | 0.001 | |||
|
| ||||||||
| Thalamus_R | 21 −25 5 | 398 | 3.14 | 0.001 | ||||
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| Memory | Frontal | Frontal_Inf_Tri_L | −40 24 26 | 154 | 2.15 | 0.017 | ||
|
| ||||||||
| Frontal_Inf_Tri_R | 40 23 28 | 586 | 3.03 | 0.002 | ||||
|
| ||||||||
| Frontal_Sup_R | 18 16 58 | 100 | 2.37 | 0.010 | ||||
|
| ||||||||
| Parietal | Parietal_lnf_R | 48 −52 46 | 180 | 2.11 | 0.019 | |||
|
| ||||||||
| Parietal_Sup_L | −18−46 64 | 225 | 2.69 | 0.004 | ||||
|
| ||||||||
| Parietal_Sup_R | 32 −50 59 | 155 | 2.11 | 0.019 | ||||
|
| ||||||||
| Postcentral_L | −58−13 23 | 654 | 2.69 | 0.004 | ||||
|
| ||||||||
| Postcentral_R | 51 −13 28 | 125 | 1.90 | 0.029 | ||||
|
| ||||||||
| Precunues_L | −18−62 34 | 841 | 2.77 | 0.003 | ||||
|
| ||||||||
| Precunues_R | 16−52 40 | 510 | 2.40 | 0.009 | ||||
|
| ||||||||
| SupraMarginal_L | −48 −49 28 | 675 | 2.51 | 0.007 | ||||
|
| ||||||||
| Temporal | Temporal_Sup_R | 63 −25 4 | 525 | 2.43 | 0.008 | |||
|
| ||||||||
| lnsula_L | −39−16 6 | 296 | 2.33 | 0.011 | ||||
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| Occipital | Occipital_Mid_L | −20 −60 35 | 213 | 2.64 | 0.005 | |||
|
| ||||||||
| Occipital_Mid_R | 34 −74 30 | 672 | 3.02 | 0.002 | ||||
|
| ||||||||
| Occipital_Sup_L | −20 −68 32 | 580 | 2.68 | 0.004 | ||||
|
| ||||||||
| Occipital_Sup_R | 24 −62 34 | 590 | 2.66 | 0.004 | ||||
|
| ||||||||
| Calcarine_L | −20 −54 4 | 123 | 2.28 | 0.012 | ||||
|
| ||||||||
| Calcarine_R | 24−49 4 | 199 | 2.26 | 0.013 | ||||
|
| ||||||||
| Cuneus_L | −18−72 34 | 328 | 2.51 | 0.006 | ||||
|
| ||||||||
| Cuneus_R | 20 −70 34 | 1050 | 2.46 | 0.007 | ||||
|
| ||||||||
| HC > OCD | Motor | Parietal | Precentral_L | −39 −28 64 | 42 | 2.91 | 0.002 | |
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| - | - | - | - | |||||
|
| ||||||||
| Memory | None | - | - | - | - | - | ||
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| - | - | - | - | - | ||||
Task Related Effects on dACC Modulation
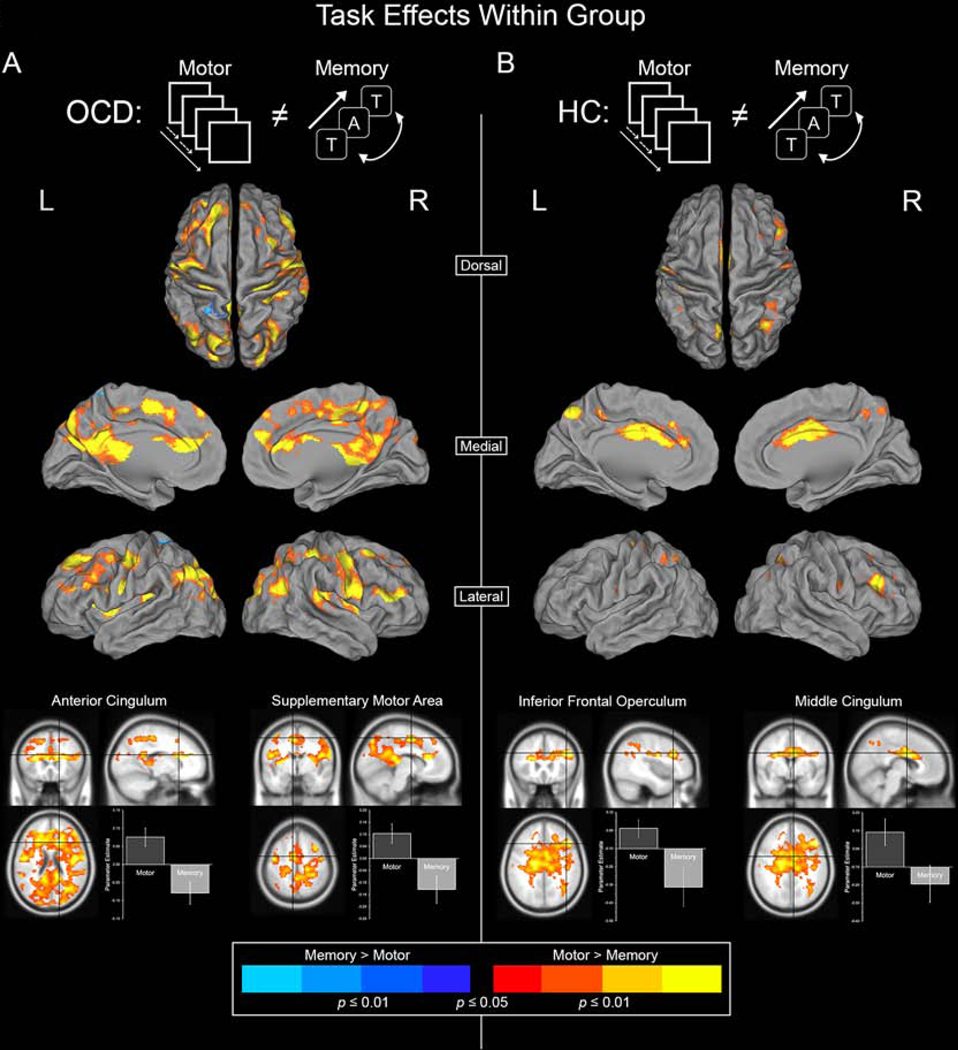
Figure 2 depicts significant clusters of differential dACC modulation between the Motor and Memory task paradigms in OCD and HC groups.
Figure 2.
Significant clusters depict areas where dACC modulation is unequal between the Motor Control and Working Memory tasks. Warm colors represent areas where dACC modulation is greater during Motor Control whereas cool colors represent areas where dACC modulation is greater during Working Memory. (A) Inter-task differences (Motor Control ≠ Working Memory) in the OCD group are depicted. (B) Inter-task differences in the HC group are depicted. Two highly significant peaks are sampled from each group to depict the specific effects (bottom).
In both groups, the motor task induced greater dACC modulation than working memory. This effect (Motor > Memory) was more salient in OCD patients, observed in areas including the Precuneus, Cingulum, Superior and Middle Occipital Lobe, Pre- and Postcentral Gyri, Inferior Parietal Lobule, and Frontal Gyrus in OCD (Figure 2A). In HC, Motor > Memory targets included the Precuneus, Cingulum, Angular Gyrus, and Inferior Parietal Lobule for HC (Figure 2B, Table 3 reports significance under the Effect of Task).
Table 3.
provides information on significant clusters under the effect of task (Motor Control ≠ Working Memory) in the OCD and HC groups. These clusters are mapped in Figure 2.
| Contrast | Lobe | Automated Anatomical Labeling (AAL) | MNI Coordinates | Cluster Extent | T Score | P (peak) | ||
|---|---|---|---|---|---|---|---|---|
| Effect of Task | Motor > Memory | OCD | Frontal | Frontal_Sup_L | −18 34 48 | 1007 | 3.03 | 0.002 |
|
| ||||||||
| Frontal_Inf_Tri_L | −32 23 14 | 752 | 3.43 | <0.001 | ||||
|
| ||||||||
| Frontal_Inf_Tri_R | 50 29 12 | 1000 | 3.28 | 0.001 | ||||
|
| ||||||||
| Frontal_Mid_L | −28 38 16 | 608 | 2.87 | 0.002 | ||||
|
| ||||||||
| Frontal_Mid_R | 45 41 22 | 713 | 3.02 | 0.002 | ||||
|
| ||||||||
| Frotal_Sup_R | 26 32 50 | 378 | 2.48 | 0.007 | ||||
|
| ||||||||
| Parietal | Parietal_Inf_L | −28 −79 41 | 250 | 2.76 | 0.003 | |||
|
| ||||||||
| Parietal_Inf_R Postcentral_L |
32−40 52 −50 −7 20 |
228 1764 |
2.70 3.18 |
0.004 0.001 |
||||
|
| ||||||||
| Postcentral_R | 62 −8 23 | 2067 | 2.90 | 0.002 | ||||
|
| ||||||||
| Precentral_L | −46 −7 20 | 2338 | 3.21 | 0.001 | ||||
|
| ||||||||
| Precentral_R | 40 −13 47 | 2194 | 3.40 | <0.001 | ||||
|
| ||||||||
| Precuneus_L | −8 −79 48 | 5243 | 2.90 | 0.002 | ||||
|
| ||||||||
| Supp_Motor_Area_L | −8 −4 53 | 2818 | 3.22 | 0.001 | ||||
|
| ||||||||
| Angular_L | −50 −67 34 | 1162 | 2.61 | 0.005 | ||||
|
| ||||||||
| Angular_R | 39 −61 23 | 1218 | 2.87 | 0.002 | ||||
|
| ||||||||
| Temporal | Temporal_Sup_L | −56 −38 18 | 309 | 2.68 | 0.004 | |||
|
| ||||||||
| Temporal_Sup_R | 52 −37 18 | 1258 | 2.56 | 0.006 | ||||
|
| ||||||||
| Cingulum_Ant_R | 10 22 22 | 2232 | 3.38 | 0.001 | ||||
|
| ||||||||
| Cingulum_Mid_L | −6 −6 50 | 3427 | 3.15 | 0.001 | ||||
|
| ||||||||
| Cingulum_Post_R | 10 −40 20 | 1666 | 3.07 | 0.001 | ||||
|
| ||||||||
| Occipital | Occipital_Mid_L | −27 −79 38 | 1692 | 2.96 | 0.002 | |||
|
| ||||||||
| Occipital_Mid_R | 32 −66 26 | 1142 | 2.91 | 0.002 | ||||
|
| ||||||||
| Occipital_Sup_L | −27 −76 40 | 1162 | 2.76 | 0.003 | ||||
|
| ||||||||
| Occipital_Sup_R | 34 −74 44 | 1417 | 2.91 | 0.002 | ||||
|
| ||||||||
| Calcarine_L | −24 −66 12 | 500 | 2.35 | 0.010 | ||||
|
| ||||||||
| Calcarine_R | 22 −54 17 | 91 | 2.45 | 0.008 | ||||
|
| ||||||||
| HC | Frontal | Frontal_Mid_L | −48 23 32 | 125 | 2.67 | 0.004 | ||
|
| ||||||||
| Frontal_Mid_R | 46 24 32 | 717 | 3.05 | 0.001 | ||||
|
| ||||||||
| Frontal_Sup_L | −16 38 29 | 132 | 2.19 | 0.015 | ||||
|
| ||||||||
| Frontal_Sup_R | 21 34 28 | 247 | 2.64 | 0.005 | ||||
|
| ||||||||
| Pareietal | Parietal_Inf_L | −40 −50 47 | 344 | 2.03 | 0.022 | |||
|
| ||||||||
| Parietal_Inf_R | 39 −56 48 | 347 | 2.03 | 0.013 | ||||
|
| ||||||||
| Postcentral_L | −45 −20 29 | 297 | 2.39 | 0.008 | ||||
|
| ||||||||
| Postcentral_R | 45 −20 32 | 266 | 2.28 | 0.012 | ||||
|
| ||||||||
| Precentral_L | −32 −26 64 | 202 | 2.28 | 0.012 | ||||
|
| ||||||||
| Precentral_R | 48 −2 28 | 160 | 2.42 | 0.009 | ||||
|
| ||||||||
| Precuneus_L | −8 −67 28 | 1362 | 3.04 | 0.001 | ||||
|
| ||||||||
| Angular_R | 33 −62 48 | 800 | 2.53 | 0.006 | ||||
|
| ||||||||
| Caudate_L | −16 6 24 | 192 | 2.87 | 0.002 | ||||
|
| ||||||||
| Temporal | Temporal_Sup_R | 60 −22 2 | 365 | 2.86 | 0.003 | |||
|
| ||||||||
| Cingulum_Ant_R | 6 5 28 | 1148 | 2.90 | 0.002 | ||||
|
| ||||||||
| Cingulum_Mid_R | 6 0 29 | 2422 | 2.77 | 0.003 | ||||
|
| ||||||||
| Memory > Motor | OCD | Parietal | Parietal_Sup_L | −18 −46 64 | 92 | 2.77 | 0.003 | |
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| - | - | - | - | - | ||||
|
| ||||||||
| HC | - | - | - | - | - | - | ||
Regression Analyses
In clusters under the group effect (OCD ≠ HC; Figure 1), did OCD symptom dimensions predict the degree of dACC modulation? To examine this question, regression analyses were employed to investigate how dACC modulation scaled as a function of two covariates of clinical interest: Lifetime Obsession and Lifetime Compulsion symptom scores measured using the CY-BOCS (see Methods). The 1st level PPI maps of each subject (Motor, Working Memory) were submitted to separate regression analyses with Obsession and Compulsion scores employed as regressors of interest. Resulting analyses were masked using significant clusters under the Effect of Group (Figure 1). This approach allowed us to specify whether clusters under the group effect were predicted by the dimensions of Obsession and/or Compulsion.
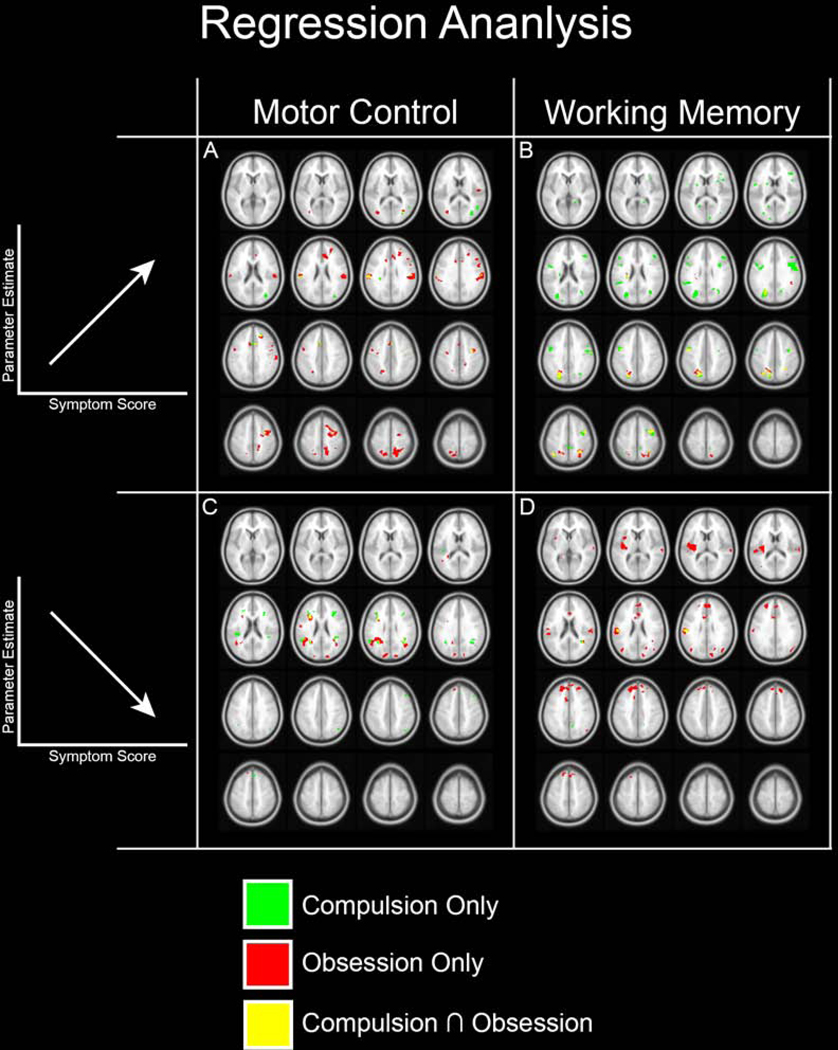
Significant clusters are depicted in Figure 3. For ease of access, clusters significantly and uniquely predicted by Compulsions are rendered in green (Compulsions Only), while those significantly and uniquely predicted by Obsessions are rendered in red (Obsessions Only). Clusters significantly predicted by both Obsessions and Compulsions are rendered in yellow (Compulsion ∩ Obsession).
Figure 3.
We investigated whether the clusters observed in Figure 1 evinced sensitivity to symptom dimensions of OCD (based on the CY-BOCS). Accordingly, within OCD regression analyses (with Compulsions and Obsessions) were masked using the significant effects in Figure 1. Sub-clusters related to Compulsions only (green) and Obsessions only (red) are color coded. Regions where clusters overlap (Compulsions Obsessions) are separately coded (yellow). Panels A and B depict sub-clusters where the degree of dACC modulation is positively correlated with symptom scores (see Supplementary Animation 1). Panels C and D depict sub-clusters where the degree of dACC modulation is negatively correlated with symptom scores (see Supplementary Animation 2).
Positive Relationships between symptom dimensions and the degree of PPI modulation:
Obsessions and Compulsions both exerted strong positive effects on dACC modulation, but the effects were confounded by task. Thus, Obsessions predicted increases in dACC modulation during the motor task, but Compulsions predicted increases in dACC modulation during the memory task (thus, Figure 3A is characterized by more red clusters, whereas Figure 3B is characterized by more green clusters). These effects were observed in areas including the Postcentral, Middle Frontal, and Supramarginal gyri (Obsessions, Motor Control; Figure 3A) and the superior occipital lobule, Precentral and Postcentral gyri (Compulsions, Working Memory; Figure 3B).
Negative Relationships between symptom dimensions and the degree of PPI modulation:
Obsessions and Compulsions both exerted strong negative effects on dACC modulation, but here too, effects differed by task. For the motor task, both Compulsions and Obsessions predicted decreases in dACC modulation. However, for the memory task it was primarily Compulsions that predicted a decrease in dACC modulation (thus, Figure 3C is characterized by both red and green clusters, whereas Figure 3D is characterized by more red clusters). For the motor task, increases in Obsessions predicted decreased dACC modulation of the Inferior frontal operculum and Angular gyrus (Obsessions, Motor Control; Figure 3C), while increases in Compulsions predicted decreased dACC modulation in the Rolandic operculum and Supramarginal gyrus (Compulsions, Motor Control; Figure 3C). For the memory task, increases in Obsessions predicted decreased dACC modulation of the Insula and Superior frontal gyrus (Obsessions, Working Memory; Figure 3D).
Discussion:
We assessed differences in dACC network profiles in OCD and HC, induced by simple motor control or working memory. Our principal results were these: a) Regardless of group, the dACC’s modulatory influence during motor control exceeded that induced during memory (Figure 2); b) For both motor control and working memory, OCD were characterized by significantly greater modulation by the dACC, with effects particularly salient during motor control (Figure 1); and c) Obsessive and Compulsive symptom dimensions predicted the degree of dACC network dysfunction in each experimental paradigm (Figure 3), but in complementary ways. During motor control, it was obsessive symptoms that were more predictive (Figure 3A), whereas during working memory, it was compulsive symptoms that were more predictive (Figure 3, B). Below we comment on how these results further elucidate the role of the dACC in the pathology of OCD, and how our results inform the experimental neuroscience of OCD.
The role of the dACC in control and performance monitoring
Previous investigations of brain activity during simple motor control and working memory have provided a rich window for exploring dACC network profiles (Bakshi et al., 2011; Diwadkar et al., 2015; Friedman et al., 2017; Weiss et al., 2018) that in part represent functional expressions of the region’s structural connectivity (Paus, 2001). Anatomical studies of the dACC note connections to areas associated with motor control including the motor cortex, premotor cortex, and spinal cord (Heilbronner and Hayden, 2016). In addition to the significant connections with motor areas, the dACC also interjects heavily with areas associated with emotion, including the amygdala, and areas associated with executive control, such as the prefrontal cortex (Stevens et al., 2011). The dACC’s functional position primes it to receive incoming sensory information, and then act on downstream motor regulation (Asemi et al., 2015; Morecraft et al., 2012). Thus, the structure is ideally positioned to translate intentions to actions, placing a heavy emphasis on the link between cognition and motor responses (Paus, 2001). Thus, the anatomy of the dACC, particularly its associations with motor areas, supports its integral role in the control and monitoring of motor function (Diwadkar et al., 2015; Friedman et al., 2017; Paus, 2001).
In addition to its role in motor control, the dACC is also associated with cognitive control and performance monitoring (Botvinick, 2007; Botvinick et al., 2001; Heilbronner and Hayden, 2016; Holroyd and Yeung, 2012). Cognitive control is generally defined as the ability to attend to relevant information while disregarding irrelevant information (Braver, 2012; McGovern and Sheth, 2017; Mennigen et al., 2014), though at the level of network interactions, it can be more generally related to how apex regions like the dACC exert effects on other regions (Breukelaar et al., 2016; Diwadkar et al., 2017; Schulz et al., 2011). Recent investigations have indeed suggested that the dACC may be biased towards motor control. For example, the dACC selectively modulates the Supplemental Motor Area (SMA) during visually-coordinated uni-manual behavior, but not during working memory (Diwadkar et al., 2017). Such results suggest that the dACC selectively potentiates motor sub-networks during simple motor tasks, an idea consistent with our results where within the healthy control group, dACC modulation during the motor task significantly exceeded that observed during working memory. Studies also implicate the dACC in performance monitoring, suggesting that the structure serves as to monitor both error (Holroyd and Coles, 2002) and conflict (Botvinick, 2007; Botvinick et al., 2001; Kerns et al., 2004; van Veen and Carter, 2002) in a continuous manner (Blanchard et al., 2015; Carter et al., 1998; Holroyd and Coles, 2002). Notably all these purported functions of the dACC interject with aspects of OCD pathology.
The dACC in Obsessive-Compulsive Disorder
An extensive literature implicates the dACC in the etiology of OCD (McGovern and Sheth, 2017; Wang et al., 2017; Zhang et al., 2017) with multiple theories speculating on the region’s contribution. A prevailing theory proposes that normative functional loops associated with cortico-striato-thalamo-cortical (CSTC) circuits are dysfunctional in OCD, in part because of altered “bottom-up” thalamic inputs and altered dACC-related top-down control (Ahmari et al., 2014; Alexander et al., 1986; Goodman et al., 2014; Rajendram et al., 2017; Wang et al., 2017). Thus, the unique role of the dACC in cognition and performance monitoring assumes significance in OCD, as the disorder has been characterized by impairments in both domains. For example, Cocchi and colleagues used a Multisource Interference Task (validated task for cognitive control) to investigate functional network correlates of performance on goal-directed cognitive control (Cocchi et al., 2012). They observed significant functional differences between the OCD and control group, reporting both relative increases and decreases in regional connectivity.
Complementary theories on dACC function have highlighted its particular role in error-monitoring (Holroyd and Coles, 2002; van Veen and Carter, 2002), suggesting that the structure uses past action outcomes to inform current action selection (Egner, 2009; Rushworth et al., 2004). OCD subjects show increased error-related negativity implying that the disorder is characterized by overactive error-monitoring (Endrass and Ullsperger, 2014; Gehring et al., 2000; Grutzmann et al., 2016; Ullsperger et al., 2014). Recent accounts also suggest that in OCD, aberrant dACC network profiles may result from its inability to differentiate task-relevant from task-irrelevant information, an inability that leads to poorly specified control signals (McGovern and Sheth, 2017). Aberrant dACC signals may then act on downstream targets that include motor areas such as the pre-SMA, and SMA, and the thalamus (Diwadkar et al., 2017; Egner, 2009; Stevens et al., 2011), and these aberrant signals are coupled with (or drive) OCD-like symptoms.
Obsessive-Compulsive symptom dimensions predict dACC dysfunction in each task domain
“Parsing” OCD by its symptom dimensions enabled us to study how specific dimensions within OCD influence dACC pathology during motor control and working memory tasks. Studies have previously shown that anterior cingulate gray matter is robustly related to obsessive but not compulsive, symptom severity (Rosenberg and Keshavan, 1998; Szeszko et al., 2004) though such structural findings are not entirely consistent (Piras et al., 2013; Szeszko et al., 1999). For example, other studies, while not observing the supposed relationship between cingulate grey matter and symptom severity, have noted that specific OCD clinical features are related to microstructural alterations in projection and association fibers to posterior brain regions (Piras et al., 2019). Such microstructural alterations may result in deficits in executive processes, including those which the dACC is purported to play a role in. These in turn may evince some form of a parametric relationship to symptom dimensions. Of course, attempts to relate clinical dimensions to any form of underlying neuroimaging measure (whether functional or structural) are highly valuable and of substantial clinical relevance (Thorsen et al., 2018), but unfortunately are characterized more by hetero-, rather than homogeneity across studies (Begue et al., 2020). This may reflect on the complexity of clinical syndromes and the indeterminacies associated with in vivo neuroimaging data, and there may not be a straightforward solution. Nevertheless, our specific observations in this regard are somewhat compelling.
The motor task we employed was relatively simple and primarily reactive. Conversely, the working memory task was more deliberative and cognitively demanding. The resultant dichotomy between obsessive and compulsive symptom dimensions and complementary relationships to each of the tasks interject with the task elements. Thus, an increase in obsessive symptoms (defined as persistent, intrusive, and/or uncontrollable thoughts or urges) predicted increased dACC modulation during the reactive motor control task, but a decrease in obsessive symptoms predicted decreased dACC modulation during the working memory task. This dissociation was preserved when assessing the relationship to compulsive symptoms. Thus, an increase in compulsive symptoms (repetitive and ritualistic behaviors and/or thoughts performed to reduce anxiety) predicted increased dACC modulation during the deliberative and challenging working memory task. The link with anxiety is compelling because anxiety and affect lead to competition with or inference on task-related processes (Eysenck and Calvo, 1992; Eysenck et al., 2007; Soloff et al., 2017; Soloff et al., 2015). Moreover, meta-analyses that studied the relationship between working memory capacity and anxiety, showed that measures of anxiety are significantly correlated with lower working memory capacity across tasks of varying complexity (Moran, 2016). By comparison, the obsessive dimension is most saliently related to the inability to control intrusive thoughts and urges (Norman et al., 2019).
As noted, aberrations in control is a common theme in the OCD literature and implicates the dACC in OCD pathology (Botvinick, 2007; Heilbronner and Hayden, 2016). Therefore, we would expect that aberrations in dACC network profiles during tasks that feature a high demand for cognitive control would be related to obsessive symptom severity. Indeed, it is precisely this correlation that was saliently observed during the motor task. The motor task is noted for relatively frequent response demands to visually presented stimuli. Both task conditions (periodic and random responding) featured higher response frequencies than did the working memory task. The expectation is that this difference in the frequency of responses led to greater response demands per unit of time, though other sub-demands would also be at play. During random epochs, the task structure induced a high propensity for false alarms and misses, plausibly evoking a greater degree of error and performance monitoring. By comparison, periodic epochs evoked the implicit perception of a temporal task structure. Thus, in OCD we found greater dACC modulation of regions associated with motor timing (the frontal gyrus, insula, and parietal cortex) (Laje et al., 2011; Paton and Buonomano, 2018) (Figure 1; Table 2).
Abnormalities in volitional motor control are strongly implicated in OCD (Takashima et al., 2019), and are almost certainly an expression of the generalized cortico-striatal dysfunction that characterizes many adolescent-onset psychiatric conditions (Kuo and Liu, 2019). Thus it is unsurprising that motor dysfunction is considered a proximal cause of various pathophysiological pathways and symptom expressions of psychiatric disorders (Hirjak et al., 2018). Considered in totality, it appears that the characteristics of our employed motor task evokes a more salient demand for control than evoked during the working memory task. Therefore, more prominent obsessive symptoms, which are most saliently related to deficits in control, are more associated with network aberrations induced during motor control.
Conclusions and Limitations
None of dACC dysfunction, deficits in motor control networks or in working memory networks are idiosyncratic to OCD. Indeed, dACC dysfunction appears to be a feature of conditions related to disordered cognitive control (Holroyd and Umemoto, 2016), across the axial diagnostic system. These effects have been documented in conditions such as schizophrenia and psychosis (Baajour et al., 2020; Woodcock et al., 2016) as well as personality disorders like borderline personality disorder (Soloff et al., 2015). Similarly, motor dysfunction is observed in conditions ranging from ADHD (Bush, 2011), autism (Lukito et al., 2020), mood disorders (Walther et al., 2012), and schizophrenia and psychosis (Abboud et al., 2017; Walther and Strik, 2012). Finally, working memory deficits are a shared characteristic across multiple diagnostic categories (Quraishi and Frangou, 2002; Tan et al., 2007). With these considerations in mind, and given that our study lacked a clinical control group, it is obvious that we can make no generalizable assertions from our results. More generally, the question of what is specific and what is shared across the biological substrates of psychiatric conditions remains for now a highly vexing question. The complexity of any definitive answers is only amplified by extant genetic (Cross-Disorder Group of the Psychiatric Genomics et al., 2013) and imaging studies (Sprooten et al., 2017). What we can suggest is that within the context of OCD, using the dACC to target in vivo properties of motor networks appears to be a more efficient strategy for identifying clinically relevant dysfunction, than targeting working memory networks. We anticipate that such competitive frameworks will be informative in other disorders as well.
Our analysis demonstrates the value of studying disease pathology through the investigation of brain network profiles. As suggested by The National Institute of Mental Health, understanding the network profiles of diseases is of particular interest to clinical neuroscience and will inform future classification schemes (Insel et al., 2010). Here, we used fMRI and PPI to model the dACC’s modulatory effects and compare between groups and tasks. While our analysis aids in understanding the punitive mechanisms of OCD, it is nevertheless precluded by the fundamental limitations that come with the use of fMRI and PPI. For example, due to the use of fMRI, our analysis could not asses the specific biochemical bases of the observed effects or differentiate between the multiple contributions to the hemodynamic response (Stephan, 2004). In addition, while the use of PPI allowed us to study the dACC’s modulatory influence, it also leaves us with relatively limited inferences that can be drawn from the analysis (Logothetis, 2008). Nevertheless, our analysis has demonstrated that these methods do reveal diseaserelated network dysfunction and provides a framework that can inform future studies.
Supplementary Material
Supplementary Animation 1. The animation depicts the dichotomy between the positive correlations of the Motor Control and Working Memory tasks (Fig 3A and 3B). Notable is the switch from predominantly Compulsion related sub-clusters during Working Memory to predominantly Obsession related sub-clusters during Motor Control.
Supplementary Animation 2. The animation depicts the dichotomy between the negative correlations of the Motor Control and Working Memory tasks (Fig 3C and 3D). Notable is the switch from predominantly Obsession related sub-clusters during Working Memory to characteristic Obsession and Compulsion related sub-clusters during Motor Control.
Table 4.
provides information on clusters under the effect of group where dACC modulation is predicted by lifetime obsessive or lifetime compulsive symptom dimensions. These clusters are represented in Figure 3.
| Compulsion / Working Memory | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Positive | Negative | |||||||||
|
|
|
|||||||||
| AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | |
| Precentral_L | −50 −1 41 | 826 | 3.77 | <0.001 | Postcentral_L | −52 −20 26 | 177 | 2.68 | 0.006 | |
|
| ||||||||||
| Occipital_Sup_L | −24 −73 32 | 1650 | 3.70 | 0.001 | Temporal_Mid_R | 33 −52 20 | 65 | 2.23 | 0.017 | |
|
| ||||||||||
| Postcentral_R | 58 −2 34 | 689 | 3.35 | 0.001 | Precuneus_R | 14 −54 38 | 54 | 2.16 | 0.020 | |
|
| ||||||||||
| Precentral_R | 42 −1 48 | 488 | 3.10 | 0.002 | - | - | - | - | - | |
|
| ||||||||||
| Frontal_Inf_Tri_R | 48 29 24 | 402 | 2.78 | 0.005 | - | - | - | - | - | |
|
| ||||||||||
| Occipital_Mid_R | 34 −67 23 | 312 | 2.72 | 0.006 | - | - | - | - | - | |
|
| ||||||||||
| SupraMarginal_L | −62 −43 29 | 201 | 2.63 | 0.007 | - | - | - | - | - | |
|
| ||||||||||
| Caudate_L | −24 −26 24 | 116 | 2.56 | 0.008 | - | - | - | - | - | |
|
| ||||||||||
| Frontal_Inf_Tri_L | −48 28 28 | 58 | 2.55 | 0.008 | - | - | - | - | - | |
|
| ||||||||||
| Parietal_Inf_R | 30 −52 52 | 159 | 2.54 | 0.009 | - | - | - | - | - | |
|
| ||||||||||
| Calcarine_R | 9 −90 11 | 58 | 2.43 | 0.011 | - | - | - | - | - | |
|
| ||||||||||
| Putamen_R | 28 8 12 | 50 | 2.40 | 0.012 | - | - | - | - | - | |
|
| ||||||||||
| Cingulum_Mid_L | 0 −40 54 | 124 | 2.26 | 0.016 | - | - | - | - | - | |
|
| ||||||||||
| Calcarine_R | 18 −48 6 | 50 | 2.23 | 0.017 | - | - | - | - | - | |
|
| ||||||||||
| Putamen_L | −24 −2 12 | 75 | 2.22 | 0.017 | - | - | - | - | - | |
|
| ||||||||||
| Frontal_Inf_Oper_R | 45 10 14 | 78 | 2.17 | 0.019 | - | - | - | - | - | |
|
| ||||||||||
| Precuneus_R | 15 −66 34 | 102 | 2.17 | 0.019 | - | - | - | - | - | |
|
| ||||||||||
| Calcarine_L | −20 −58 14 | 87 | 2.17 | 0.019 | - | - | - | - | - | |
|
| ||||||||||
| Obsession / Working Memory | ||||||||||
|
| ||||||||||
| Positive | Negative | |||||||||
|
|
|
|||||||||
| AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | |
|
|
|
|||||||||
| Precuneus_L | −14 −60 50 | 1113 | 3.80 | <0.001 | Occipital_Mid_R | 44 −76 29 | 195 | 3.49 | 0.001 | |
|
| ||||||||||
| Frontal_Mid_R | 46 2 52 | 192 | 3.17 | 0.002 | Insula_L | −34 −19 11 | 1552 | 3.48 | 0.001 | |
|
| ||||||||||
| Parietal_Inf_R | 30 −52 52 | 454 | 3.08 | 0.002 | Frontal_Sup_Medial_L | −6 42 48 | 1614 | 2.95 | 0.003 | |
|
| ||||||||||
| Precentral_L | −45 −2 42 | 92 | 2.77 | 0.005 | Temporal_Sup_R | 66 −25 16 | 285 | 2.62 | 0.007 | |
|
| ||||||||||
| Insula_L | −24 −30 26 | 65 | 2.70 | 0.006 | Cingulum_Ant_L | −8 11 28 | 416 | 2.40 | 0.012 | |
|
| ||||||||||
| - | - | - | - | - | Occipital_Mid_L | −40 −73 26 | 143 | 2.26 | 0.016 | |
|
| ||||||||||
| - | - | - | - | - | Cuneus_R | 15 −86 26 | 139 | 2.25 | 0.017 | |
|
| ||||||||||
| - | - | - | - | - | Insula_L | −34 5 8 | 115 | 2.24 | 0.017 | |
|
| ||||||||||
| - | - | - | - | - | Angular_R | 38−48 22 | 78 | 2.20 | 0.018 | |
|
| ||||||||||
| - | - | - | - | - | Cuneus_R | 22 −70 26 | 64 | 2.04 | 0.025 | |
|
| ||||||||||
| Compulsion / Motor Control | ||||||||||
|
| ||||||||||
| Positive | Negative | |||||||||
|
|
|
|||||||||
| AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | |
|
|
|
|||||||||
| Precentral_R | 33 −4 44 | 127 | 2.91 | 0.004 | Frontal_Mid_R | 42 23 47 | 66 | 3.08 | 0.002 | |
|
| ||||||||||
| Cingulum_Mid_R | 4 16 40 | 169 | 2.90 | 0.004 | SupraMarginal_R | 44 −38 23 | 394 | 2.61 | 0.007 | |
|
| ||||||||||
| Frontal_Sup_R | 20 36 35 | 50 | 2.76 | 0.005 | Rolandic_Oper_L | −40 −31 20 | 181 | 2.60 | 0.008 | |
|
| ||||||||||
| SupraMarginal_L | −56 −25 26 | 149 | 2.47 | 0.010 | Frontal_Inf_Oper_L | −28 14 26 | 318 | 2.59 | 0.008 | |
|
| ||||||||||
| SupraMarginal_R | 54 −24 30 | 73 | 2.46 | 0.010 | SupraMarginal_L | −54 −48 24 | 168 | 2.47 | 0.010 | |
|
| ||||||||||
| Temporal_Mid_R | 50 −60 16 | 118 | 2.34 | 0.014 | Frontal_Inf_Tri_R | 32 22 23 | 250 | 2.43 | 0.011 | |
|
| ||||||||||
| Occipital_Mid_R | 30 −76 | 328 | 2.10 | 0.023 | Angular_L | −30 −54 28 | 201 | 2.40 | 0.012 | |
|
| ||||||||||
| - | - | - | - | - | Frontal_Sup_Medial_R | 4 32 53 | 75 | 2.28 | 0.015 | |
|
| ||||||||||
| - | - | - | - | - | Angular_R | 46 −64 38 | 96 | 2.02 | 0.026 | |
|
| ||||||||||
| Obsession / Motor Control | ||||||||||
|
| ||||||||||
| Positive | Negative | |||||||||
|
|
|
|||||||||
| AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | AAL | MNI Coordinates | Cluster Extent | T Score | P (peak) | |
|
|
|
|||||||||
| Postcentral_R | 57 −22 34 | 981 | 4.02 | <0.001 | Frontal_Inf_Oper_L | −28 10 26 | 306 | 3.84 | <0.001 | |
|
| ||||||||||
| Frontal_Mid_R | 38 −2 52 | 820 | 3.67 | 0.001 | Angular_L | −36 −44 26 | 889 | 2.97 | 0.003 | |
|
| ||||||||||
| SupraMarginal_L | −60 −24 24 | 361 | 3.62 | 0.001 | Occipital_Sup_R | 18 −91 28 | 144 | 2.47 | 0.010 | |
|
| ||||||||||
| Cingulum_Mid_R | 8 12 35 | 347 | 3.45 | 0.001 | Cuneus | −10 −85 30 | 164 | 2.45 | 0.011 | |
|
| ||||||||||
| Postcentral_R | 12 −43 60 | 762 | 3.43 | 0.001 | Angular_R | 40 −61 26 | 259 | 2.44 | 0.011 | |
|
| ||||||||||
| Parietal_Sup_L | −26 −52 64 | 458 | 3.35 | 0.001 | - | - | - | - | - | |
|
| ||||||||||
| Frontal_Sup_R | 20 36 35 | 319 | 3.24 | 0.002 | - | - | - | - | ||
|
| ||||||||||
| Precentral_L | −33 −10 44 | 58 | 3.17 | 0.002 | - | - | - | - | - | |
|
| ||||||||||
| Precentral_L | −50 −2 42 | 124 | 3.08 | 0.002 | - | - | - | - | - | |
|
| ||||||||||
| Cingulum_Ant_R | 9 28 24 | 257 | 3.07 | 0.002 | - | - | - | - | - | |
|
| ||||||||||
| Precentral_R | 52 5 34 | 131 | 2.53 | 0.009 | - | - | - | - | - | |
|
| ||||||||||
| Occipial_Mid_L | −32 −73 12 | 161 | 2.51 | 0.009 | - | - | - | - | - | |
|
| ||||||||||
| Frontal_Inf_Oper_R | 45 20 30 | 116 | 2.38 | 0.012 | - | - | - | - | - | |
Highlights.
OCD is characterized by increased dACC modulation during Motor Control and Working Memory.
OCD symptom dimensions predict the degree of dACC dysfunction during Motor Control and Working Memory.
In OCD, Motor Control induces greater dACC network dysfunction than does Working Memory.
Studying disease pathology through the investigation of network profiles yields insights that cannot be assessed by simple activation based analyses.
Acknowledgements
This work was supported by the National Institute of Mental Health (MH059299), the Children’s Foundation of Michigan, the Prechter World Bipolar Foundation, the Lykacki-Young Fund from the State of Michigan, the Miriam Hamburger Endowed Chair of Psychiatry, the Paul and Anita Strauss Endowment, the Donald and Mary Kosch Foundation, the Elliott Luby Endowed Professorship, and the Detroit Wayne County Authority. The funding agencies played no role in the analyses or reporting of the data.
Footnotes
Author Statement
TM conducted the analyses, created the figures and wrote the paper
AC assisted in analyses and statistical methods
PE assisted in data collection and clinical analyses
TA assisted in data analyses
EK assisted in data analyses
GH assisted in clinical characterization and data collection
PA assisted in clinical issues and data collection
DR assisted in clinical characterization and data collection
VAD oversaw all aspects of the analyses, writing and presentation
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Taylor S, McKay D, 2009. Obsessive-compulsive disorder. Lancet 374(9688), 491–499. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Eich T, Cebenoyan D, Smith EE, Blair Simpson H, 2014. Assessing neurocognitive function in psychiatric disorders: a roadmap for enhancing consensus. Neurobiol Learn Mem 115, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL, 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Apter A., Fallon TJ Jr., King RA., Ratzoni G., Zohar AH., Binder M., Weizman A., Leckman JF., Pauls DL., Kron S., Cohen DJ., 1996. Obsessive-compulsive characteristics: from symptoms to syndrome. Journal of the American Academy of Child and Adolescent Psychiatry 35(7), 907–912. [DOI] [PubMed] [Google Scholar]
- Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL, 2015. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci 9, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA, 2011. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res 45(8), 1067–1076. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW, 2013. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Begue I, Kaiser S, Kirschner M, 2020. Pathophysiology of negative symptom dimensions of schizophrenia - Current developments and implications for treatment. Neuroscience and biobehavioral reviews 116, 74–88. [DOI] [PubMed] [Google Scholar]
- Berg CZ, Whitaker A, Davies M, Flament MF, Rapoport JL, 1988. The survey form of the Leyton Obsessional Inventory-Child Version: norms from an epidemiological study. Journal of the American Academy of Child and Adolescent Psychiatry 27(6), 759–763. [DOI] [PubMed] [Google Scholar]
- Blanchard TC, Strait CE, Hayden BY, 2015. Ramping ensemble activity in dorsal anterior cingulate neurons during persistent commitment to a decision. J Neurophysiol 114(4), 2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, 2007. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, affective & behavioral neuroscience 7(4), 356–366. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD, 2001. Conflict monitoring and cognitive control. Psychol Rev 108(3), 624–652. [DOI] [PubMed] [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci 16(2), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukelaar IA, Antees C, Grieve SM, Foster SL, Gomes L, Williams LM, Korgaonkar MS, 2016. Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Human brain mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America 99(1), 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD, 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science (New York, N.Y 280(5364), 747–749. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL, 1995. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage 2(3), 221–229. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS, 2000. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol 83(3), 1550–1566. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ, 2005. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev 29(3), 399–419. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W, 2005. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res Cogn Brain Res 25(3), 607–623. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yucel M, 2012. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum Brain Mapp 33(5), 1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL, 2017. Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS ONE 12(3), e0172531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA., Bellani M., Chowdury A., Savazzi S., Perlini C., Marinelli V., Zoccatelli G., Alessandrini F., Ciceri E., Rambaldelli G., Ruggieri M., Carlo Altamura A., Marzi CA., Brambilla P., 2018. Activations in gray and white matter are modulated by uni-manual responses during within and inter-hemispheric transfer: effects of response hand and right-handedness. Brain imaging and behavior 12(4), 942–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Burgess A, Hong E, Rix C, Arnold PD, Hanna GL, Rosenberg DR, 2015. Dysfunctional Activation and Brain Network Profiles in Youth with Obsessive-Compulsive Disorder: A Focus on the Dorsal Anterior Cingulate during Working Memory. Front Hum Neurosci 9, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A, Zajac-Benitez C, Rajan U, Keshavan MS, 2011. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Progress in neuro-psychopharmacology & biological psychiatry 35(5), 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, 2009. Prefrontal cortex and cognitive control: motivating functional hierarchies. Nat Neurosci 12(7), 821–822. [DOI] [PubMed] [Google Scholar]
- Endrass T, Ullsperger M, 2014. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehav Rev 46 Pt 1, 124–138. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG, 1992. Anxiety and performance: The processing efficiency theory. Cognition and Emotion 6(6), 409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG, 2007. Anxiety and cognitive performance: attentional control theory. Emotion 7(2), 336–353. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Shekar S, Gorwood PA, Voon V, Morein-Zamir S, Denys D, Sahakian BJ, Moeller FG, Robbins TW, Potenza MN, 2014. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 19(1), 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AL., Burgess A., Ramaseshan K., Easter P., Khatib D., Chowdury A., Arnold PD., Hanna GL., Rosenberg DR., Diwadkar VA., 2017. Brain network dysfunction in youth with obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Res Neuroimaging 260, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, 1997. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6(3), 218–229. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG, 2000. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci 11(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Gilbert KE, Barclay ME, Tillman R, Barch DM, Luby JL, 2018. Associations of Observed Performance Monitoring During Preschool With Obsessive-Compulsive Disorder and Anterior Cingulate Cortex Volume Over 12 Years. JAMA Psychiatry 75(9), 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Grice DE, Lapidus KA, Coffey BJ, 2014. Obsessive-compulsive disorder. Psychiatr Clin North Am 37(3), 257–267. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS, 1989. The Yale-Brown Obsessive Compulsive Scale: I. Development, Use, and Reliability. Archives of General Psychiatry 46(11), 1006–1011. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N, 2016. Presupplementary Motor Area Contributes to Altered Error Monitoring in Obsessive-Compulsive Disorder. Biol Psychiatry 80(7), 562–571. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Hayden BY, 2016. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu Rev Neurosci 39, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak D, Meyer-Lindenberg A, Fritze S, Sambataro F, Kubera KM, Wolf RC, 2018. Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neurosci Biobehav Rev 95, 315–335. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH, 2002. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, 2012. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci 16(2), 122–128. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS, 2004. Anterior cingulate conflict monitoring and adjustments in control. Science (New York, N.Y 303(5660), 1023–1026. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J, 2009. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29(47), 14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HY, Liu FC, 2019. Synaptic Wiring of Corticostriatal Circuits in Basal Ganglia: Insights into the Pathogenesis of Neuropsychiatric Disorders. eNeuro 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje R, Cheng K, Buonomano DV, 2011. Learning of temporal motor patterns: an analysis of continuous versus reset timing. Front Integr Neurosci 5, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A., McIntosh AR., 2005. The Role of Anterior Cingulate Cortex in Working Memory is Shaped by Functional Connectivity. Journal of cognitive neuroscience 17(7), 1026–1042. [DOI] [PubMed] [Google Scholar]
- Li B, Mody M, 2016. Cortico-Striato-Thalamo-Cortical Circuitry, Working Memory, and Obsessive-Compulsive Disorder. Front Psychiatry 7, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R, 2010. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc 16(6), 1064–1076. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453(7197), 869–878. [DOI] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS, 2000. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science (New York, N.Y 288(5472), 1835–1838. [DOI] [PubMed] [Google Scholar]
- Mars RB, Grol MJ, 2007. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J Neurosci 27(8), 1801–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P, 1997. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. Journal of neurophysiology 77(3), 1630–1634. [DOI] [PubMed] [Google Scholar]
- McGovern RA, Sheth SA, 2017. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg 126(1), 132–147. [DOI] [PubMed] [Google Scholar]
- Mennigen E, Rodehacke S, Muller KU, Ripke S, Goschke T, Smolka MN, 2014. Exploring adolescent cognitive control in a combined interference switching task. Neuropsychologia 61, 175–189. [DOI] [PubMed] [Google Scholar]
- Moran TP, 2016. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol Bull 142(8), 831–864. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN, 2012. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull 87(4–5), 457–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Ravishankar M, Pivetta L, Chowdury A, Falco D, Damoiseaux JS, Rosenberg DR, Bressler SL, Diwadkar VA, 2018. Response Hand and Motor Set Differentially Modulate the Connectivity of Brain Pathways During Simple Uni-manual Motor Behavior. Brain Topogr 31(6), 985–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan A, Richardson T, Anney RJ, Gill M, 2009. The Social Communication Questionnaire in a sample of the general population of school-going children. Ir J Med Sci 178(2), 193–199. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR, 2005. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. Journal of neurophysiology 93(2), 884–908. [DOI] [PubMed] [Google Scholar]
- Norman LJ., Taylor SF., Liu Y., Radua J., Chye Y., De Wit SJ., Huyser C., Karahanoglu FI., Luks T., Manoach D., Mathews C., Rubia K., Suo C., van den Heuvel OA., Yucel M., Fitzgerald K., 2019. Error Processing and Inhibitory Control in Obsessive-Compulsive Disorder: A Meta-analysis Using Statistical Parametric Maps. Biol Psychiatry 85(9), 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H, 2012. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci 7(5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Buonomano DV, 2018. The Neural Basis of Timing: Distributed Mechanisms for Diverse Functions. Neuron 98(4), 687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, 2001. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2(6), 417–424. [DOI] [PubMed] [Google Scholar]
- Piras F, Piras F, Abe Y, Agarwal SM, Anticevic A, Ameis S, Arnold P, Bargalló N, Batistuzzo MC, Benedetti F, Beucke J-C, Boedhoe PSW, Bollettini I, Brem S, Calvo A, Kevin Cho KI, Dallaspezia S, Dickie E, Ely BA, Fan S, Fouche J-P, Gruner P, Gürsel DA, Hauser T, Hirano Y, Hoexter MQ, Iorio M, James A, Reddy J, Kaufmann C, Koch K, Kochunov P, Kwon JS, Lazaro L, Lochner C, Marsh R, Nakagawa A, Nakamae T, Narayanaswamy JC, Sakai Y, Shimizu E, Simon D, Simpson HB, Soreni N, Stämpfli P, Stern ER, Szeszko P, Takahashi J, Venkatasubramanian G, Wang Z, Yun J-Y, Stein DJ, Jahanshad N, Thompson PM, van den Heuvel OA, Spalletta G, 2019. White Matter Microstructure and its Relation to Clinical Features of Obsessive-Compulsive Disorder: Findings from the ENIGMA OCD Working Group. bioRxiv, 855916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Piras F, Caltagirone C, Spalletta G, 2013. Brain circuitries of obsessive compulsive disorder: a systematic review and meta-analysis of diffusion tensor imaging studies. Neuroscience and biobehavioral reviews 37(10 Pt 2), 2856–2877. [DOI] [PubMed] [Google Scholar]
- Rajendram R, Kronenberg S, Burton CL, Arnold PD, 2017. Glutamate Genetics in Obsessive-Compulsive Disorder: A Review. J Can Acad Child Adolesc Psychiatry 26(3), 205–213. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, 1998. A.E. Bennett Research Award. Toward a neurodevelopmental model of of obsessive--compulsive disorder. Biological psychiatry 43(9), 623–640. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM, 2004. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8(9), 410–417. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, 2003. Social Communication Questionnaire, in: Services, W.P. (Ed.). Los Angeles. [Google Scholar]
- Scahill L, Riddle MA, McSWIGGIN-HARDIN M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF, 1997. Children's Yale-Brown Obsessive Compulsive Scale: Reliability and Validity. Journal of the American Academy of Child & Adolescent Psychiatry 36(6), 844–852. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Bedard AC, Czarnecki R, Fan J, 2011. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. NeuroImage 57(1), 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD, 2013. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79(2), 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein BH, Bressler SL, Diwadkar VA, 2016. Inferring the Dysconnection Syndrome in Schizophrenia: Interpretational Considerations on Methods for the Network Analyses of fMRI Data. Frontiers in psychiatry 7, 132–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Ramaseshan K, Burgess A, Diwadkar VA, 2017. Hyper-modulation of brain networks by the amygdala among women with Borderline Personality Disorder: Network signatures of affective interference during cognitive processing. J Psychiatr Res 88, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA, 2015. Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry research 233(1), 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, 2004. On the role of general system theory for functional neuroimaging. J Anat 205(6), 443–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens FL, Hurley RA, Taber KH, 2011. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 23(2), 121–125. [DOI] [PubMed] [Google Scholar]
- Szeszko PR., MacMillan S., McMeniman M., Chen S., Baribault K., Li KO., Ivey J., Rose M., Banerjee SP., Bhandari R., Moore GJ., Rosenberg DR., 2004. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. The American journal of psychiatry 161(6), 1049–1056. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B, 1999. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry 56(10), 913–919. [DOI] [PubMed] [Google Scholar]
- Takashima S, Najman FA, Ramos RT, 2019. Disruption of volitional control in obsessive-compulsive disorder: Evidence from the Bereitschaftspotential. Psychiatry Res Neuroimaging 290, 30–37. [DOI] [PubMed] [Google Scholar]
- Thorsen AL, Kvale G, Hansen B, van den Heuvel OA, 2018. Symptom dimensions in obsessive-compulsive disorder as predictors of neurobiology and treatment response. Curr Treat Options Psychiatry 5(1), 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To WT, De Ridder D, Menovsky T, Hart J, Vanneste S, 2017. The role of the dorsal Anterior Cingulate Cortex (dACC) in a cognitive and emotional counting Stroop task: Two cases. Restor Neurol Neurosci 35(3), 333–345. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Fischer AG, Nigbur R, Endrass T, 2014. Neural mechanisms and temporal dynamics of performance monitoring. Trends Cogn Sci 18(5), 259–267. [DOI] [PubMed] [Google Scholar]
- Valleni-Basile LA, Garrison CZ, Waller JL, Addy CL, McKeown RE, Jackson KL, Cuffe SP, 1996. Incidence of obsessive-compulsive disorder in a community sample of young adolescents. J Am Acad Child Adolesc Psychiatry 35(7), 898–906. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS, 2002. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77(4–5), 477–482. [DOI] [PubMed] [Google Scholar]
- van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA, 2014. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci 8, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Fan Q, Zhang Z, Chen Y, Tong S, Li Y, 2017. White matter integrity correlates with choline level in dorsal anterior cingulate cortex of obsessive compulsive disorder patients: A combined DTI-MRS study. Conf Proc IEEE Eng Med Biol Soc 2017, 3521–3524. [DOI] [PubMed] [Google Scholar]
- Ward BD, 2000. Simultaneous inference for fMRI data. Medical College of Wisconsin, Milwaukee, WI. [Google Scholar]
- Weiss AR., Gillies MJ., Philiastides MG., Apps MA., Whittington MA., FitzGerald JJ., Boccard SG., Aziz TZ., Green AL., 2018. Dorsal Anterior Cingulate Cortices Differentially Lateralize Prediction Errors and Outcome Valence in a Decision-Making Task. Front Hum Neurosci 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff RP, Wolff LS, 1991. Assessment and treatment of obsessive-compulsive disorder in children. Behav Modif 15(3), 372–393. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fan Q, Zhu Y, Tan L, Chen Y, Gao R, Zhang H, Li Y, Xiao Z, 2017. Intrinsic functional connectivity alteration of dorsal and rostral anterior cingulate cortex in obsessive-compulsive disorder: A resting fMRI study. Neurosci Lett 654, 86–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Animation 1. The animation depicts the dichotomy between the positive correlations of the Motor Control and Working Memory tasks (Fig 3A and 3B). Notable is the switch from predominantly Compulsion related sub-clusters during Working Memory to predominantly Obsession related sub-clusters during Motor Control.
Supplementary Animation 2. The animation depicts the dichotomy between the negative correlations of the Motor Control and Working Memory tasks (Fig 3C and 3D). Notable is the switch from predominantly Obsession related sub-clusters during Working Memory to characteristic Obsession and Compulsion related sub-clusters during Motor Control.