Stopp and Sixt preview work from the Sarris lab showing that neutophrils follow a two-step search-and-run strategy to approach alarm gradients within complex tissue environments in living organisms.
Abstract
Reading, interpreting and crawling along gradients of chemotactic cues is one of the most complex questions in cell biology. In this issue, Georgantzoglou et al. (2022. J. Cell. Biol. https://doi.org/10.1083/jcb.202103207) use in vivo models to map the temporal sequence of how neutrophils respond to an acutely arising gradient of chemoattractant.
The ability to follow gradients of guidance cues is fundamental for almost all living systems, from the cellular to the organismal scale. There are distinctive strategies to sense molecule concentration differences of which prokaryotic chemotaxis is the best understood biological example. Bacteria interrupt phases of straight, flagella-driven swimming by tumbling phases where the bacterium randomly changes its direction. Tumbling is more likely to happen when the concentration of the guidance cue—usually a nutrient—has dropped compared to previous time-points. The resulting “biased random walk” leads the cell toward the higher concentration (1). In general, such a temporal strategy is effective for moving entities that are fast and small or if the gradient is shallow or fluctuating. With bigger cell size, slower migratory speed and steeper gradients, a spatial sensing strategy becomes an option. Eukaryotic cells do integrate spatial signals—they can effectively sense concentration changes across their surface. By polarizing their deformable cell body toward the higher concentration, they can crawl up-gradient. Spatial sensing has been established in many eukaryotic cells, most prominently in single celled amoeba of Dictyostelium discoideum but also in amoeboid animal leukocytes that share many of the signaling pathways established in Dictyostelium (2). While it is clear from controlled in vitro experiments that leukocytes can integrate spatial information, it is not well understood if and under which circumstances they use the temporal domain.
There are few studies where chemotactic gradients are sufficiently controlled to quantitatively address eukaryotic temporal gradient sensing. Some of them suggest that chemotaxis encompasses consecutive abrupt phases of motility and stagnation rather than a continuous phase and propose that the breaks between the motile and stationary states allow cells to adapt their receptors to the rising concentrations of the guidance cue (3, 4). While these works do not assess whether cells compare concentrations over time, they show that eukaryotic chemotaxis is not necessarily an uninterrupted process.
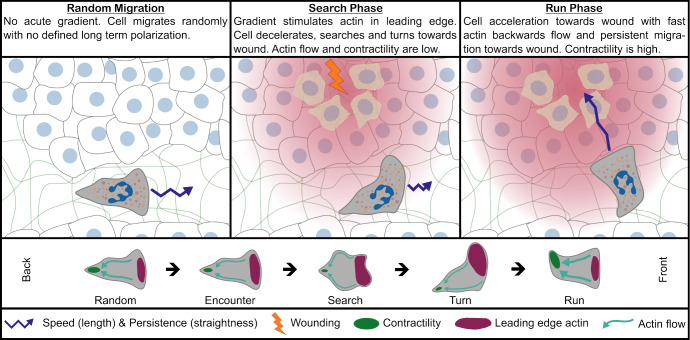
In their new study, Georgantzoglou et al. examine the chemotactic behavior of neutrophils in the living organism. They devised experiments where a chemotactic gradient can be abruptly induced in the tissue and the cytoskeletal response of the cells precisely monitored. Their data suggest that chemotaxis is a two-stage process which begins with a search phase where the cell samples its surrounding and continues with a run phase where it rushes toward the target (Fig. 1).
Figure 1.
Model for neutrophil search-and-run strategy in vivo. After neutrophils are recruited into tissue, they migrate randomly. Once they encounter an acute gradient, they slow down and actin is enriched at the cell front to expand the leading edge, while cell contractility and actin flow are low. The dynamic expansion of the leading edge helps neutrophils to sense the direction of the gradient, leading to a biased turn into the direction of the wound and ending the search phase. At the onset of the run phase, contractility is increased, leading to a high retrograde actin flow, which results in rapid cell movement. The contractility creates an inertia effect on cell polarity, locking the cell into a forward movement toward the source with high directional persistence.
The authors used neutrophils in fins of zebrafish and ears of mice as back-to-back in vivo model systems. Using intravital microscopy they tracked the trajectories of endogenous or previously transferred neutrophils in response to an acutely administered laser-wound. Upon wounding, the cells transition from random migration to a directed, collective movement toward the site of damage, where the chemoattractant is released (5, 6). In a first set of experiments, they found that, once exposed to the gradient, neutrophils are faster whenever their path is pointing toward the wound (7). At the same time, neutrophils get more directionally persistent (i.e., they make less directional changes) the closer they are to the wound; thus, once they are on the right track, they speed up and go straight.
Georgantzoglou et al. next linked cellular behavior with cytoskeletal changes by using reporters of actin and myosin and cross-correlated cellular trajectories with cytoskeletal polarity and flow. Somewhat surprisingly, they found that low speed correlated with actin enrichment in the front and high speed with actin enrichment in the back. In turn, front actin enrichment correlated positively with ongoing directional changes, meaning that a cell with strong protrusive activity is slow and prone to turn. Fast and directionally persistent cells had their actin mainly at the back, the site where actomyosin contractility is high.
They then turned their attention to the phase immediately before and after wounding and found that the acute response of a randomly wandering neutrophil to an abruptly emerging gradient is to slow down and increase protrusive activity at the cell front. At the end of this hesitation time, which takes a few minutes, the neutrophil makes a turn toward the wound. Then, it polarizes the actin to the back and starts to accelerate toward the site of damage. This suggested a model where the initial response of a cell is to ramp up the formation of exploratory protrusion which sample for concentration differences. When reading out downstream signals (i.e., PIP3 as a marker for intracellular polarization) in cells where all actin dynamics were pharmacologically abolished with LatrunculinB, the authors found that successful polarization relied on shape changes. Hence, it is possible (but remains to be shown) that during the search phase, the cells do not only integrate spatial information, but also create a temporal domain by “sniffing around” in their immediate vicinity.
After the search phase, myosin II at the back of the cell became dominant and with it, actin relocalized to the trailing edge. When the authors dynamically imaged actomyosin, they saw a rapidly increasing flow directed from the front to the back. Actin flows are the mechanical force that, once coupled to the substrate by transmembrane receptors or shape-changes, drive the cell forward. Increased actin flow was in line with the cell trajectories, suggesting that after the search phase, neutrophils entered the run phase, where they head straight to the target. Blocking myosin activity with Blebbistatin led to extended search times and impaired run phases, confirming that polymerization-driven actin protrusions characterized the search phase, while contraction-driven actin flows were responsible for the run phase (Fig. 1).
From these data it seems plausible that during the transition from the search to the run phase, the cell consolidates all the information it previously collected and translates it into a well-defined front-back axis. How this works mechanistically remains to be shown. One possible scenario is that when myosin II ramps up its contractility and pulls all filaments toward the back-pole of the cell, this rectifies the cell along the axis where most actin has been polymerized during the search phase. Thus, the flow patterns of a retracting actin cortex might act as an integrator of the previously collected information.
Another open question is, how the search-and-run strategy translates in an environment, where the source of the gradient is either further away than a few cell body lengths or when other motile cells release the attractant themselves, as is often seen when cells migrate along cascades of relay factors. Sampling once and running straight toward these sources will not suffice here, as the cell will have to reorient along local anatomical obstacles and re-adjust its path along the changing gradient, respectively. One possible solution is that the search-and-run process might repeat itself, possibly in an oscillatory manner, similarly to what has been suggested in the germ cells and neurons (3, 4). To better understand the strategies that different cells use to navigate in complex environments we will need the combination of tractable in vivo models, as pioneered by the authors, and reconstituted in vitro models that allow to precisely manipulate and monitor both gradients and cellular responses.
Acknowledgments
The authors declare no competing financial interests.
References
- 1.Colin, R., et al. 2021. FEMS Microbiol. Rev. 10.1093/femsre/fuab038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal, D.S., et al. 2019. Int. J. Dev. Biol. 10.1387/ijdb.190265pd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ming, G.L., et al. 2002. Nature. 10.1038/nature745 [DOI] [Google Scholar]
- 4.Minina, S., et al. 2007. Curr. Biol. 10.1016/j.cub.2007.05.073 [DOI] [PubMed] [Google Scholar]
- 5.Lämmermann, T., et al. 2013. Nature. 10.1038/nature12175 [DOI] [Google Scholar]
- 6.Poplimont, H., et al. 2020. Curr. Biol. 10.1016/j.cub.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgantzoglou, A., et al. 2022. J. Cell Biol. 10.1083/jcb.202103207 [DOI] [PMC free article] [PubMed] [Google Scholar]