Abstract
Hypercholesterolemia is a well-established risk factor for atherosclerotic cardiovascular disease (ASCVD). How cholesterol and its carrier lipoproteins are involved in ASCVD is still under extensive investigation. Satins are thus far the best-proven class of cholesterol-lowering medications to improve the clinical outcomes of ASCVD. Statins specifically inhibit the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase of the mevalonate pathway for cholesterol biosynthesis. The widely accepted theory is that statins inhibit the hepatic cholesterol synthesis causing upregulation of hepatocyte low-density lipoprotein (LDL) receptor; receptor-mediated LDL uptake and metabolism in the liver results in reduction of circulating LDL cholesterol, which subsequently reduces vascular deposition and retention of cholesterol or LDL in atherogenesis. Nevertheless, cholesterol biosynthesis is ubiquitous, also in extrahepatic cells including those in vascular wall, under tight regulation by sterol regulatory element-binding protein (SREBP), the master gene transcription factor governing cholesterol biosynthesis. Studies have shown that SREBP can be upregulated in vascular wall subject to injury or stent implantation. SREBP can be activated by proinflammatory and mitogenic factors in vascular cells, leading to hyperactive mevalonate pathway, which promotes vascular cell mobilization, further proinflammatory and mitogenic factor release from vascular cells, and vascular inflammation. In this article, we review the cellular cholesterol homeostasis regulation by SREBP and SREBP-mediated vascular hyperactive cholesterol biosynthesis, we term vascular hypercholesterolism, in the pathogenesis of ASCVD and vasculopathy. SREBP functions as a platform bridging cholesterol, inflammation, and vascular cell mobilization in ASCVD pathogenesis. Targeting vascular hypercholesterolism could open a new avenue in fighting against ASCVD.
Keywords: atherosclerotic cardiovascular disease, vasculopathy, cholesterol, vascular cell, inflammation, sterol regulatory element-binding protein
Hypercholesterolemia is a well-established risk factor for atherosclerotic cardiovascular disease (ASCVD). In the past decades since the cholesterol hypothesis was put forward to explain ASCVD, great efforts have been made in lowering the blood level of cholesterol, mostly focused on low-density lipoprotein (LDL) cholesterol (LDL-C) level. For a long time, LDL-C has been labeled as “bad” cholesterol and high-density lipoprotein cholesterol as “good” cholesterol. Many approaches have been studied and applied to lower LDL-C level or manipulate the levels of lipoprotein or apolipoproteins, including bile acid sequestrants, fibrates, niacin, cholesteryl ester transfer protein inhibitors, apolipoprotein A-I and high-density lipoprotein mimetics, apolipoprotein B (ApoB) regulators, acyl coenzyme A:cholesterol acyltransferase inhibitors, cholesterol absorption inhibitors, statins, proprotein convertase subtilisin kexin 9 inhibitors, and ATP-citrate lyase inhibitor. Some approaches have been successful in improving ASCVD clinical outcomes, such as statins, but many have not been as rewarding and even ended in failures. Although it is not yet fully understood why different cholesterol lowering or lipoprotein regulators result in variable clinical outcomes for ASCVD, their mechanism of action may be relevant.
Thus far, statins remain the best-proven class of cholesterol-lowering medications in improving clinical outcomes including reducing cardiovascular and all-cause mortality in patient with ASCVD. Statins uniquely inhibit the rate-limiting enzyme, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), in the mevalonate (MVA) pathway for cholesterol biosynthesis (Fig. 1). It is generally believed that by decreasing hepatic cholesterol synthesis, statins upregulate the hepatic LDL receptors (LDLRs) resulting in more LDLR-mediated clearance of apoB-containing lipoproteins, particularly LDL. This reduces circulating LDL-C and thus LDL vascular deposition in ASCVD. Statins can also directly reduce the hepatic assembly and secretion of apoB lipoproteins.
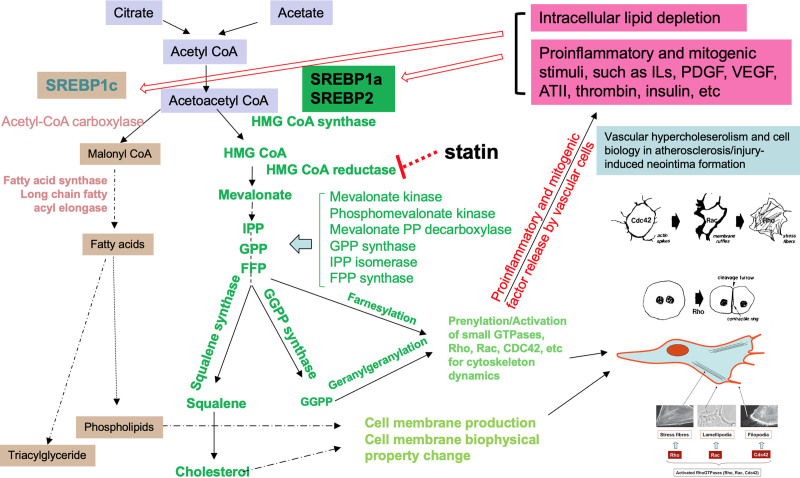
FIGURE 1.
Gene regulation by SREBPs and vascular cell biology in vasculopathy. The diagram shows some of the genes regulated by SREBPs in the biosyntheses of cholesterol and fatty acids and their roles in atherosclerosis and injury-induced neointima formation. SREBP governs cholesterol and fatty acid biosyntheses and homeostasis. In response to proinflammatory or mitogenic factors, SREBP in vascular wall is upregulated (at gene transcription and protein level) and activated at protein level. The cholesterol biosynthesis in vascular cells such as endothelial cells and VSMCs synthesize cholesterol and phospholipids for cell membrane production and for membrane biophysical property adaptation needed for cell migration and proliferation. The cholesterol biosynthesis also produces isoprenoids such as FPP and GGPP for prenylation of small GTPases including Rho, Rac, and CDC42, among others to drive the cytoskeletal dynamics for cell functional change, migration, and proliferation, leading to atherosclerosis or injury-induced neointima formation. AT II indicates angiotensin II; bFGF, basic fibroblast growth factor; FPP, farnesyl diphosphate; GGPP, geranylgeranyl pyrophosphate; GPP, geranylgeranyl pyrophosphate synthase; HMG CoA, 3-hydroxy-3-methylglutaryl-CoA; ILs, interleukins; IPP, isopentenyl diphosphate; LPA, lysophosphatidic acid; PDGF, platelet-derived growth factor; SREBP, sterol regulatory element-binding protein; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
A body of evidence has shown that statins have pleiotropic effects, which is related to the MVA pathway, and this is believed to at least partly contribute to the beneficial effect of statins for ASCVD.1,2 In humans, the 27-carbon cholesterol is synthesized with all of the carbon atoms originally from acetate through the MVA pathway.3,4 The enzymes catalyzing the MVA pathway are tightly regulated by the master gene transcription factor, sterol regulatory element-binding protein (SREBP), in response to cellular cholesterol contents and homeostasis not only in hepatic cells but also in nonhepatic ones such as vascular cells (Fig. 1).
In this article, we review cellular cholesterol homeostasis and its regulation by SREBP and role in bridging cholesterol, inflammation, and vascular cell mobilization, and its implication in vasculopathy.
CHOLESTEROL HOMEOSTASIS VIA MVA PATHWAY GOVERNED BY SREBP
In 1933, a landmark study using mice sealed in ventilated bottles by Schoenheimer and Breusch5 first demonstrated that dietary cholesterol could inhibit endogenous cholesterol biosynthesis. When fed a cholesterol-free diet, mice would make more endogenous cholesterol but when fed a cholesterol-containing diet, endogenous cholesterol synthesis was no longer observed. This was the first demonstration that an end-product could inhibit a biosynthetic pathway through a feedback mechanism. The feedback regulation of cholesterol biosynthesis is present in various animal species including humans.6–8 The feedback suppression and regulation of cholesterol biosynthesis was more recently discovered to be a coordination of gene product expression via the key gene transcription factor SREBP.4
The mammalian genome harbors 2 SREBP isoform genes, SREBP-1 and SREBP-2. SREBP-1 gene encodes SREBP-1a and SREBP-1c derived from a single gene with different N-termini due to alternative promoters and different first exons.9 SREBP-1 was first isolated in 1993 by the Goldstein and Brown laboratory when studying cellular cholesterol homeostasis.10 The group further elucidated the SREBP activation pathway.9,11 SREBP-1a activates the genes encoding enzymes for cholesterol biosynthesis, LDL-R, and the enzymes for fatty acid synthesis. SREBP-1c mainly activates the genes for fatty acid synthesis. SREBP-2 is encoded by a different gene, which more specifically activates the genes of the cholesterol biosynthetic enzymes and LDL-R. SREBP can also upregulate themselves at the gene expression level. All 3 SREBP molecules require processing at the protein level for activation (Figs. 1 and 2).
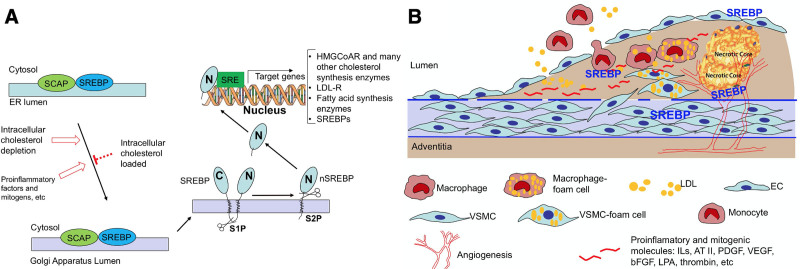
FIGURE 2.
SREBP activation and vasculopathy. SREBP activation in the regulation of cholesterol biosynthesis (A) and its implication in vascular hypercholesterolemia, cell mobilization, and inflammation in the pathogenesis of vasculopathy (B). A, When cells are depleted of lipid/cholesterol or in response to proinflammatory or mitogenic factors, SREBP is escorted by SCAP from the ER to the Golgi apparatus and the SREBP is cleaved by S1P. S1P cleaved SREBP is then further cleaved by S2P to release the active nSREBP from the membrane. The nSREBP enters the nucleus and binds to the SRE of target genes to turn on the expression of the downstream genes including those for cholesterol and fatty acid biosyntheses, LDL-R, and SREBP itself, among others. B, Atherosclerosis or injury-induced neointima formation begins with endothelial cell dysfunction or injury. Mitogenic, chemotactic, and proinflammatory factors from the blood circulation and produced locally by dysfunctional vascular cells and inflammatory cells cause further cell dysfunction such as endothelium layer permeability increase, recruitment of circulating monocytes and lymphocytes to subendothelial space, intimal migration of VSMC from the media, formation of foam cells from macrophages and VSMC, and angiogenesis, culminating in intimal hyperplasia and plaque formation. SREBP plays an important role linking inflammation and cellular cholesterol biosynthesis process in the pathogenesis of vasculopathy. AT II indicates angiotensin II; bFGF; basic fibroblast growth factor; EC, endothelial cell; ER, endoplasmic reticulum; HMG CoAR, 3-hydroxy-3-methylglutaryl-CoA reductase; ILs, interleukins; LDL, low-density lipoprotein; LDL-R, low-density lipoprotein receptor; LPA, lysophosphatidic acid; nSREBP, N-terminal sterol regulatory element-binding protein; PDGF, platelet-derived growth factor; S1P, site-1 protease; S2P, site-2 protease; SCAP, SREBP cleavage-activating protein; SRE, sterol regulatory element; SREBP, sterol regulatory element-binding protein; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
SREBPs are endoplasmic reticulum (ER) membrane-bound, with the N- and C-termini protruding into the cytoplasm and the central hydrophilic domain spanning the ER lumen. In sterol-loaded cells, SREBPs form a complex with the ER membrane-bound SREBP cleavage-activating protein and SREBPs remain inactivated. When the cells sense deficiency in lipids or sterols, SREBP cleavage-activating protein escorts SREBPs from the ER to the Golgi, where SREBPs are cleaved by site 1 and site 2 proteases, a process named activation (Fig. 2). This SREBP activation process is followed by release and subsequent nuclear translocation of the SREBP N-terminal leucine zipper transcription factor to direct the transcriptional activation of target genes including those for the enzymes in MVA pathway, such as HMGCR, as well as LDLR, and fatty acid synthase, among others.12
CHOLESTEROL PARADIGM, INFLAMMATION, AND VASCULAR CELL MOBILIZATION, BEYOND CHOLESTEROL IN ASCVD
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin trial is a randomized placebo-controlled large-scale study investigating the effects of rosuvastatin in traditionally normocholesterolemic, apparently healthy subjects with high levels of inflammatory marker high-sensitivity C-reactive protein (hs-CRP). A total of 17,802 subjects with LDL-C levels below 130 mg/dL (3.4 mmol/L) but hs-CRP levels of 2.0 mg/dL or higher were assigned to rosuvastatin or placebo. Rosuvastatin decreased LDL-C levels by 50% and hs-CRP levels by 37%. Rosuvastatin treatment led to a significant reduction in primary end point including significant decrease, respectively in myocardial infarction (MI), stroke, revascularization or unstable angina, and combined end point of MI, stroke, or death from cardiovascular causes, as well as in death from any cause.13 The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin trial indicated that a proinflammatory state as reflected by high levels of hs-CRP is a risk factor for ASCVD and rosuvastatin has anti-inflammatory and LDL-C lowering effect, suggesting a link between inflammation and cholesterol homeostasis, more specifically the MVA pathway.
Proinflammatory and Mitogenic Factors Cause Vascular Cell SREBP Activation and Hyperactive Cholesterol Biosynthesis
The cholesterol hypothesis implies that hepatic cholesterol biosynthesis is the cause of hypercholesterolemia, and excessive vascular deposition and retention of cholesterol or ApoB-containing lipoproteins from blood circulation drives atherogenesis. However, cholesterol biosynthesis is ubiquitous, not only in hepatic cells but also in vascular wall and other nonhepatic cells. Atherosclerosis carries pathological features of inflammation.14 Proinflammatory state with elevated levels of interleukins (ILs) and other inflammatory markers in patients with ASCVD including ACS has been well recognized.15–18 Insulin resistance and hyperinsulinemia are a hallmark of type II diabetes mellitus, an equivalent of ASCVD, which is considered an inflammatory disease characterized by high blood levels of proinflammatory cytokines.19
Studies showed that SREBP could be activated in vascular cells such as endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), by many proinflammatory and mitogenic factors known to be involved in atherosclerosis and neointima formation, such as vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, thrombin, IL-8, IL-1β, angiotensin II, insulin, phenylephrine, and lysophosphatidic acid.20–24 These factors can be from the systematic circulation or produced locally in the injured vascular wall (Figs. 1 and 2). The SREBP activation is followed by upregulation of its downstream genes such as HMGCR and other enzymes for MVA pathway, LDLR, and enzymes for fatty acid biosynthesis.
SREBP-Mediated Vascular Hyperactive Cholesterol Biosynthesis Promotes Vascular Cell Proliferation and Migration and Vascular Inflammation
Cholesterol and phospholipids are the major components of cell membrane and therefore cholesterol and fatty acid production is needed when a cell divides into daughter cells during proliferation. During cell migration, extension of pseudopodia is followed by cell body movement, membrane detachment, and retraction at the rear edge. During cell migration, such as in ECs, plasma membrane microviscosity increases at the leading edge, and cholesterol enrichment in the front edge is responsible for the generation of this microviscosity gradient, which affects actin function during cell migration.25,26
The MVA pathway is essential in the de novo biosynthesis of cholesterol. MVA is synthesized from 3-hydroxy-3-methylglutaryl-CoA in an irreversible step via the rate-limiting HMGCR and is then further metabolized to the isoprenoids, farnesyl pyrophosphate, and geranylgeranyl pyrophosphate, among other molecules, either as precursors for cholesterol production or as “side products” upstream or away from cholesterol formation (Fig. 1). Isoprenoids farnesyl pyrophosphate and geranylgeranyl pyrophosphate derived from the MVA pathway are critical in the isoprenylation and thus activation of small GTPases (Rho, Ras, Rac, and Cdc42). When isoprenylated, these small GTPases anchor in cell membranes enabling signaling and regulation of actin and other cell cytoskeleton dynamics to form actin stress fibers and provides the driving force of cell movement through actin cytoskeletal processes called lamellipodia and filopodia.27,28 Activated small GTPases play an important role in the migration and proliferation of vascular EC and VSMC. The activated small GTPases also play an important role in inflammatory cell migration into vascular wall and inflammatory mediator production by VSMC, EC, and inflammatory cells in vascular wall.29
Cholesterol biosynthesis process mediated by SREBP and the resultant intermediates may play a role well beyond cholesterol in ASCVD pathogenesis (Fig. 1). Studies showed that SREBP is important in regulating vascular EC function and angiogenesis, both in vitro and in vivo,20,21,24 which is an important process in atheroma formation, inflammation, and post-intervention vascular intimal hyperplasia.30 Angiogenic factors of different classes, such as basic fibroblast growth factor, thrombin, IL-8, insulin, and vascular endothelial growth factor could upregulate the expression and stimulate the activation of SREBP in EC.20,21,24 SREBP-mediated MVA pathway activation and resultant isoprenoids-mediated small GTPase RhoA activation in EC played an indispensable role in vascular EC proliferation, migration, and angiogenesis, as well as increased endothelial layer permeability in response to proinflammatory and mitogenic stimuli. Inhibition of SREBP activation can modify EC behavior resulting in suppression of endothelial migration and proliferation and angiogenesis.20
Aberrant migration of VSMC from medium into intima and proliferation play an important role in atherogenesis and injury-induced intimal hyperplasia in vasculopathy. SREBP can be upregulated in VSMCs as well, at both gene and protein levels in response to multiple stimuli known to cause atherosclerosis, inflammation, and post-intervention restenosis.20–22 The SREBP activation, that is, its cleavage to release N-terminal active protein form, is followed by upregulation of its target gene products involved in cholesterol biosynthesis.20–22 Targeted inhibition of SREBP also led to decreased VSMC migration and proliferation in response to proinflammatory and mitogenic stimuli.22
VASCULAR HYPERCHOLESTEROLISM AND VASCULOPATHY
VSMC migration and proliferation, foamy macrophage accumulation, and inflammatory cell infiltration in vascular wall are among the key features of atherosclerosis of the native coronary artery and vein grafts and of the neoatherosclerosis and neointima formation for in-stent restenosis.30,31 In addition to their circulating blood source, inflammatory and mitogenic factors local to the vascular wall also derive from infiltrating inflammatory cells, locally adhering platelets, and dysfunctional vascular EC and VSMC.32,33 These proinflammatory and mitogenic molecules can then activate cellular SREBP and subsequently the MVA pathway for cholesterol biosynthesis. Hyperactive MVA pathway and subsequent accelerated production of pleiotropic molecules cause prenylation and activation of the small GTPases, such as Rho, Rac, CDC42, and Ras proteins, which in turn further promotes proinflammatory cytokine release by vascular cells and increase in endothelial/leukocyte adhesion, endothelial permeability, and inflammatory cell accumulation, as well as in vascular cell migration and proliferation.34 This self-perpetuating mechanism, composed of inflammatory and mitogenic factors—SREBP activation—MVA pathway—vascular inflammation and cellular mitogenesis—inflammatory and mitogenic factor release—SREBP activation, may play a critical role in the pathogenesis of ASCVD and vasculopathy (Figs. 1 and 2). In injury-induced arterial intimal hyperplasia and in-stent restenosis animal models, SREBP was found to be overexpressed abundantly in arterial wall, strikingly prominent in the VSMCs of the neointima as demonstrated by histological analysis with immunofluorescence and immunohistochemistry studies, reflecting a hyperactive cholesterol biosynthesis mechanism (Figs. 3 and 4).22
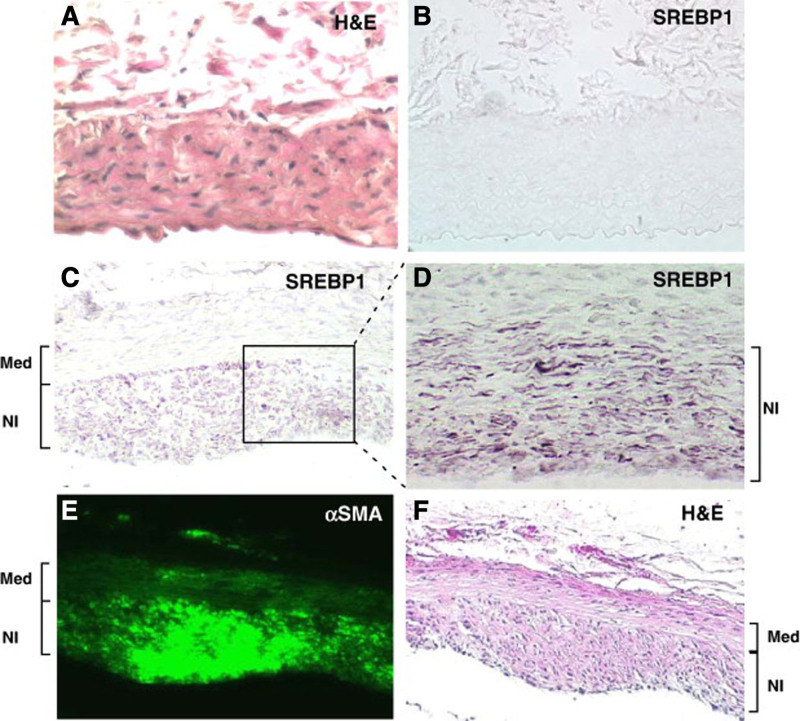
FIGURE 3.
SREBP-1 is increased in the neointima following stent placement. A stent was deployed in rat abdominal aortas for 4 weeks. Subsequently, vessels were harvested and analyzed by immunohistochemistry and immunofluorescence. Six μm sections were stained as described below. A, Control normal rat abdominal aorta stained with H&E reveals no neointima but a medial and adventitial (top) layer. B, Immunohistochemical staining for SREBP-1 in normal control untreated rat abdominal aorta is undetectable. C, Following stent placement for 4 weeks, a neointima layer is now visible and robust staining of SREBP-1 is detected. D, Higher magnification of SREBP-1 staining within the neointima. E, Immunofluorescence of FITC-anti-αSMA reveals robust staining within the neointima. F, H&E staining of the abdominal aorta following 4 weeks of stent placement. Reprinted with permission from Zhou et al.22 Copyright American College of Cardiology Foundation. All permission requests for this image should be made to the copyright holder. FITC indicates fluorescein isothiocyanate, H&E, hematoxylin and eosin; NI, neointima; SREBP1, sterol regulatory element-binding protein 1; αSMA, α-smooth muscle actin.
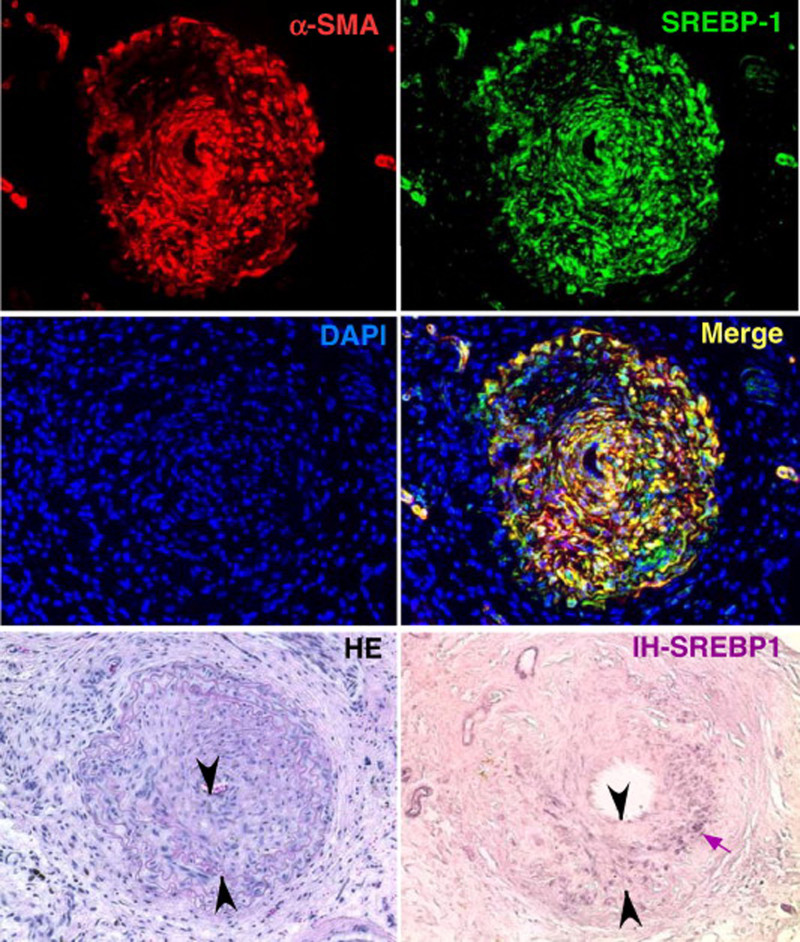
FIGURE 4.
SREBP-1 is increased in both the neointima and VSMC layer of mouse carotid arteries following vascular injury using a model of angioplasty. Four weeks after probe injury, carotid arteries were harvested and subjected to immunofluorescence or immunohistochemistry as indicated. Sections of 6 μm were stained with anti-SREBP-1 or Cy3-anti-α-SMA or stained using H&E. Nuclei were stained with DAPI in blue. Merge image of SREBP-1 (green) and α-SMA (red) is shown in yellow. SREBP-1 detected using immunohistochemistry is represented in purple and indicated by the small arrow. Neointima is indicated as the area between arrowheads in the H&E-stained and immunohistochemistry sections. In uninjured arteries from the same animals, SREBP-1 was barely detected under the same conditions by both methods (data not shown). Reprinted with permission from Zhou et al.22 Copyright American College of Cardiology Foundation. All permission requests for this image should be made to the copyright holder. DAPI indicates 4',6-diamidino-2-phenylindole; H&E, hematoxylin and eosin; IH-SREBP-1, immunohistochemistry for SREBP-1; SREBP1, sterol regulatory element-binding protein 1; VSMC, vascular smooth muscle cell; αSMA, α-smooth muscle actin.
The hyperactive cellular cholesterol biosynthesis in vascular wall, here we label as vascular hypercholesterolism, in response to the proinflammatory and mitogenic milieu created by circulating or locally produced factors could play a crucial “pleiotropic” role beyond cholesterol itself in the pathogenesis of ASCVD. Given that the vascular cells are not secretory cells for cholesterol, it is conceivable that vascular hypercholesterolism would not influence the blood level of cholesterol. The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) trial is thus far the only positive trial showing that directly targeting inflammation reduces cardiovascular events without affecting blood LDL-C level.35 In the CANTOS trial, IL-1β inhibition by canakinumab administration in addition to optimal medical treatment in patients with a history of MI and levels of hs-CRP greater than 2 mg/L led to reduction of hs-CRP levels without affecting the lipid levels and resulted in a 15% reduction in the risk of the primary composite end point of nonfatal MI, nonfatal stroke, or cardiovascular death, as compared with placebo-treated patients.35 Further analysis showed that in those patients who achieved lower on-treatment hs-CRP concentrations (<2 mg/L), cardiovascular mortality and all-cause mortality were significantly reduced by 31%, whereas these benefits were not observed in those with a hs-CRP concentrations of 2 mg/L or above.36 Based on the CANTOS trial data, it was found that reduction of the cardiovascular events and total mortality was associated with IL-6 blood level reduction independent of blood lipid level.37 Of note, IL-1β is among the factors known to activate SREBP and upregulate cholesterol biosynthesis in VSMC.23 Sirolimus, an immunosuppressant used in renal transplant recipients and in the first generation drug-eluting stents to minimize intimal hyperplasia and reduce repeat revascularization, can inhibit IL-1β–induced SREBP activation and thus endogenous cholesterol synthesis in human VSMC.23
CONCLUSIONS
Statins are currently the best-proven cholesterol-lowering medications to improve the clinical outcomes of the patients with ASCVD and their unique mechanism of action suggests that endogenous cholesterol biosynthesis MVA pathway plays an important role in ASCVD. MVA pathway is tightly regulated ubiquitously in all cell types by the master gene, SREBP, for cellular cholesterol homeostasis. SREBP is overexpressed in vascular wall in response to injury or stent implantation and SREBP can be activated in vascular cells, in response to proinflammatory and mitogenic factors causing hyperactive MVA pathway activity, here we termed vascular hypercholesterolism, as a new concept. SREBP functions as a crosslink platform bridging inflammation, cholesterol biosynthesis, and cell mobilization in vascular wall. Vascular hypercholesterolism through pleiotropic molecules promotes vascular cell mobilization and further proinflammatory and mitogenic factors production, forming a self-perpetuating mechanism to participate in the pathogenesis of atherosclerosis or vasculopathy (Figs. 1 and 2); therefore, targeting vascular hypercholesterolism to break the self-perpetuating mechanism could become a new avenue in the prevention and treatment of ASCVD.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
REFERENCES
- 1.Yeganeh B, Wiechec E, Ande SR, et al. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol Ther. 2014;143:87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs WJ, Olivier LM, Krisans SK. Central role of peroxisomes in isoprenoid biosynthesis. Prog Lipid Res. 2002;41:369–391. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. [DOI] [PubMed] [Google Scholar]
- 5.Schoenheimer R, Breusch F. Synthesis and destruction of cholesterol in the organism. J Biol Chem. 1933;103:439–448. [Google Scholar]
- 6.Shimomura I, Bashmakov Y, Shimano H, et al. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci U S A. 1997;94:12354–12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould RG, Taylor CB, Hagerman JS, et al. Cholesterol metabolism. I. Effect of dietary cholesterol on the synthesis of cholesterol in dog tissue in vitro. J Biol Chem. 1953;201:519–528. [PubMed] [Google Scholar]
- 8.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 9.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama C, Wang X, Briggs MR, et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 11.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. [DOI] [PubMed] [Google Scholar]
- 12.Horton JD, Shah NA, Warrington JA, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 15.Zhou RH, Shi Q, Gao HQ, et al. Changes in serum interleukin-8 and interleukin-12 levels in patients with ischemic heart disease in a Chinese population. J Atheroscler Thromb. 2001;8:30–32. [DOI] [PubMed] [Google Scholar]
- 16.Fanola CL, Morrow DA, Cannon CP, et al. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) trial. J Am Heart Assoc. 2017;6:e005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Held C, White HD, Stewart RAH, et al. ; STABILITY Investigators. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J Am Heart Assoc. 2017;6:e005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escárcega RO, Lipinski MJ, García-Carrasco M, et al. Inflammation and atherosclerosis: cardiovascular evaluation in patients with autoimmune diseases. Autoimmun Rev. 2018;17:703–708. [DOI] [PubMed] [Google Scholar]
- 19.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- 20.Yao M, Zhou RH, Petreaca M, et al. Activation of sterol regulatory element-binding proteins (SREBPs) is critical in IL-8-induced angiogenesis. J Leukoc Biol. 2006;80:608–620. [DOI] [PubMed] [Google Scholar]
- 21.Zhou RH, Yao M, Lee TS, et al. Vascular endothelial growth factor activation of sterol regulatory element binding protein: a potential role in angiogenesis. Circ Res. 2004;95:471–478. [DOI] [PubMed] [Google Scholar]
- 22.Zhou RH, Pesant S, Cohn HI, et al. Enhanced sterol response element-binding protein in postintervention restenotic blood vessels plays an important role in vascular smooth muscle proliferation. Life Sci. 2008;82:174–181. [DOI] [PubMed] [Google Scholar]
- 23.Ma KL, Varghese Z, Ku Y, et al. Sirolimus inhibits endogenous cholesterol synthesis induced by inflammatory stress in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2010;298:H1646–H1651. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanisms of insulin-induced angiogenesis. J Cell Mol Med. 2009;13:4492–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasanji A, Ghosh PK, Graham LM, et al. Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell. 2004;6:29–41. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh PK, Vasanji A, Murugesan G, et al. Membrane microviscosity regulates endothelial cell motility. Nat Cell Biol. 2002;4:894–900. [DOI] [PubMed] [Google Scholar]
- 27.Lamarche N, Tapon N, Stowers L, et al. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. [DOI] [PubMed] [Google Scholar]
- 28.Mackay DJ, Esch F, Furthmayr H, et al. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strassheim D, Gerasimovskaya E, Irwin D, et al. RhoGTPase in vascular disease. Cells. 2019;8:E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Cresswell N, Tavora F, et al. In-stent restenosis is associated with neointimal angiogenesis and macrophage infiltrates. Pathol Res Pract. 2014;210:1026–1030. [DOI] [PubMed] [Google Scholar]
- 31.Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. [DOI] [PubMed] [Google Scholar]
- 32.Helkin A, Stein JJ, Lin S, et al. Dyslipidemia part 1–review of lipid metabolism and vascular cell physiology. Vasc Endovascular Surg. 2016;50:107–118. [DOI] [PubMed] [Google Scholar]
- 33.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tousoulis D, Psarros C, Demosthenous M, et al. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol. 2014;63:2491–2502. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, MacFadyen JG, Everett BM, et al. ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]