Abstract
3-Methoxybenzamide (3-MBA), which is known to be an inhibitor of ADP-ribosyltransferase, inhibits cell division in Bacillus subtilis, leading to filamentation and eventually lysis of cells. Our genetic analysis of 3-MBA-resistant mutants indicated that the primary target of the drug is the cell division system involving FtsZ function during both vegetative growth and sporulation.
ADP-ribosylation is one of the mechanisms of posttranslational regulation of proteins by which the ADP-ribose moiety of NAD+ is covalently bound to target proteins by ADP-ribosyltransferase (ADPRT) (13, 26). The well-known ADPRTs are bacterial toxins that catalyze the ADP-ribosylation of GTP-binding proteins within mammalian cells (23). Recently, it has been reported that endogenous ADP-ribosylation plays an important role in cell differentiation in Streptomyces species (19, 20, 22). Furthermore, Huh et al. (8) have reported that the profile of ADP-ribosylated proteins changes markedly during sporulation in Bacillus subtilis. These workers have also isolated mutants that can grow in the presence of the benzamide derivative 3-methoxybenzamide (3-MBA), which is the most commonly used inhibitor of ADPRTs (25, 26). In the present study, we demonstrated that the lethal effect of 3-MBA can be suppressed by a mutation in the ftsZ gene of B. subtilis. The physiological effects of 3-MBA on cell growth and sporulation were also examined by using wild-type and ftsZ mutant strains.
3-MBA inhibits cell division, leading to filamentation and lysis.
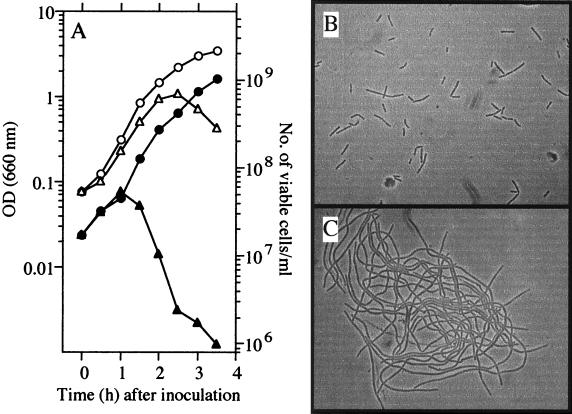
UOT1285 (trpC2 lys-1 nprR2 nprE18 aprEΔ3), which has been used as the wild-type strain in our laboratory, could not grow on Luria-Bertani (LB) plates containing more than 5 mM 3-MBA (Table 1). We monitored the effects of the drug on the kinetics of growth and cell morphology in LB liquid medium containing 10 mM 3-MBA. As shown in Fig. 1A, the optical density at 660 nm (OD660) of a culture of UOT1285 increased more than 10-fold during the initial 2 h of growth at 37°C and then rapidly decreased, regardless of the cell density at inoculation (data not shown). The number of viable cells (CFU) in the culture is also shown in Fig. 1A. Whereas the OD660 increased until 2 h after inoculation into LB medium containing 10 mM 3-MBA, the number of viable cells increased until 1 h after inoculation and then rapidly decreased, indicating that colony-forming ability was lost 1 h before cell lysis occurred. These results suggest that cell growth in the presence of 3-MBA resulted in a filamentous morphology. This was ascertained by microscopic observation (Fig. 1B and C). These results indicate that 3-MBA had an ability to inhibit cell division, resulting in filamentation of cells during vegetative growth.
TABLE 1.
Growth characteristics of the ftsZ mutant strains in the presence of various concentrations of 3-MBAa
| Strain | Relevant genotype | Growth at 3-MBA concn (mM)
|
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 20 | 30 | ||
| UOT1285 | ftsZ+ | + | + | − | − | − | − |
| RIK7 | ftsZ7 (brgA1) | − | − | − | − | + | + |
| RIK8 | ftsZ8 (spb-1) | + | + | + | + | + | + |
| RIK9 | ftsZ7 ftsZ8 (brgA1 spb-1) | + | + | + | + | + | + |
Strains were grown on LB plates at 37°C.
FIG. 1.
Effect of 3-MBA on vegetative cell division. UOT1285 cells were grown in LB medium with or without 10 mM 3-MBA at 37°C. (A) OD660 (open symbols) and number of viable cells per milliliter (closed symbols). The number of viable cells is shown as the number of CFU per milliliter on an LB plate. Circles and triangles show results for cultivation without and with 10 mM 3-MBA, respectively. (B and C) Effect of 3-MBA on cell morphology. Cells grown in the absence (B) or presence (C) of 3-MBA until 2 h after inoculation were photographed under light microscopy at a magnification of ×600.
ftsZ mutation elicits cell growth in the presence of 3-MBA.
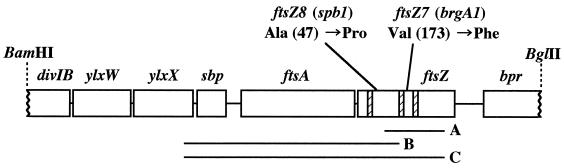
The brgA1 mutation was introduced by transformation into UOT1285 by using chromosomal DNA extracted from strain 168-1, which has been reported by Huh et al. to be resistant to 3-MBA (8). We found that the resulting transformant, RIK7 (trpC2 lys-1 nprR2 nprE18 aprEΔ3 brgA1), cannot grow on LB plates without 3-MBA but can grow in the presence of 3-MBA at a concentration of 20 mM or more (Table 1), indicating that brgA1 was a 3-MBA-dependent mutation. In LB liquid medium containing 3-MBA at a concentration of 10 mM or less, the OD660 of the RIK7 culture began to decrease 2 h after inoculation and the cells showed a filamentous morphology (data not shown). To facilitate further studies of this mutant, we isolated several spontaneous suppressor mutants (spb; suppressor of brgA1) which can grow on LB medium with or without 3-MBA. RIK7 cells grown on LB plates containing 35 mM 3-MBA at 30°C overnight were inoculated into LB medium at an OD660 of 0.1, incubated overnight at 37°C with shaking, and then spread onto LB plates. After incubation at 37°C overnight, 10 colonies appeared, all of which could grow on LB plates containing 35 mM 3-MBA; these were further analyzed. One of these suppressor mutations (designated spb-1) was found to be cotransformed with the brgA1 marker at a frequency of 94%, suggesting that spb-1 is an intracistronic suppressor mutation. During our genetic analysis, we found that 2% of 3-MBA-resistant transformants formed smaller colonies than the brgA1 mutant on LB plates containing 35 mM 3-MBA, suggesting that these transformants carry an spb-1 mutation alone. We have cloned the drug resistance determinant on the chromosome of the suppressed mutant, RIK9 (trpC2 lys-1 nprR2 nprE18 aprEΔ3 brgA1 spb-1), as described in the legend to Fig. 2. Two mutations were detected within the coding region of the ftsZ gene which would result in amino acid substitutions of Pro for Ala at position 47 (A47P) and of Phe for Val at position 173 (V173F). To determine which mutation was brgA1 or spb-1, we constructed strains carrying each mutation by transformation with PCR products (Fig. 2). The strain having the V173F mutation (ftsZ7) did not grow on LB plates but grew in the presence of 20 mM 3-MBA (Table 1). On the other hand, the strain having both mutations (ftsZ7 and ftsZ8) grew and showed normal morphology (data not shown) regardless of the presence or absence of the drug. The mutations brgA1 and spb-1 were therefore renamed ftsZ7 and ftsZ8, respectively (Fig. 2). As expected from the result obtained above, cells carrying ftsZ8 (spb-1) alone exhibited a 3-MBA-resistant phenotype (Table 1) and formed shorter filaments than UOT1285 in liquid medium with 10 mM 3-MBA (data not shown). ftsZ is an essential gene for cell division or cytokinesis in Escherichia coli and B. subtilis, and it is believed that the FtsZ protein forms a ring structure (the Z ring) at the cell division site (1, 3, 6, 11, 14). Moreover, FtsZ shows tubulin-like properties, including a GTPase activity and an ability to polymerize in vitro (5, 16, 21, 28). Since one of two mutation sites, A47P (ftsZ8), is located next to an Asp residue that has been shown to participate in GTPase activity in E. coli (27) and is believed to be within the active center of FtsZ in Methanococcus jannaschii (12), it would affect the structure of active site allowing the cell to tolerate 3-MBA. Polymerization and ring formation of FtsZ are regulated in a GTP-dependent manner (4, 7, 17, 18). It is therefore possible that 3-MBA changes directly or indirectly the ability of FtsZ to bind or hydrolyze the guanine nucleotide, ultimately leading to the inhibition of Z-ring formation.
FIG. 2.
ftsZ mutation sites of 3-MBA-resistant strains. To define the mutation sites, a 13-kb BamHI fragment of the RIK9 (ftsZ7 ftsZ8) chromosome was cloned into a λEMBL vector (Stratagene). The fragment containing the mutations was judged by its brgA1 transformation activity. In all transformation experiments, the 3-MBA-resistant transformants were selected on LB plates containing 35 mM 3-MBA. A 6-kb BamHI-BglII fragment within the 13-kb fragment showed transformation activity and significant linkage with the spoIIG gene. The 6-kb region was assigned to the divIB-bpr region by reference to the complete sequence of B. subtilis (10). The mutation sites were assigned to the ftsZ gene as judged by the transformation activity of fragments which were prepared by digestion with various restriction enzymes and then sequenced. To characterize the two mutations, three DNA fragments, A, B, and C, were prepared by PCR with primers MBA-2 (5′-CGGCGCAAAGCTGACTAGAGG-3′) and MBA-4 (5′-GCTTCGTCACGTCCTTCTCTTG-3′), primers MBA-1 (5′-CGTACAACAGCTGAAACTGC-3′) and MBA-3 (5′-CAAGGATACGGTCGTTCGGG-3′), and primers MBA-1 and MBA-4, respectively. Three strains, RIK7, RIK8, and RIK9, were constructed by transformation of the parental strain UOT1285 with fragments A, B, and C, respectively. Since RIK8 formed smaller colonies than RIK9 cells regardless of the 3-MBA concentration, strain RIK9 was judged by the colony morphology of transformants. Hatched rectangles indicate the positions corresponding to conserved amino acid residues among bacterial FtsZ proteins which participate in GTPase activity (27).
Sporulation of 3-MBA-resistant mutants.
Septation during sporulation differs from septation during vegetative growth in occurring at an asymmetric position in the cell. ftsZ plays an essential role during both cell division events (2). Sporulation of three ftsZ mutants, RIK7 (ftsZ7), RIK8 (ftsZ8), and RIK9 (ftsZ7 ftsZ8), was examined, and the results are shown in Table 2. RIK8 as well as RIK9 sporulated, producing 107 spores per ml, in the absence of the drug. On the other hand, both RIK8 and the 3-MBA-dependent mutant, RIK7, were extremely unstable genetically through the sporulation-germination process in the presence of the drug (see footnote c of Table 2). However, 3-MBA did not affect sporulation of double mutant RIK9. These results indicated that the ftsZ7 and ftsZ8 mutations cooperatively suppressed the pressure of 3-MBA during the sporulation-germination process.
TABLE 2.
Sporulation of ftsZ mutantsa
| Strain | Relevant genotype | 3-MBA concn (mM) | No. of spores/mlb |
|---|---|---|---|
| UOT1285 | ftsZ+ | 0 | 7.3 × 108 |
| RIK7 | ftsZ7 | 20 | 5.9 × 105c |
| RIK8 | ftsZ8 | 0 | 2.4 × 107 |
| 10 | 7.6 × 107c | ||
| RIK9 | ftsZ7 ftsZ8 | 0 | 1.4 × 107 |
| 10 | 1.5 × 107 |
Cells were incubated at 37°C in 2× SG medium containing 0.1% glucose with the indicated concentrations of 3-MBA until 24 h after the end of logarithmic growth.
Number of CFU per milliliter after heating at 80°C for 10 min.
Colony morphology generated from the heat-resistant spores was clearly distinguishable from those from the original ftsZ mutants (RIK7 or RIK8), indicating that the spores formed from these ftsZ mutants in the presence of 3-MBA should have had an additional mutation which could suppress sporulation deficiency.
3-MBA inhibits asymmetric septation.
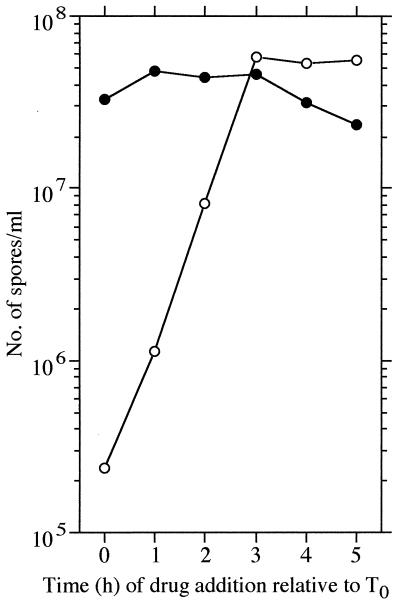
To determine whether 3-MBA affects asymmetric septation for sporulation, we examined the effects of 3-MBA during sporulation. UOT1285 cells were grown in 2× SG medium, and 3-MBA was added at a final concentration of 10 mM at the times indicated in Fig. 3. Sporulation was drastically inhibited by 3-MBA when added before 3 h after the end of vegetative growth (T3) but was slightly affected when added after T3. In the case of the RIK9 strain, sporulation was unaffected regardless of the time of drug addition. These results clearly indicate that 3-MBA affects sporulation events that precede T3.
FIG. 3.
Effect of 3-MBA on sporulation of wild-type and ftsZ mutant strains. Cells were grown in 2× SG medium containing 0.1% glucose at 37°C, and 10 mM 3-MBA (solid powder) was added to the medium at the indicated times after the end of vegetative growth (T0). The number of heat-resistant spores is shown as CFU per milliliter on LB plates. Open and closed symbols represent UOT1285 (ftsZ+) and RIK9 (ftsZ7 ftsZ8), respectively.
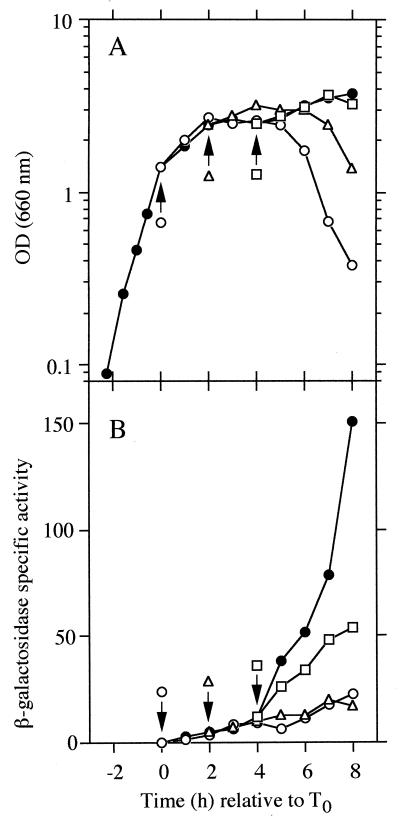
We next examined the effect of 3-MBA on spoIID expression by using a spoIID-lacZ fusion in UOT1285 (Fig. 4). Transcription of the spoIID gene has been shown to depend on ςE, which is processed and activated after completion of asymmetric septation (9, 24). In fact, spoIID expression is not induced in a strain in which expression of ftsZ is repressed (2). As Fig. 4 shows, the expression of spoIID was clearly inhibited by addition of 3-MBA until T2, indicating that ςE is not activated. These results indicate that 3-MBA affects asymmetric septation and are consistent with the previous work of Beall and Lutkenhaus (2) showing that spoIID expression depends on ftsZ expression. On the other hand, addition of the drug after T4 delayed spoIID expression. This result, in combination with the observation that sporulation of strain UOT1285 was affected partially by the addition of 3-MBA after T3 (see above and Fig. 3), indicates that 3-MBA also inhibits stages later than asymmetric septation.
FIG. 4.
Effect of 3-MBA on spoIID-lacZ expression. UOT1285 carrying plasmid pKZIID-1 (29) was grown in 2× SG medium containing 0.1% glucose and 5 μg of kanamycin per ml at 37°C. At the times indicated by the arrows, 3-MBA was added to the medium as described in the legend to Fig. 3. (A) Growth measured by the OD660. (B) Expression of spoIID-lacZ-directed β-galactosidase activity. Since most of cells eventually lysed (decreasing the OD660 values) when 3-MBA was added before T2, few developing cells were present after cell lysis occurred. Hence, 1 U of the β-galactosidase activity in the medium was expressed as the A420 per milliliter per minute × 1,000 (A420 is absorbance at 420 nm). Aliquots of 0.2 ml were withdrawn from the medium at the various times, mixed with 0.3 ml of 5/3× Z buffer, and treated with a few drops of toluene. Subsequent steps for assay of the β-galactosidase activity were carried out as described previously (15). Closed circles in both panels indicate the controls (for which no drug was added).
In summary, the present study indicates that 3-MBA affects septation via the FtsZ system during both vegetative growth and sporulation. Since 3-MBA is known to be an inhibitor of ADPRT, it is possible that ADP-ribosylation of FtsZ protein occurs, as is the case for GTP-binding proteins within mammalian cells (23). It is, however, not clear whether 3-MBA inhibits the FtsZ function directly or indirectly, since we do not have at present direct evidence for ADP-ribosylation of FtsZ.
Acknowledgments
We are grateful to Roy H. Doi and Jeffery Errington for helpful discussion and critical reading of the manuscript.
This work is supported in part by a grant from the Joint Basic Research Cooperation (Japan).
REFERENCES
- 1.Beall B, Lowe M, Lutkenhaus J. Cloning and characterization of Bacillus subtilis homologs of Escherichia coli cell division genes ftsZ and ftsA. J Bacteriol. 1988;170:4855–4864. doi: 10.1128/jb.170.10.4855-4864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 3.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 4.Bramhill D, Thompson C M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci USA. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 6.Erickson H P. FtsZ, a tubulin homologue in prokaryote cell division. Trends Cell Biol. 1997;7:362–367. doi: 10.1016/S0962-8924(97)01108-2. [DOI] [PubMed] [Google Scholar]
- 7.Erickson H P, Taylor D W, Taylor K A, Bramhill D. Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc Natl Acad Sci USA. 1996;93:519–523. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh J-W, Shima J, Ochi K. ADP-ribosylation of proteins in Bacillus subtilis and its possible importance in sporulation. J Bacteriol. 1996;178:4935–4941. doi: 10.1128/jb.178.16.4935-4941.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonas R M, Weaver E A, Kenney T J, Moran C P, Jr, Haldenwang W G. The Bacillus subtilis spoIIG operon encodes both ςE and a gene necessary for ςE activation. J Bacteriol. 1988;170:507–511. doi: 10.1128/jb.170.2.507-511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunst F, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 11.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 12.Löwe J, Amos L A. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 13.Lowery R G, Ludden P W. Endogenous ADP ribosylation in procaryotes. In: Moss J, Vaughan M, editors. ADP-ribosylating toxins and G proteins: insights into signal transduction. Washington, D.C: American Society for Microbiology; 1990. pp. 459–477. [Google Scholar]
- 14.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z-ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 16.Mukherjee A, Dai K, Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochi K, Penyige A, Barabas G. The possible role of ADP-ribosylation in sporulation and streptomycin production by Streptomyces griseus. J Gen Microbiol. 1992;138:1745–1750. doi: 10.1099/00221287-138-8-1745. [DOI] [PubMed] [Google Scholar]
- 20.Penyige A, Saido-Sakanaka H, Ochi K. Endogenous ADP-ribosylation of proteins during development of Streptomyces griseus. Actinomycetologica. 1996;10:98–103. [Google Scholar]
- 21.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 22.Shima J, Penyige A, Ochi K. Changes in patterns of ADP-ribosylated proteins during differentiation of Streptomyces coelicolor A3(2) and its developmental mutants. J Bacteriol. 1996;178:3785–3790. doi: 10.1128/jb.178.13.3785-3790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stragier P, Bonamy C, Karmazyn-Campeli C. Processing of a sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988;52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 25.Ueda K. Poly(ADP-ribose) synthetase. In: Moss J, Vaughan M, editors. ADP-ribosylating toxins and G proteins: insight into signal transduction. Washington, D.C: American Society for Microbiology; 1990. pp. 525–542. [Google Scholar]
- 26.Ueda K, Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Huang J, Mukherjee A, Cao C, Lutkenhaus J. Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J Bacteriol. 1997;179:5551–5559. doi: 10.1128/jb.179.17.5551-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Lutkenhaus J. The FtsZ protein of Bacillus subtilis is localized at the division site and has GTPase activity that is dependent upon FtsZ concentration. Mol Microbiol. 1993;9:435–442. doi: 10.1111/j.1365-2958.1993.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K. Ph.D. thesis. Hiroshima, Japan: Hiroshima University; 1989. [Google Scholar]