Abstract
Sargassum hemiphyllum is a common plant found on the coasts of Taiwan; it has been used as an anti-inflammatory agent in traditional herbal medicine. This study aimed to evaluate the anti-inflammatory effects of S. hemiphyllum sulfated polysaccharide (SHSP) using two different mouse models. In both arachidonic acid-induced ear inflammatory gavage and paint models, SHSP decreased ear swelling and erythema. In addition, SHSP decreased the production of myeloperoxidase, nitric oxide, interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α in a dose-dependent manner. Histological examination results showed that SHSP reduced the area of neutrophilic infiltration in inflamed ears. The anti-inflammatory activity of SHSP has already been demonstrated in vitro. In this study, SHSP extracted from the same species of brown seaweed exhibited anti-inflammatory activity in both oral and topical applications in vivo. Therefore, SHSP may play a role in the treatment of inflammatory diseases.
Keywords: animal model, anti-inflammation, cytokines, myeloperoxidase, Sargassum hemiphyllum
1. Introduction
Inflammation is a physical response that protects against injury, infection, and stress through multiple mechanisms. Inflammation can be classified into two separate conditions, namely, acute inflammation and chronic inflammation. Acute inflammation is the initial response to stress that brings about rapid and short-term immune responses [1]. Chronic inflammation is initiated by an inappropriate or excessive acute inflammatory immune response and is a symptom of various pathological conditions [2]. It is well-known that inflammation can facilitate tumor progression [3], increase the risk of atherosclerosis [4], cause lesions of Alzheimer’s disease [5], and promote insulin resistance and diabetes [6]. In such diseases, inflammation is a primary cause, and therefore, treatment of the inflammatory condition may be an effective therapeutic approach.
Prior to the 1950s, seaweeds were used as traditional and folk medicines [7]. However, biologically active compounds from brown seaweed were not discovered until the 1990s. Previous studies have shown that extracts from the brown seaweeds Dictyota dichotoma [8], Ecklonia cava [9,10], Fucus vesiculosus [11], Sargassum hemiphyllum [12,13], Sargassum vulgare [14], and Eisenia bicyclis [15] exhibit anti-inflammatory activity in vitro. Moreover, extracts from the brown seaweeds Laminaria saccharina, Laminaria digitata, Cladosiphon okamuranus, Fucus evanescens, F. vesiculosus, Fucus serratus, Fucus distichus, Fucus spiralis, Ascophyllum nodosum [16], Sargassum fulvellum, Sargassum thunbergii [17], Sargassum wightii [18], Turbinaria ornata [19], Lobophora variegata [20], E. cava [21], and Padina tetrastromatica [22] also possess powerful anti-inflammatory activities in vivo. In two separate studies that used different extraction processes, E. cava elicited anti-inflammatory activity (separately) in vitro and in vivo; however, there is no evidence in previous reports indicating that single brown seaweed extract has anti-inflammatory activity in both in vitro and in vivo.
The brown seaweed S. hemiphyllum is commonly found in east Asian coastlines and its extracts (obtained by boiling the seaweed in water) are used as a traditional herbal anti-inflammatory medicine. Our previous study demonstrated that S. hemiphyllum sulfated polysaccharide (SHSP), which is extracted using hot water, is central to the anti-inflammatory activity of the plant. Inside the cell, SHSP causes decreased cytosolic and nuclear expression of nuclear factor-κB (NF-κB) p65, thereby inhibiting cytokine secretions of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), and the nitric oxide (NO) and messenger RNA expression of IL-β, inducible NO synthase, and cyclooxygenase-2. It was observed in vitro that the inhibitory effect of SHSP is through the NF-κB signaling pathway [13]. However, because in vitro studies are conducted in simulated organic conditions and are not in the living organism, their success is limited. To verify these observations in vivo, we determined the anti-inflammatory activity of SHSP using two arachidonic acid (AA)-induced ear inflammation animal models.
2. Materials and methods
2.1. Preparation of SHSP
SHSP was extracted and isolated as described earlier [13]. In brief, the dried S. hemiphyllum powder (100 g) was mixed with 5 L of distilled water and boiled at 100°C for 30 minutes. The hot-water extract was centrifuged at 10,000g for 20 minutes and the supernatant was lyophilized under reduced pressure. To the lyophilized hot-water extract, 4 volumes of 95% ethanol were added and the mixture was allowed to precipitate overnight at 4°C. The precipitated polysaccharides were collected by centrifugation and lyophilized. These lyophilized polysaccharides are the SHSP samples used in the experiments.
2.2. Chemical analysis
Sulfate content was determined according to the gelatin–barium method [23] using sodium sulfate (1 mg/mL) as standard and after acid hydrolysis of the polysaccharides (6N HCl, 100°C, 6 hours). Monosaccharide fractions of the polysaccharide extract hydrolysates were separated by high-performance anion-exchange chromatography (Dionex BioLC, Sunnyvale, CA, USA) with an anion-exchange column (CarboPac PA10, 4.6 × 250 mm; Dionex BioLC). The monosaccharide was analyzed at an isocratic NaOH concentration of 18 mM at ambient temperature [24]. The fucose and sulfate compositions of SHSP are 221.40 ± 0.42 μmol/g and 44.2% ± 3.6% (wt/wt), respectively.
2.3. Mice
Male BALB/c mice (8 weeks old, weight, 20–25 g) were purchased from the National Taiwan University College of Medicine Laboratory Animal Center (Taipei, Taiwan). All animals aged 12 weeks at the start of the experiment and were housed in a normal, environmentally controlled animal room with free access to pathogen-free feed and water ad libitum.
2.4. AA-induced ear inflammatory animal models
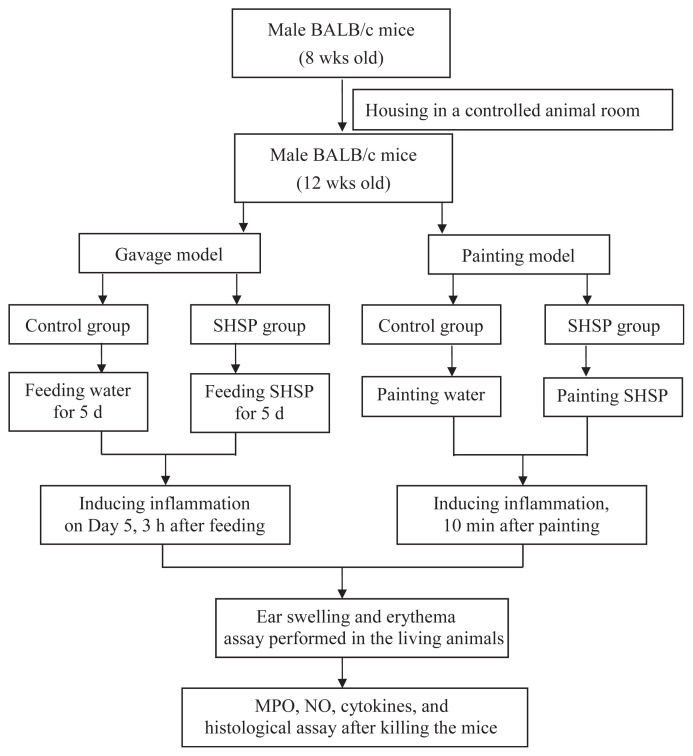
The flowchart of AA-induced ear inflammatory animal models used in this study is shown in Fig. 1.
Fig. 1.
Flowchart of arachidonic acid (AA)-induced ear inflammatory animal models. MPO = myeloperoxidase; NO = nitric oxide; SHSP = Sargassum hemiphyllum sulfated polysaccharide.
2.4.1. Gavage model
The mice were given 500 μL of SHSP at doses of 20 mg/kg, 40 mg/kg, and 80 mg/kg body weight (BW) for 5 consecutive days (Day 1–5). Inflammation was induced on Day 5 by AA administration (2 mg AA dissolved in 5 μL acetone), 3 hours after SHSP gavage. AA was applied to both the anterior and posterior surfaces of the right ear of mice. Mice in the control group received the same volume of vehicle (500 μL) using the same schedule as that followed for the SHSP group. There were six mice in each group.
2.4.2. Painting model
Both the anterior and posterior surfaces of the right ear of mice were painted with 500 μL of SHSP (doses: 1 mg/mL, 1.5 mg/mL, and 3 mg/mL). Inflammation was induced by AA injection, 10 minutes after painting with SHSP. Mice in the control group were painted with the same volume of vehicle (500 μL) using the same schedule as that followed for the SHSP group. There were six mice in each group.
2.5. Ear swelling assay
The ear swelling was measured prior to the experiment and at 1-, 3-, 6-, and 24-hour time points after AA treatment. The ear thickness was measured using a micrometer (Model 293-561; Mitutoyo Digimatic, Veenendaal, The Netherlands) [25]. The ear swelling% is calculated as:
2.6. Ear erythema assay
The ear erythema was measured prior to the experiment and at 1-, 3-, 6-, and 24-hour time points after AA treatment. The ear erythema was measured using the tissue viability (TiVi) polarized light spectroscopy method (Tissue Viability Imager TiVi600; WheelsBridge AB, Linköping, Sweden) to detect ear red blood cell concentration (RBCconc). The measurement was carried in a dark-room environment at an ambient temperature of 21–23°C and a relative humidity of 30–40%. The distance between the camera and ear surface was 25 cm [26]. The ear erythema% is calculated as:
After the last measurement, the mice were sacrificed. The ears were collected for myeloperoxidase (MPO), NO, inflammatory cytokines, and histology assays.
2.7. MPO and NO assays
Ears were homogenized in 80 mM sodium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide for 45 seconds at 0°C, followed by centrifugation at 9000g for 20 minutes at 4°C. The supernatant was collected and stored at −80°C until MPO analysis. The concentration of MPO was determined using the InnoZyme Myeloperoxidase Activity enzyme-linked immunosorbent assay (ELISA) kit (Calbiochem, Darmstadt, Germany). NO was determined using the Griess method [27].
2.8. Cytokines assay
The ears were homogenized and dissolved in 1% Triton X-100 lysis buffer (250 μL; Sigma, Zwijndrecht, The Netherlands) and enzyme inhibitor mix (Roche Diagnostics, Almere, The Netherlands) in phosphate-buffered saline. The homogenates were centrifuged at 13,500 rpm for 20 minutes at 4°C. The supernatants were collected and stored at −80°C until cytokine analysis. The concentration of IL-1β, IL-6, and TNF-α was determined using ELISA kits (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
2.9. Histological assay
The ears were fixed with 10% (v/v) paraformaldehyde, dehydrated, and embedded in paraffin. For histological analysis, 4-mm sections of fixed embedded tissues were cut on a microtome (Model 2165 rotary microtome; Leica, Nußloch, Germany). The sections were then placed on glass slides, deparaffinized, and sequentially stained with hematoxylin and eosin (Richard-Allan Scientific, Kalamazoo, MI, USA).
2.10. Statistical analysis
The data were presented as mean ± standard deviation of three determinations. Significant between-group differences were determined by analysis of variance, and p < 0.05 was considered as statistically significant.
3. Results and discussion
In traditional herbal medicine formulations, both oral and topical applications are common. To investigate differences between these two modes of application, oral gavage and topical paint models were used; in addition, we also determined whether SHSP is effective as both oral and topical applications using these two models.
3.1. Effect of SHSP on AA-induced ear swelling
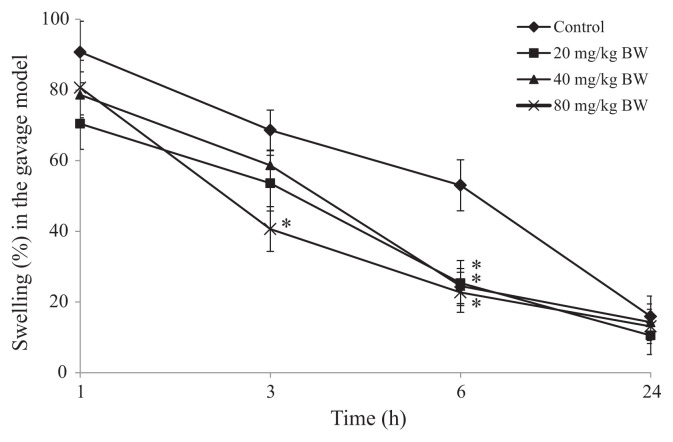
The AA metabolism pathway is one of the main mechanisms for the induction of inflammation. Therefore, AA was used to initiate inflammation in mouse ears. For the gavage model, SHSP was orally administered for 5 days. One hour after AA-induced inflammation, no significant difference between mice that received SHSP and the control group was noted. However, after 3 hours, treatment with SHSP administered at a dose of 80 mg/kg BW significantly inhibited ear swelling from 68.64% ± 5.65% (control group) to 40.64% ± 6.32%. In the majority of tested groups, inhibition of ear swelling was measured at the 6-hour time point. After 6 hours, SHSP administered at doses of 20 mg/kg, 40 mg/kg, and 80 mg/kg BW inhibited ear swelling from 53.03 ± 7.21% (control group) to 25.35 ± 6.37%, 24.52 ± 8.94%, and 22.72 ± 5.65%, respectively (Fig. 2). This result complied with the test results obtained using methanol extracts of Sargassum swartzii administered at doses of 175 mg/kg and 350 mg/kg BW [28] and water-soluble crude polysaccharide of T. ornata administered orally at a dose of 2.5–20 mg/kg BW [19], which showed inhibition of paw edema in carrageenan-induced rats.
Fig. 2.
Effect of Sargassum hemiphyllum sulfated polysaccharide (SHSP) on arachidonic acid-induced ear swelling in the gavage model. Vehicle (control), 20 mg/kg, 40 mg/kg, and 80 mg/kg body weight (BW) of SHSP were administered orally for 5 constitutive days. Results are shown as mean ± standard deviation (n = 6). *p < 0.05 compared with the control.
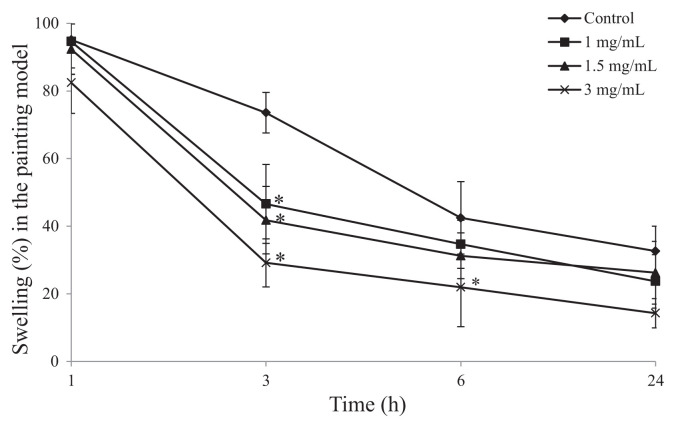
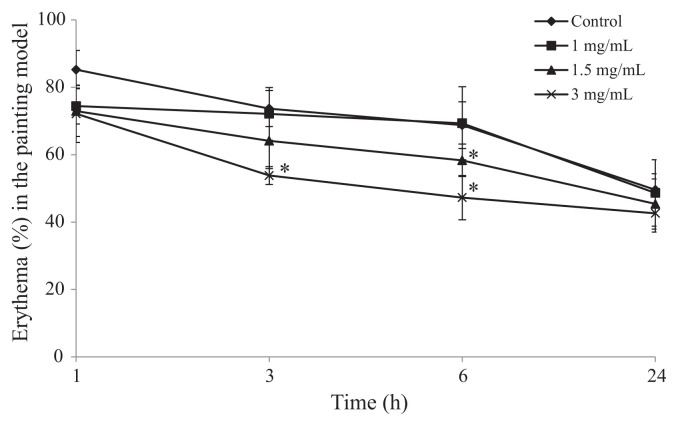
To investigate the effects of topical SHSP on epidermis, SHSP was painted on the right ear 10 minutes after the administration of AA. Compared with the control group mice, significant inhibition of ear swelling was observed after 3 hours. At this time after AA-induced inflammation, 1 mg/mL, 1.5 mg/mL, and 3 mg/mL of SHSP inhibited ear swelling in a dose-dependent manner from 73.56 ± 5.99% (control group) to 46.59 ± 11.67%, 41.78 ± 9.97%, and 29.16 ± 7.12%, respectively (Fig. 3). In further experiments, extracts of S. thunbergii, S. fulvellum [17], and Turbinaria conoides [29], which were obtained from boiling water, also inhibited ear edema and inflammation. The biological properties of these brown seaweed water extracts are quite similar to those of SHSP. These data, and those of previous studies, suggest that extraction by boiling in water effectively isolates an anti-inflammatory substance from S. hemiphyllum, and that SHSP may be the key active ingredient.
Fig. 3.
Effect of Sargassum hemiphyllum sulfated polysaccharide (SHSP) on arachidonic acid (AA)-induced ear swelling in the painting model. Vehicle (control), 1 mg/mL, 1.5 mg/mL, and 3 mg/mL of SHSP were painted after the application of AA. Results are shown as mean ± standard deviation (n = 6). *p < 0.05 compared with control.
3.2. Effect of SHSP on AA-induced ear erythema
Erythema is one of the major visible inflammatory symptoms on skin, which is influenced by the regional blood flow rate, the concentration of RBC, and the thickness and pigmentation of skin. The clinical symptoms of skin erythema are usually analyzed subjectively with the naked eye. However, when clinical symptoms need to be graded (quantified), methods of skin reflectance spectrophotometry [30], erythema meter, and laser Doppler flow meter [31] have been used. These methods are noninvasive and touch free and can be used to measure erythema in living animals without physical interference. In this study, we used TiVi polarized light spectroscopy to investigate the effect of SHSP on ear erythema.
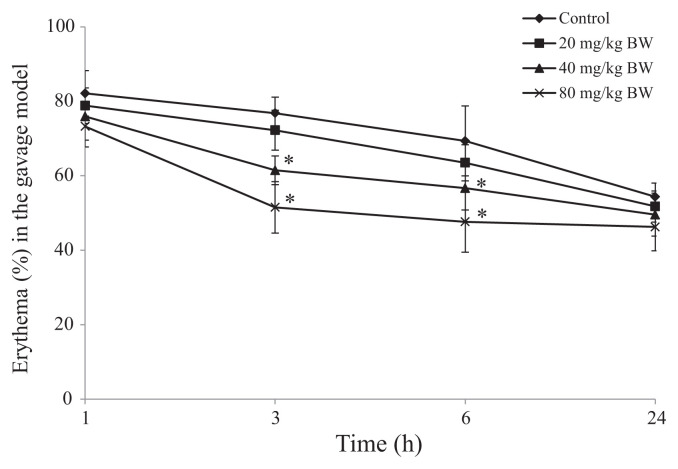
In the gavage model, SHSP administered at doses of 40 and 80 mg/kg BW inhibited erythema from 76.81 ± 4.31% (control group) to 61.49 ± 3.83% and 47.64 ± 6.94%, respectively, at the 3-hour time point. At the 6-hour time point, SHSP administered at doses of 40 mg/kg and 80 mg/kg BW also inhibited erythema from 69.35 ± 9.36% (control group) to 61.67 ± 5.84% and 51.52 ± 8.16%, respectively. However, no inhibition of erythema was observed when SHSP was administered at the dose of 20 mg/kg BW (Fig. 4). In the paint model, 3 mg/mL of SHSP inhibited erythema from 73.67 ± 5.35% (control group) to 63.81 ± 2.66% at the 3-hour time point, and 1.5 mg/mL of SHSP showed slight inhibition at the 6-hour time point; however, 1 mg/mL of SHSP had no effect on erythema (Fig. 5).
Fig. 4.
Effect of Sargassum hemiphyllum sulfated polysaccharide (SHSP) on arachidonic acid-induced ear erythema in the gavage model. Vehicle (control), 20 mg/kg, 40 mg/kg, and 80 mg/kg body weight (BW) of SHSP were administered orally for 5 constitutive days. Results are shown as mean ± standard deviation (n = 6). *p < 0.05 compared with control.
Fig. 5.
Effect of Sargassum hemiphyllum sulfated polysaccharide (SHSP) on arachidonic acid (AA)-induced ear erythema in the painting model. Vehicle (control), 1 mg/mL, 1.5 mg/mL, and 3 mg/mL of SHSP were painted after the application of AA. Results are shown as mean ± standard deviation (n = 6). *p < 0.05 compared with control.
Some types of inflammatory tissue injury are mediated by free radicals. These free radicals injure cells and tissues directly by causing oxidative degradation of essential cellular components and by exacerbating inflammation [32]. Antioxidants, such as sulfated polysaccharides from brown seaweed, protect against oxidant-mediated inflammation by scavenging free radicals. Indeed, the anti-inflammatory effect of sulfated polysaccharide is improved with the degree of sulfation [33]. Our previous study indicated that S. hemiphyllum contains compounds with potent antioxidant activity [34] and high sulfate content [13]. Thus, it was suggested that the effect of SHSP on animal models might be due to the presence of such compounds with high antioxidative activity and sulfate content.
3.3. Effect of SHSP on inflammatory MPO, NO, and cytokine levels in ears
The neutrophil leukocyte marker MPO is known to indicate chemotaxis of neutrophil leukocytes in inflamed tissues [35]. Elevated MPO release in the inflammation site is correlated with NO, which is derived from MPO [36]. Therefore, we analyzed the effect of SHSP on MPO and NO production. In the gavage model, MPO and NO were present in AA-induced inflamed tissues at levels of 34.22 ± 3.55 ng/mL and 29.35 ± 2.97 μM, respectively. Among mice treated with an SHSP dose of 80 mg/kg BW, MPO and NO levels decreased to 20.63 ± 2.49 ng/mL and 18.42 ± 3.38 μM, respectively. Treatment with SHSP at doses of 40 mg/kg and 80 mg/kg BW was more effective than at a dose of 20 mg/kg BW in causing inhibition of MPO and NO production. Moreover, SHSP showed an inhibitory effect on these molecules in the paint model. We also evaluated the production of the proinflammatory cytokines IL-1, IL-6, and TNF-α in inflamed ears. These data also demonstrate that SHSP inhibits IL-1, IL-6, and TNF-α production in both gavage and paint models (Table 1). The results comply with those from previous studies in which C. okamuranus ameliorated inflammation by inhibiting MPO, IL-4, IL-6, and IL-10 [37]. Therefore, the anti-inflammatory activity of SHSP can be attributed to suppression of the release of proinflammatory cytokines, low production of MPO and NO, and maybe inhibition of leukocyte influx [38].
Table 1.
Effect of SHSP on AA-induced cytokine production in the ear.
| Gavage model | Painting model | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Control | 20 | 40 | 80 | Control | 1 | 1.5 | 3 | |
|
|
|
|
|
|||||
| mg/kg BW | mg/mL | |||||||
| MPO (ng/mL) | 34.22 ± 3.55 | 32.40 ± 4.37 | 22.34 ± 4.16* | 20.63 ± 2.49* | 39.14 ± 3.14 | 36.84 ± 2.58 | 22.76 ± 1.94* | 18.76 ± 2.14* |
| NO (μM) | 29.35 ± 2.97 | 29.11 ± 3.41 | 20.58 ± 3.71* | 18.42 ± 3.38* | 30.13 ± 1.78 | 27.12 ± 2.56 | 19.94 ± 3.62* | 16.86 ± 3.23* |
| IL-1β (ng/mL) | 5.17 ± 0.23 | 5.72 ± 1.29 | 5.15 ± 1.01 | 3.21 ± 0.84* | 5.68 ± 0.88 | 5.41 ± 0.71 | 3.21 ± 0.84* | 2.01 ± 0.44* |
| IL-6 (ng/mL) | 0.92 ± 0.04 | 0.84 ± 0.10 | 0.62 ± 0.09* | 0.60 ± 0.10* | 0.95 ± 0.03 | 0.82 ± 0.19 | 0.85 ± 0.11 | 0.71 ± 0.02* |
| TNF-α (ng/mL) | 0.22 ± 0.04 | 0.21 ± 0.01 | 0.22 ± 0.03 | 0.11 ± 0.05* | 0.25 ± 0.02 | 0.24 ± 0.05 | 0.22 ± 0.04 | 0.23 ± 0.06 |
Results are shown as mean ± standard deviation (n = 6).
p < 0.05 compared with control.
AA = arachidonic acid; BW = body weight; IL = interleukin; MPO = myeloperoxidase; NO = nitric oxide; SHSP = Sargassum hemiphyllum sulfated polysaccharide; TNF-α = tumor necrosis factor-α.
3.4. Effect of SHSP on histological changes in ears
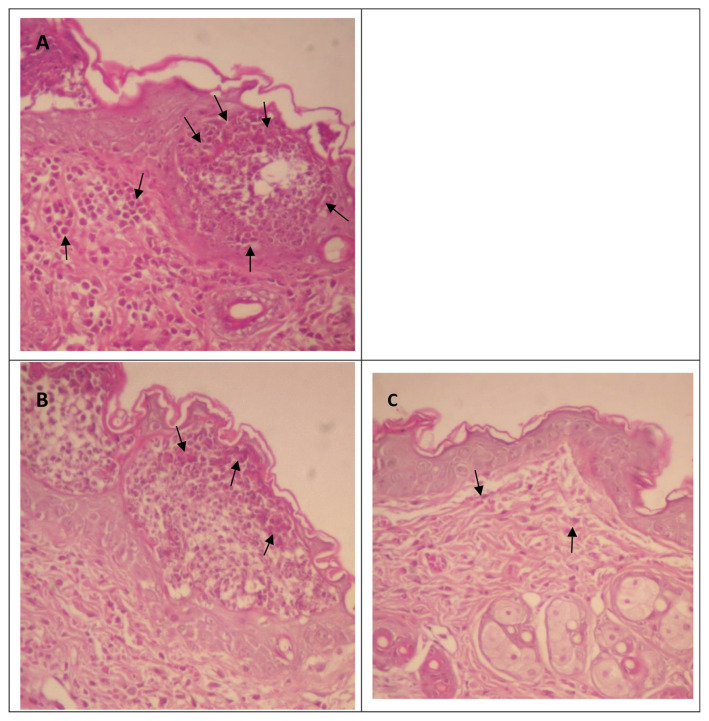
In the inflammatory process, neutrophils are the first type of leukocytes to infiltrate inflammatory sites, thereby the most common histological feature of inflammation [39]. Thus, we conducted a histological evaluation of AA-induced inflammatory neutrophilic infiltration (Fig. 6A). After oral administration of SHSP at a dose of 80 mg/kg BW or topical administration by painting with 3 mg/mL SHSP, neutrophilic infiltration was clearly decreased (Fig. 6B and C). Importantly, these histological data correspond with the observed dose–response relationship to ear swelling, erythema, and measurements of MPO, NO, and proinflammatory cytokines.
Fig. 6.
Effect of Sargassum hemiphyllum sulfated polysaccharide (SHSP) on histological changes as a result of arachidonic acid-induced inflammation. (A) Control, (B) 80 mg/kg body weight of SHSP in the gavage model, and (C) 3 mg/mL of SHSP in the painting model. Sections of the ears were stained by hematoxylin and eosin staining (200×). The arrows indicate neutrophilic infiltration.
In conclusion, SHSP is the major anti-inflammatory agent present in S. hemiphyllum preparations, and it acts by suppressing the production of inflammatory mediators. To our knowledge, this is the first study to demonstrate that SHSP, obtained from a brown seaweed using a single extraction process, has anti-inflammatory activity in an animal model and is effective in both oral and topical applications. Hence, SHSP may have promise in the treatment of inflammatory disease.
Acknowledgments
We would like to thank Enago (www.enago.tw) for the English language review. This research was kindly supported by grants from the Council of Agriculture, Executive Yuan, Taiwan, R.O.C.
Funding Statement
This research was kindly supported by grants from the Council of Agriculture, Executive Yuan, Taiwan, R.O.C.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Ulich TR, Yin SM, Guo KZ, del Castillo J, Eisenberg SP, Thompson RC. The intratracheal administration of endotoxin and cytokines. III. The interleukin-1 (IL-1) receptor antagonist inhibits endotoxin- and IL-1-induced acute inflammation. Am J Pathol. 1991;138:521–4. [PMC free article] [PubMed] [Google Scholar]
- 2. Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 3. Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 4. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5. McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 6. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lincoln RA, Strupinski K, Walker JM. Bioactive compounds from algae. Life Chem Rep. 1991;8:97–183. [Google Scholar]
- 8. Yoon WJ, Ham YM, Kim KN. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW264.7 cells. J Med Plants Res. 2009;3:1–8. [Google Scholar]
- 9. Kang S-M, Kim K-N, Lee S-H, Ahn G, Cha S-H, Kim A-D, Yang X-D, Kang M-C, Jeon Y-J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr Polym. 2011;85:80–5. [Google Scholar]
- 10. Lee S-H, Ko C-I, Ahn G, You SG, Kim J-S, Heu MS, Kim J, Jee Y, Jeon Y-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr Polym. 2012;89:599–606. doi: 10.1016/j.carbpol.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 11. Yang JW, Yoon SY, Oh SJ, Kim SK, Kang KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2006;346:345–50. doi: 10.1016/j.bbrc.2006.05.135. [DOI] [PubMed] [Google Scholar]
- 12. Na HJ, Moon PD, Ko SG, Lee HJ, Jung HA, Hong SH, Seo Y, Oh JM, Lee BH, Choi BW, Kim HM. Sargassum hemiphyllum inhibits atopic allergic reaction via the regulation of inflammatory mediators. J Pharmacol Sci. 2005;97:219–26. doi: 10.1254/jphs.fp0040326. [DOI] [PubMed] [Google Scholar]
- 13. Hwang PA, Chien SY, Chan YL, Lu MK, Wu CH, Kong ZL, Wu CJ. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J Agric Food Chem. 2011;59:2062–8. doi: 10.1021/jf1043647. [DOI] [PubMed] [Google Scholar]
- 14. Dore CM, das C, Faustino Alves MG, Will LS, Costa TG, Sabry DA, de Souza Rêgo LA, Accardo CM, Rocha HA, Filgueira LG, Leite EL. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym. 2013;91:467–75. doi: 10.1016/j.carbpol.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 15. Kang YM, Eom SH, Kim YM. Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ Toxicol Pharmacol. 2013;35:395–401. doi: 10.1016/j.etap.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16. Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI, Ustyuzhanina NE, Grachev AA, Sanderson CJ, Kelly M, Rabinovich GA, Iacobelli S, Nifantiev NE. Consorzio Interuniversitario Nazionale per la Bio-Oncologia, Italy. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–52. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 17. Kang JY, Khan MN, Park NH, Cho JY, Lee MC, Fujii H, Hong YK. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 2008;116:187–90. doi: 10.1016/j.jep.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 18. Sarithakumari CH, Renju GL, Kurup GM. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology. 2013;21:261–8. doi: 10.1007/s10787-012-0159-z. [DOI] [PubMed] [Google Scholar]
- 19. Ananthi S, Raghavendran HR, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga) Food Chem Toxicol. 2010;48:187–92. doi: 10.1016/j.fct.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 20. Medeiros VP, Queiroz KC, Cardoso ML, Monteiro GR, Oliveira FW, Chavante SF, Guimaraes LA, Rocha HA, Leite EL. Sulfated galactofucan from Lobophora variegata: anticoagulant and anti-inflammatory properties. Biochemistry (Mosc) 2008;73:1018–24. doi: 10.1134/s0006297908090095. [DOI] [PubMed] [Google Scholar]
- 21. Kim SK, Lee DY, Jung WK, Kim JH, Choi I, Park SG, Seo SK, Lee SW, Lee CM, Yea SS, Choi YH, Choi IW. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: role of suppressor of cytokine signaling. Biomed Pharmacother. 2008;62:289–96. doi: 10.1016/j.biopha.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22. Mohsin S, Kurup GM, Mahadevan R. Effect of ascophyllan from brown algae Padina tetrastromatica on inflammation and oxidative stress in carrageenan-induced rats. Inflammation. 2013;36:1268–78. doi: 10.1007/s10753-013-9665-4. [DOI] [PubMed] [Google Scholar]
- 23. Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1963;84:106–10. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee IH, Huang RL, Chen CT, Chen HC, Hsu WC, Lu MK. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol Lett. 2002;209:63–7. doi: 10.1111/j.1574-6968.2002.tb11110.x. [DOI] [PubMed] [Google Scholar]
- 25. Hartog A, Leenders I, van der Kraan PM, Garssen J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: evidence for synergistic activity. Int Immunopharmacol. 2007;7:1784–92. doi: 10.1016/j.intimp.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 26. O’Doherty J, Henricson J, Anderson C, Leahy MJ, Nilsson GE, Sjöberg F. Sub-epidermal imaging using polarized light spectroscopy for assessment of skin microcirculation. Skin Res Technol. 2007;13:472–84. doi: 10.1111/j.1600-0846.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 27. Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase pathways. Prostaglandins Leukot Essent Fatty Acids. 1996;54:229–38. doi: 10.1016/s0952-3278(96)90053-8. [DOI] [PubMed] [Google Scholar]
- 28. Hong DD, Hien HM, Anh HTL. Studies on the analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulata Forsskal (Chlorophyta) in experiment animal models. Afr J Biotechnol. 2011;10:2308–14. [Google Scholar]
- 29. Boonchum W, Peerapornpisal Y, Kanjanapothi D, Pekkoh J, Amornlerdpison D, Pumas C, Sangpaiboon P, Vacharapiyasophon P. Antimicrobial and anti-inflammatory properties of various seaweeds from the Gulf of Thailand. Int J Agric Biol. 2011;13:100–4. [Google Scholar]
- 30. Bjerring P, Andersen PH. Skin reflectance spectrophotometry. Photodermatol. 1987;4:167–71. [PubMed] [Google Scholar]
- 31. Lahti A, Kopola H, Harila A, Myllylä R, Hannuksela M. Assessment of skin erythema by eye, laser Doppler flowmeter, spectroradiometer, two-channel erythema meter and Minolta chroma meter. Arch Dermatol Res. 1993;285:278–82. doi: 10.1007/BF00371596. [DOI] [PubMed] [Google Scholar]
- 32. Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–7. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 33. Ji SL, Du HY, Chi YQ, Cui HF, Cao JC, Geng MY, Guan HS. Effects of dermatan sulfate derivatives on platelet surface P-selectin expression and protein C activity in blood of inflammatory bowel disease patients. World J Gastroenterol. 2004;10:3485–9. doi: 10.3748/wjg.v10.i23.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hwang P-A, Wu C-H, Gau S-Y, Chien S-Y, Hwang D-F. Antioxidant and immune-stimulating activities of hot-water extract from seaweed Sargassum hemiphyllum. J Mar Sci Tech. 2010;18:41–6. [Google Scholar]
- 35. Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–86. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 36. Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–8. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto S, Nagaoka M, Hara T, Kimura-Takagi I, Mistuyama K, Ueyama S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin Exp Immunol. 2004;136:432–9. doi: 10.1111/j.1365-2249.2004.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montanher AB, Zucolotto SM, Schenkel EP, Fröde TS. Evidence of anti-inflammatory effects of Passiflora edulis in an inflammation model. J Ethnopharmacol. 2007;109:281–8. doi: 10.1016/j.jep.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 39. Engeman T, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J Leukoc Biol. 2004;76:941–9. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]