Abstract
In this study, the antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz fruits were evaluated. Total phenolic and flavonoid compounds, 2,2′-azino-bis (3-ethylbenzothioazoline-6-sulfonic acid), 2,2′-diphenyl-1-picrylhydrazyl, and superoxide anion radicals scavenging activities and ferric-reducing antioxidant power of water, ethyl acetate, acetone, and methanol extracts were determined for the measurement of the antioxidant activity. Quercetin and α-tocopherol were used as standard antioxidants. The inhibitory effect of the water extract on acetylcholinesterase (AChE) was evaluated using the Ellman method and galantamine was used as a standard. Water extract had the highest total phenolic concentration and the strongest antioxidant activity followed by ethyl acetate and acetone extracts whereas methanol extract has the lowest phenolics and weakest antioxidant activity. Moreover, water extract showed moderate ability to inhibit AChE. It was concluded that fruits of S. torminalis have antioxidant and anti-AChE activities and that the plant might be a natural source of antioxidants and AChE inhibitors.
Keywords: antiacetylcholinesterase, antioxidant activity, free radicals, Sorbus torminalis (L.) Crantz, wild service tree
1. Introduction
Free radicals can be defined as molecules or molecular fragments containing one or more unpaired electrons in orbitals [1]. The most important class of radical species generated in living systems is reactive oxygen species, which include superoxide anion ( ), hydroxyl radical (OH•) and hydrogen peroxide (H2O2) [2]. Oxidative stress is a term commonly used to denote the imbalance between the concentrations of reactive oxygen and nitrogen species and the antioxidative defense mechanisms. Such an imbalance plays a pivotal role in many pathologies such as atherosclerosis, diabetes, cancer, rheumatoid arthritis, cataract, and Parkinson’s diseases [3].
Antioxidants are substances that are capable of inhibiting oxidation. They protect the key cell components by neutralizing the damaging effects of free radicals [4]. In plant tissues, many phenolic compounds (in addition to tocopherols) are potential antioxidants; flavonoids, tannins, and lignin precursors may work as reactive oxygen species scavenging compounds [5]. Recently, there has been worldwide interest in finding new and safe antioxidants from natural sources, to prevent oxidative stress and to minimize oxidative injury of living cells.
Acetylcholinesterase (AChE; EC 3.1.1.7) catalyzes the hydrolysis of the neurotransmitter acetylcholine to terminate signaling events across cholinergic synapses, including those at neuromuscular junctions. Inhibition of AChE serves as a strategy for the treatment of neurodegenerative disorders such as Alzheimer’s disease [6]. Alzheimer’s disease is associated with aging and characterized by progressive memory loss and cognitive deterioration [7]. For the treatment of Alzheimer’s disease, the molecular basis of the drugs used to date is as AChE inhibitors. Therefore the search for sources of effective anti-AChE compounds is extremely important. Oxidative stress has also been associated with the progression of Alzheimer’s disease [8]. The brains of patients with Alzheimer’s disease show a significant extent of oxidative damage associated with a marked accumulation of amyloid-β peptide [9]. Some studies suggest that dietary supplements with antioxidants and free radical scavengers such as vitamin E may display some benefits in slowing the mild cognitive impairment of Alzheimer’s disease [8,10].
Sorbus torminalis (L.) Crantz (Rosaceae) otherwise known as the “wild service tree” is a medium-sized deciduous tree native to central and southern Europe, north-western Africa, the Balkan peninsula, Asia Minor, the Crimea, Caucasus, and Transcaucasia [11,12]. The fruits of S. torminalis are used in traditional medicine for treatment of cardiac diseases and its astringet effects [13,14]. Also, its fruits are eaten raw [15] as well as consumed as jam, syrups, and wines [16]. In Kirklareli, Turkey, S. torminalis leaves are consumed by boiling, for treatment of diabetes and stomach ache [17].
The antioxidant potential of some Sorbus species such as Sorbus aucuparia, Sorbus domestica, Sorbus aria, etc. has been demonstrated [16,18–21]. However, there is no report about antioxidant activities of water, ethyl acetate, acetone, and methanol extracts from S. torminalis fruits. Currently, many natural antioxidants have been investigated for their inhibitory effect on AChE. In this study, anti-AChE activity of S. torminalis was examined for the first time. The aim of this study was to assess the possible antioxidant and AChE inhibitory potential of extracts of S. torminalis fruits.
2. Materials and methods
2.1. Chemicals
Alpha-tocopherol, β-nicotinamide-adenine dinucleotide (NADH), 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATChI), AChE, galantamine hydrobromide, gallic acid, and phenazine methosulfate (PMS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2,2′-azino-bis(3-ethylbenzothioazoline-6-sulphonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), catechin, nitroblue tetrazolium (NBT), and quercetin were purchased from Fluka Chemical Co. (Buchs, Switzerland). 2,4,6-tripyridyl-s-triazine (TPTZ) was purchased from Merck Chemical Co. (Darmstadt, Germany). All other chemicals were analytical grade.
2.2. Plant material
Fruits of S. torminalis (L.) Crantz were collected in 2010 from the Belgrad Forest, Istanbul, Turkey and identified by Associate Professor Sükran Kültür. Voucher specimen has been deposited at the Herbarium of the Faculty of Pharmacy, Istanbul University, Istanbul, Turkey (ISTE 99256).
2.3. Preparation of fruit extracts
Fruits of S. torminalis were washed and cut into small pieces, the seeds were carefully removed and fruits were stored at −20°C (Bosch, Stuttgart, Germany) until extraction. For water extract, a 15 g portion of fruit was refluxed with 150 mL distilled water for 3 hours. For ethyl acetate, acetone and methanol extractions, a 30 g portion of fruit was extracted by Soxhlet apparatus with 150 mL of the respective solvent for 24 hours. All of the extracts were filtered and evaporated to dryness in a rotary evaporator (Buchi, Switzerland). The crude extracts were kept at −20°C until used. For the assessment of antioxidant and anti-AChE activities, the extracts were dissolved in solvents by ultrasonic water bath (Elma, Singen, Germany). All the analyses were performed using a microplate reader (Biotek, Winooski, VT, USA).
2.4. Determination of antioxidant capacity
2.4.1. Total phenolic and flavonoid contents
Total phenolic contents (PC) of the extracts were determined using Folin–Ciocalteu reagent using a modification of the method of Slinkard and Singleton [22]. An 8 μL sample of solution was mixed with 260 μL distilled water, 8 μL Folin–Ciocalteu reagent (previously diluted with distilled water 1:2; v:v), and 24 μL 2% sodium carbonate solution. The mixture was incubated in the dark at room temperature for 2 hours. The absorbance of the mixture was measured at 760 nm. PC in the extracts were expressed in terms of mg gallic acid equivalents/g extract using equation of a regression curve obtained using standard gallic acid solutions (range, 0.04–0.5 mg/mL).
A standard colorimetric assay [23] with slight modifications was used to quantify total flavonoid contents (FC) of the extracts. A 25 μL aliquot of sample solution was mixed with 125 μL distilled water and 7.5 μL 5% sodium nitrate solution and allowed to stand for 5 minutes. Then, 15 μL 10% aluminum chloride solution was added and after 5 minutes of incubation, 50 μL 1M sodium hydroxide solution was added and diluted with 27.5 μL distilled water. The absorbance was measured at 510 nm. FC of the extracts were expressed as mg catechin equivalents/g extract using a regression curve obtained from standard catechin solutions (range, 0.016–0.25 mg/mL).
2.4.2. ABTS radical scavenging activity
The ABTS radical (ABTS•) scavenging activities of the extracts were determined by modifying the method of Re et al [24]. ABTS• stock solution (7 mM) was produced by reacting the ABTS with potassium persulfate solution (final concentration 2.45 mM) and the mixture was kept in dark at room temperature for 16 hours prior to use. ABTS• stock solution was diluted with absolute ethanol to get ABTS• working solution and the absorbance of this solution was set as 0.70 ± 0.02 at 734 nm. A 5 μL sample of solution was mixed with 245 μL of ABTS• working solution and, after 6 minutes, decolorization was measured at 734 nm. Alpha-tocopherol and quercetin were used as standards and 96% absolute ethanol was used as a control. The percentage of ABTS• scavenging potential was calculated according to following formula:
Also ABTS• scavenging ability of the extracts was expressed as Trolox equivalents antioxidant capacity (TEAC) by using the Trolox standard regression curve. TEAC (ABTS) values were defined as equivalent antioxidant capacity in terms of mM of Trolox.
2.4.3. DPPH radical scavenging activity
The DPPH radical (DPPH•) scavenging activities of the extracts were determined by modifying the method of Brand-Williams et al [25]. A 10 μL sample of solution was mixed with 240 μL 0.1 mM DPPH• solution in methanol. The mixture was shaken and kept in the dark at room temperature for 30 minutes, and then the decrease in the absorbance was measured at 517 nm. Alpha-tocopherol and quercetin were used as standards and methanol was used as a control. The percentage of DPPH• scavenging activity was calculated according to the following formula:
DPPH• scavenging ability of the extracts was expressed as TEAC value by using the Trolox standard regression curve. TEAC (DPPH) values were defined as equivalent antioxidant capacity in terms of mM of Trolox.
2.4.4. Superoxide anion radical scavenging activity
The superoxide anion radical ( ) scavenging activities of the extracts were measured by modifying NBT reduction method [26]. A 10 μL sample of solution was mixed with 100 μL 468 μM NADH solution and 100 μL 150 μM NBT solution. The reaction was started by adding 10 μL 60 μM PMS solution. After incubation of the mixture at 25°C for 5 minutes, the decrease in the absorbance was measured at 560 nm. Quercetin was used as a standard and distilled water was used as a control. The percentage of scavenging activity was calculated according to the following formula:
2.4.5. Ferric-reducing antioxidant power
The antioxidant activities of the extracts were quantified by ferric reducing antioxidant power (FRAP) assay [27]. The FRAP reagent was freshly prepared by mixing 0.3 M acetate buffer (pH 3.6), 10 mM TPTZ solution (in 40 mM HCl), and 20 mM ferric chloride solution at a ratio 10:1:1 (v:v:v). A 10 μL sample of solution was mixed with 20 μL solvent, and after 10 minutes incubation at 37°C, 270 μL FRAP reagent was added (the reagent was warmed to 37°C before using). After 4 minutes incubation, increase in the absorbance was measured at 593 nm. The increased absorbance indicates increased reducing power. Alpha-tocopherol and quercetin were used as standards. Reducing power of the extracts was expressed as FRAP value (mM Fe2+ equivalents) using equation of standard regression curve using FeSO4.7H2O solutions (range, 0.2–1.5 mM).
2.5. AChE inhibition assay
The inhibitory activity of the water extract on AChE was determined using our modification of the method of Ellman et al [28]. The Ellman solution consisted of phosphate buffer (pH 7.5) with 318 μM DTNB and 955 μM ATChI was prepared. A 20 μL sample of solution and 220 μL Ellman solution were mixed and the absorbance was measured at 412 nm for 10 minutes. After 10 μL of AChE solution (0.5 U/mL) was added, the reaction rate was monitored at 412 nm for 10 minutes. Any increase in absorbance due to the spontaneous hydrolysis of substrate was corrected by subtracting the rate of the reaction prior to adding the enzyme from the rate after adding the enzyme. Galantamine was used as a standard AChE inhibitor and distilled water was used as a control. The percent inhibition of the enzyme activity due to the presence of increasing test compound concentration was calculated according to the following formula:
2.6. Statistical analysis
All measurements were made in triplicate. The results were evaluated using unpaired t test with NCSS statistical computer package and expressed as mean ± standard deviation. Differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Total phenolic and flavonoid contents
Phenols and polyphenolic compounds, such as flavonoids, are widely found in food products derived from plant sources and they have been shown to possess significant antioxidant activities [29]. It has been reported that various extracts from Sorbus species have a wide range of PC [18,20]. PC (as gallic acid equivalents) and FC (as catechin equivalents) of S. torminalis extracts are shown in Table 1. The results showed that the water extract has the highest PC and FC followed by ethyl acetate and acetone extracts. Also, it was found that methanol extract contained the lowest concentration of phenolic. Phytochemical constituents of S. torminalis leaves [30] and inflorescences [31] have been reported previously.
Table 1.
Total phenolic contents (PC; as gallic acid equivalents), total flavonoid contents (FC; as catechin equivalents) of Sorbus torminalis extracts.
| Extract | PC (mg/g extract) | FC (mg/g extract) |
|---|---|---|
| Water | 20.44 ± 0.910a | 12.19 ± 2.005a |
| Ethyl acetate | 10.36 ± 1.547b | 1.61 ± 0.410b |
| Methanol | 3.83 ± 0.164c | 1.73 ± 0.612b |
| Acetone | 8.38 ± 1.151b | 2.00 ± 0.214b |
Data are presented as the mean of three replicates ± standard deviation. Different superscript letters in the same column indicate a significant difference (p < 0.05).
In general, efficiency of extraction of polyphenols from plant materials are influenced by multiple parameters. Solvent polarity will play a key role in increasing phenolic solubility [32]. Our results showed that water is the best solvent for the extraction of phenolics from S. torminalis fruits.
3.2. Antioxidant potential of the extracts
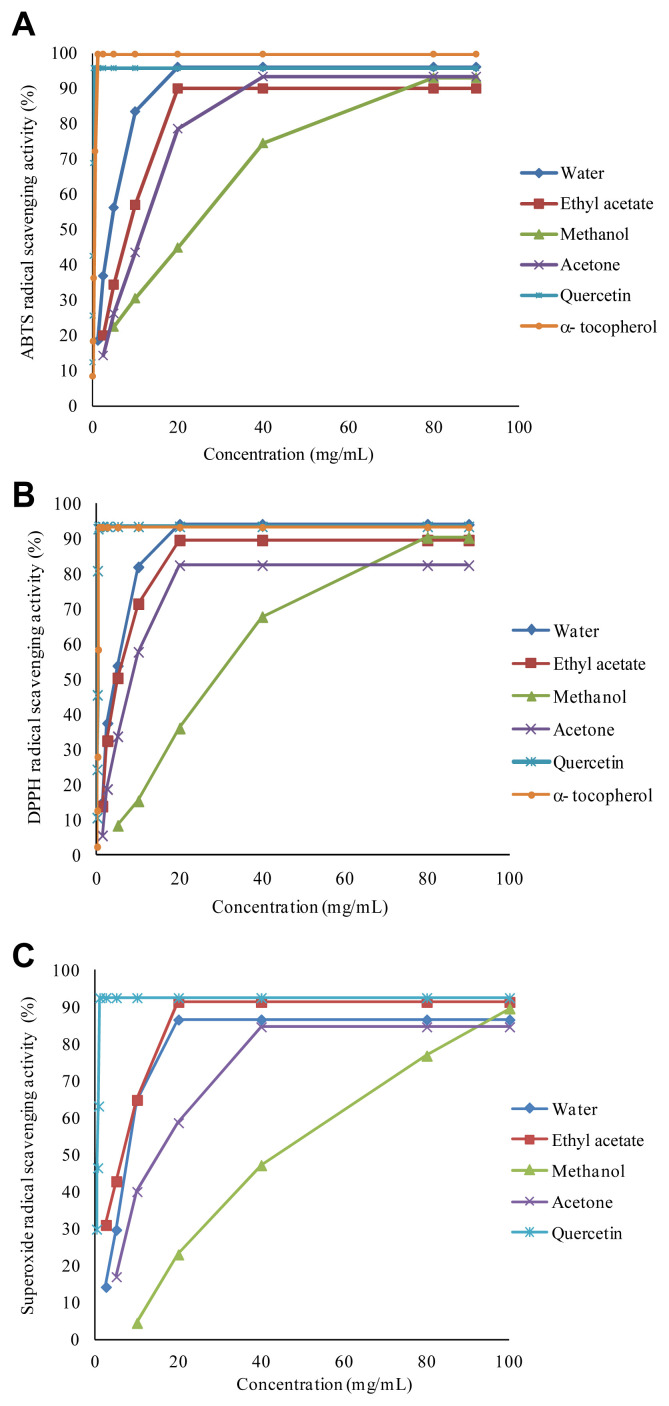
Several methods have been developed to evaluate the antioxidant activity, using the scavenging of synthetic or generated radicals. Both ABTS and DPPH assays are the most commonly used antioxidants methods due to excellent reproducibility under certain assay conditions [33]. In this study, free radical scavenging activities of S. torminalis extracts were determined by using ABTS, DPPH, and radicals. Fig. 1 shows that dose-dependent scavenging activities of the extracts on these free radicals. Also, in order to quantify the antioxidant activity, the half maximal effective concentration (EC50) and TEAC values were calculated and are summarized in Table 2. Lower (EC50) values indicate greater free radical scavenging activity. The highest ABTS• scavenging rate was found for water extract followed by ethyl acetate and acetone extracts. The stable radical DPPH has been used widely for the determination of primary antioxidant activity. Water and ethyl acetate extracts showed higher DPPH• and scavenging activity than acetone extract. The results obtained from all assays showed that, methanol extract has significantly less radical scavenging activity compared to other extracts.
Fig. 1.
(A) 2,2′-azino-bis(3-ethylbenzothioazoline-6-sulphonic acid) (ABTS) radical cation scavenging activity, and (B) 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, and (C) Superoxide anion radical scavenging activity of Sorbus torminalis extracts and standard antioxidants.
Table 2.
Half maximal effective concentration (EC50), Trolox equivalents antioxidant capacity (TEAC) and ferric reducing antioxidant power (FRAP) values of Sorbus torminalis extracts and standards.
| Extract | EC50 ABTS (mg/mL) | TEAC (ABTS) (mM) | EC50 DPPH (mg/mL) | TEAC (DPPH) (mM) | EC50 O2−(mg/mL) | FRAP (mM) |
|---|---|---|---|---|---|---|
| Water | 5.30 ± 0.166a | 1.37 ± 0.051a | 5.69 ± 0.364a | 0.85 ± 0.019a | 9.01 ± 1.025a | 3.51 ± 0.060a |
| Ethyl acetate | 9.30 ± 0.449b | 0.94 ± 0.0470b | 6.08 ± 1.263a | 0.73 ± 0.033b | 7.41 ± 0.887a | 1.60 ± 0.060b |
| Acetone | 13.23 ± 0.341c | 0.71 ± 0.091c | 10.21 ± 0.230b | 0.59 ± 0.015c | 18.64 ± 1.162b | 1.17 ± 0.027c |
| Methanol | 27.53 ± 4.097d | 0.50 ± 0.084d | 32.31 ± 2.615c | 0.19 ± 0.052d | 48.47 ± 4.779c | 0.45 ± 0.020d |
| Quercetin | 0.12 ± 0.001e | 1.42 ± 0.028e | 0.07 ± 0.001d | 0.97 ± 0.002e | 0.53 ± 0.037d | 3.24 ± 0.136e |
| α-tocopherol | 0.49 ± 0.035f | 1.64 ± 0.004f | 0.24 ± 0.018e | 0.97 ± 0.009f | — | 4.10 ± 0.240f |
Data are presented as the mean of three replicates ± standard deviation. Different superscript letters in the same column indicate a significant difference (p < 0.05). EC50 is the effective concentration for which the antioxidant activity was 50% and was obtained by interpolation from linear regression analysis. TEAC and FRAP values; for extracts at 10 mg/mL and for quercetin at 0.25 mg/mL concentration. For α-tocopherol TEAC (ABTS) and FRAP values at 1.25 mg/mL; and TEAC (DPPH) value at 0.5 mg/mL concentration.
ABTS = 2,2′-azino-bis(3-ethylbenzothioazoline-6-sulphonic acid); DPPH = 2,2′-diphenyl-1-picrylhydrazyl.
Apart from two studies performed using only aqueous methanol (70%) extract, there is no published report on antioxidant activity of S. torminalis. Our results (weak antioxidant activity of methanol extract) are in accordance with the findings of these studies. Olszewska [16] reported that DPPH• and ABTS• scavenging capacity of S. torminalis aqueous methanol extract was lower than that of S. aucuparia. In the second study, the antioxidant activity of methanol extracts of 38 plants growing in Turkey, including S. torminalis, were evaluated by using different antioxidant assays, in result of DPPH• scavenging assay, S. torminalis aqueous methanol extract was one of the five lowest plants with respect to radical scavenging [34].
The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity [35]. In this study, the ability of the extracts to reduce ferric ions was determined using the FRAP assay. This method based on the presence of antioxidant in the sample would result in reducing Fe3+ to Fe2+ by donating an electron. Reducing power of the S. torminalis extracts was expressed as FRAP value (mM Fe2+ equivalents) and the FRAP values decreased in the order of: water > ethyl acetate > acetone > methanol (Table 2). High reducing power of different species of Sorbus has been reported by Olszewska and Michel [36] and Hukkanen et al [18]. Vitamin C has strongly reducing properties and the reducing power of many plant extracts is mainly due to this substance. Vitamin C contents of different Sorbus species (S. domestica and S. aucuparia) have been demonstrated by Egea et al [37] and Jablońska-Ryś et al [38]. Reducing properties of S. torminalis may be related to its vitamin C content.
In this study strong correlations were observed between PC and antioxidant activity (Table 3). It can be suggested that polyphenolics in the S. torminalis fruits may play an important role in free radical scavenging. These results are consistent with previous observations that indicated a direct correlation between the antioxidant capacity of Sorbus extracts and PC [14,15]. Higher total phenol and flavonoid contents lead to better radical scavenging activity [39]. The antioxidant activity of phenolic compounds is mainly due to the redox properties of their hydroxyl groups, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides [40].
Table 3.
Correlation coefficients, r, for relationships between phenolic contents (PC) and antioxidant activity.a
| PC | ABTS | DPPH | O2− | |
|---|---|---|---|---|
| ABTS | −0.855 | |||
| DPPH | −0.728 | 0.977 | ||
| O2− | −0.753 | 0.984 | 0.997 | |
| FRAP | 0.999 | −0.837 | −0.703 | −0.731 |
ABTS = 2,2′-azino-bis (3-ethylbenzothioazoline-6-sulphonic acid); DPPH = 2,2-diphenyl-1-picrylhydrazyl; FRAP = ferric reducing antioxidant power.
Correlation coefficients were calculated using EC50 values for ABTS, DPPH and O2−.
The antioxidant activities of the S. torminalis extracts and reference antioxidants (quercetin and α-tocopherol) increased in a dose-dependent manner. However, all the tested extracts exhibited lower antioxidant activity than reference antioxidants, quercetin, and α-tocopherol.
3.3. AChE inhibitory potential of the water extract
Alzheimer’s disease is the most common cause of dementia in aged populations. Inhibition of AChE can restore the level of acetylcholine in the brain and AChE inhibitors are pharmacological therapies for Alzheimer’s disease. In recent years, the research for new AChE inhibitors has been of great interest. This is the first study examining the AChE inhibitory activity of S. torminalis fruits. Table 4 depicts the AChE inhibitory activity of S. torminalis water extract and galantamine. The results show that the water extract of S. torminalis possesses the dose-dependent ability to inhibit AChE, but it was not as good as galantamine. This effect may be explained by its antioxidant activity due to the presence of polyphenols. Many natural antioxidant have been proposed as alternative therapeutic agents for Alzheimer’s disease [41].
Table 4.
The acetylcholinesterase inhibitory activity (%) of Sorbus torminalis water extract and galantamine.
| 20 mg/mL | 10 mg/mL | 5 mg/mL | 0.05 mg/mL | |
|---|---|---|---|---|
| S. torminalis | 62.67 ± 5.09 | 52.31 ± 4.93 | 38.84 ± 4.79 | |
| Galantamine | 84.01 ± 4.48 |
It can be concluded that the water extract of S. torminalis fruits showed antioxidant activity together with inhibitory effect on AChE, indicating that the water extract may be used as a source of natural antioxidant and AChE inhibitors. This result is important and beneficial because of antioxidant components and AChE inhibitors in the fruit can be easily taken to the organism with the diet. Further studies are needed to characterize fully the active principles in S. torminalis fruits.
Acknowledgments
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University, Project No. 26844. The authors are thankful to Associate Professor Sükran Kültür from the identification of S. torminalis (L.) Crantz.
Funding Statement
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University, Project No. 26844.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Miller DM, Buettner GR, Aust SD. Transition metals as catalysts of “autoxidation” reactions. Free Radical Bio Med. 1990;8:95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- 2. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 3. Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Method Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 4. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev. 1987;87:955–79. [Google Scholar]
- 7. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–52. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grundman M. Vitamin E and Al zheimer disease: the basis for additional clinical trials. Am J Clin Nutr. 2000;71:630S–6S. doi: 10.1093/ajcn/71.2.630s. [DOI] [PubMed] [Google Scholar]
- 9. Butterfield DA, Castegna A, Lauderback CM, et al. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neuorobiol Aging. 2002;23:655–64. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 10. Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–7. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 11. Aldasoro JJ, Aedo C, Garmendia FM, et al. Revision of Sorbus subgenera Aria and Torminaria (Rosaceae-Maloideae) Syst Bot Monogr. 2004;69:1–148. [Google Scholar]
- 12. Oršanić M, Drvodelić D, Jemrić T, et al. Variability of morphological and biological characteristics of wild service tree [Sorbus torminalis (L.) Crantz] fruits and seeds from different altitudes. Period Biol. 2009;111:495–504. [Google Scholar]
- 13. Tuzlaci E, Erol MK. Turkish folk medicinal plants. Part II: Eğirdir (Isparta) Fitoterapia. 1999;70:593–610. [Google Scholar]
- 14. Rivera D, Obon C, Inocencio C, et al. The ethnobotanical study of local Mediterranean food plants as medicinal resources in Southern Spain. J Physiol Pharmacol. 2005;56:97–114. [PubMed] [Google Scholar]
- 15. Mustafa B, Hajdari A, Pajazita Q, et al. An ethnobotanical survey of the Gollak region, Kosovo. Genet Resour Crop Evol. 2012;59:739–54. [Google Scholar]
- 16. Olszewska MA. In vitro antioxidant activity and total phenolic content of the inflorescences, leaves and fruits of Sorbus torminalis (L.) Crantz. Acta Pol Pharm. 2011;68:945–53. [PubMed] [Google Scholar]
- 17. Kültür S. Medicinal plants used in Kirklareli Province (Turkey) J Ethnopharmacol. 2007;111:341–64. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 18. Hukkanen AT, Polonen SS, Karenlampi SO, et al. Antioxidant capacity and phenolic content of sweet rowanberries. J Agr Food Chem. 2006;54:112–9. doi: 10.1021/jf051697g. [DOI] [PubMed] [Google Scholar]
- 19. Termentzi A, Kefalas P, Kokkalou E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruit at different maturity stages. Food Chem. 2006;98:599–608. [Google Scholar]
- 20. Olszewska MA, Nowak S, Michel P, et al. Assessment of the content of phenolics and antioxidant action of inflorescences and leaves of selected species from the genus Sorbus sensu stricto. Molecules. 2010;15:8769–83. doi: 10.3390/molecules15128769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olszewska MA, Presler A, Michel P. Profiling of phenolic compounds and antioxidant activity of dry extracts from selected Sorbus species. Molecules. 2012;17:3093–113. doi: 10.3390/molecules17033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55. [Google Scholar]
- 23. Kim DO, Chun OK, Kim YJ, et al. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agr Food Chem. 2003;51:6509–15. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 24. Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 25. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. [Google Scholar]
- 26. Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and moleculer oxygen. Biochem Biophys Res Commun. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 27. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28. Ellman GL, Courtney KD, Andres V, Jr, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 29. Nabavi SM, Nabavi SF, Ebrahimzadeh MA. Free radical scavenging and antioxidant activities of Dorema aitchisonii. J Food Drug Anal. 2012;20:34–40. [Google Scholar]
- 30. Tsitsa-Tzardi E, Loukis A, Philianos S. Constituents of Sorbus torminalis leaves. Fitoterapia. 1992;63:189–90. [Google Scholar]
- 31. Olszewska MA, Roj JM. Phenolic constituents of the inflorescence of Sorbus torminalis (L.) Crantz. Phytochem Lett. 2011;4:151–7. [Google Scholar]
- 32. Naczk M, Shadidi F. Phenolics in cereals, fruits and vegetables: occurrence extraction and analysis. J Pharmaceut Biomed. 2006;41:1523–42. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33. Nithiyanantham S, Varadharajan S, Siddhuraju P. Differential effects of processing methods on total phenolic content, antioxidant and antimicrobial activities of three species of Solanum. J Food Drug Anal. 2012;20:844–54. [Google Scholar]
- 34. Serteser A, Kargioğlu M, Gök V, et al. Antioxidant properties of some plants growing wild in Turkey. Grasas Aceites. 2009;60:147–54. [Google Scholar]
- 35. Meir S, Kanner J, Akiri B, et al. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agr Food Chem. 1995;43:1813–9. [Google Scholar]
- 36. Olszewska MA, Michel P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat Prod Res. 2009;23:1507–21. doi: 10.1080/14786410802636177. [DOI] [PubMed] [Google Scholar]
- 37. Egea I, Sánchez-Bel P, Romojaro F, et al. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Food Hum Nutr. 2010;65:121–9. doi: 10.1007/s11130-010-0159-3. [DOI] [PubMed] [Google Scholar]
- 38. Jablońska-Ryś E, Zalewska-Korona M, Kalbarczyk J. Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J Fruit Ornam Plant Res. 2009;172:115–20. [Google Scholar]
- 39. Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agr Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 40. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agr Food Chem. 2001;49:5165–70. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 41. Mancuso C, Bates TE, Butterfield DA, et al. Natural antioxidants in Alzheimer’s disease. Expert Opin Investig Drugs. 2007;16:1921–31. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]