Abstract
Citrus essential oils are widely applied in food industry as the backbone of citrus flavors. Unfortunately, due to relatively simple chemical composition and tremendous price differences among citrus species, adulteration has been plaguing the industry since its inception. Skilled blenders are capable of making blends that are almost indistinguishable from authentic oils through conventional gas chromatography analysis. A reversed-phase high performance liquid chromatography (HPLC) method was developed for compositional study of nonvolatile constituents in essential oils from major citrus species. The nonvolatile oxygenated heterocyclic components identified in citrus oils were proved to be more effective as markers in adulteration detection than the volatile components. Authors are hoping such an analysis procedure can be served as a routine quality control test for authenticity evaluation in citrus essential oils.
Keywords: adulteration, authenticity, citrus oil, HPLC, oxygenated heterocyclic, compounds
1. Introduction
Citrus essential oils have been gradually gaining popularity for the past century in the flavor industry [1]. Such a rise in popularity is due to their globally accepted flavor profile and consumers' craving for naturalness [2]. Citrus essential oils, as the backbone of citrus flavors, will be in great demand judging by the current trend. As a result, to ensure decent and consistent quality of citrus oils is a great challenge to quality control groups in flavor companies.
Unfortunately, unscrupulous players have been tempering citrus essential oils for a long time. It is not an easy task to bring forth an analytical approach which is effective in adulteration detection, given the fact that the perpetrators are equipped with as much knowledge as we are [3,4].
The nonvolatile fraction of citrus essential oils has been overlooked for quite a long time, due to its insignificant contribution to the flavor profile of citrus oils. Recently there has been a rise in the interest of polymethoxyflavones (PMFs) found in the nonvolatile fraction of orange oil due to their proposed antiinflammatory and anticarcinogenic effects [5–8]. Such components are regarded as nonvolatile because regular gas chromatography conditions are insufficient to vaporize them. These nonvolatile components can be the key in the development of an ideal analytical approach that the flavor industry has been demanding.
Unlike most other citrus oil studies in which samples were extracted in laboratories, this study focused on the industrial situation. All the samples in this study were of industrial origin. The goal of this study is to establish an efficient, sensitive, and economical procedure which can serve as a routine quality control test for incoming citrus essential oils.
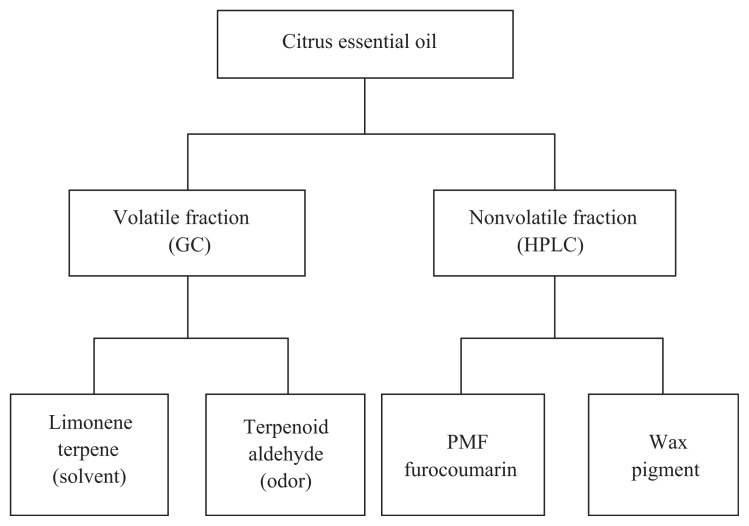
Citrus essential oils are complex natural mixtures of a wide range of compounds from diverse chemical groups. To date, > 200 components have been successfully identified from citrus essential oils and this number is still growing [9]. All of these components can be divided into two subgroups: a volatile fraction and a nonvolatile fraction. The criterion for their volatilities is under regular GC conditions (i.e., maximum 320°C). Fig. 1 shows the two main fractions of citrus oil and their subgroups.
Fig. 1.
Typical citrus oil composition. GC = gas chromatography; HPLC = high performance liquid chromatography; PMF = polymethoxyflavones.
The volatile fraction is responsible for 85–99% of the whole oil on a weight basis [9,10]. The majority of this fraction is short chain alcohols, aldehydes, esters, acids, monoterpenes (C10H16), sesquiterpenes (C15H24), and their corresponding terpenoids (oxygen containing derivatives). There are also trace levels of sulfur- and nitrogen-containing compounds which contribute to the aroma character of citrus oils. It was the development of GC and the capillary column technique that makes comprehensive compositional analysis possible. The majority of the identified volatile compounds are common among citrus species.
For the majority of the volatile components, their odor contributions are not proportional to their relative abundance. Hydrocarbon compounds constitute an overwhelming proportion in citrus oils, yet they contribute very little to the “citrus note”, as one might expect. Because of their extremely hydrophobic nature, terpenes are responsible for the poor aqueous solubility observed in citrus oils. Functionally speaking, terpenes serve as a natural solvent that dissolves the rest of the components which have greater contribution to the odor profile.
The aroma of citrus oil is mainly characterized by aldehydes, alcohols, esters, and trace amounts of sulfur- or nitrogen-containing compounds. Such a trait distinguishes citrus oils from noncitrus essential oils whose flavor profiles are usually defined by their abundant components. It is also these less abundant components that define the unique flavor profiles of each citrus species.
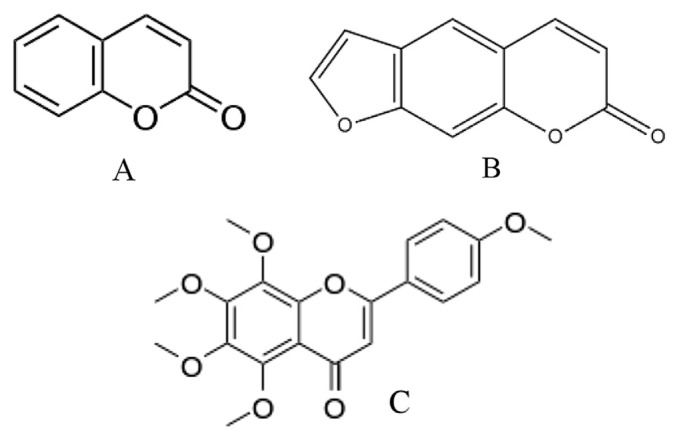
The nonvolatile fraction in citrus essential oils ranges between 1% in some sweet orange oils, and 15% in key lime expressed oil [9]. This fraction is composed of long chain hydrocarbons, fatty acids, sterols, carotenoids, and oxygenated heterocyclic compounds (Fig. 2). Compared to the volatile fraction, the nonvolatile fraction in citrus oils has been much less explored for multiple reasons. However, the oxygenated heterocyclic components are gradually gaining attention due to their biological activity and role in authenticity analysis.
Fig. 2.
Crucial nonvolatile compounds found in citrus essential oils: (A) coumarin; (B) furocoumarin; (C) polymethoxyflavone (PMF).
Because of their nonvolatile nature, studies on oxygenated heterocyclic compounds are usually carried out by normal or reversed-phase high performance liquid chromatography (HPLC) with ultraviolet or fluorescence detectors [11]. Opposed to the similar patterns of volatile components from different citrus oils, the nonvolatile components are much more species-specific. For example, orange, mandarin, and tangerine oil contain exclusively PMFs, while lemon and lime oils are solely comprised of furocoumarins. In grapefruit oil and bitter orange oil, both PMFs and furocoumarins have been identified [12]. Therefore oxygenated heterocyclic components can serve as markers in revealing interspecies adulteration.
From a practical point of view, HPLC analysis bears several advantages over GC analysis when employed for citrus oil adulteration studies. Firstly, citrus oils are more prone to volatile fraction adulteration than nonvolatile fraction adulteration. Citrus oils are valued due to their unique aroma, which is mainly conferred by their volatile fractions. The nonvolatile fractions, due to their limited volatilities, can only contribute moderately to the taste of the citrus oils. It is obvious that adulterators would choose not to invest their time and resources on the nonvolatile fraction, which has little impact on the odor of citrus oils. Secondly, the GC profiles of citrus oils have long been established and are readily accessible to both buyers and producers. Most of the crucial volatile components are well studied and can be purchased from chemical plants or flavor houses at only a fraction of the oil price. Such a fact has unintentionally encouraged perpetrators to reconstitute their diluted or extended oils with aroma chemicals in order to pass through GC screening. The nonvolatile components, by contrast, are structurally more complicated and therefore difficult to synthesize. As a result, the oxygenated heterocyclic compounds are either commercially unavailable or can only be purchased at formidable prices compared to citrus oils themselves. Thus, it is not possible or economically feasible for adulterators to perform the same reconstitution trick on the nonvolatile fraction as they are doing to the volatile fraction. Lastly, the nonvolatile fraction is generally more stable against oxidation reactions than the volatile fraction. Thus, HPLC analysis provides a more helpful and accurate method for quality control toward aged or abused samples.
2. Materials and methods
2.1. Citrus essential oil samples
A total of > 300 citrus oil samples were collected from Flavor Materials International (Avenel, NJ, USA) from the past 3 years.
2.2. Equipment and GC conditions
Phenomenex ZB-1MS phase capillary column (100% dimethylpolysiloxane, 20 m × 0.10 mm × 0.10 μm, Phenomenex, Torrance, CA, USA) was used in GC analysis. Citrus oil samples were directly injected without any pretreatment. The injection volume was 0.1 μL. The carrier gas (helium) flow rate was 0.33 mL/minute. The temperature ranged from 70°C to 300°C and was programmed as: (1) 70°C hold for 1 minute; (2) 70–110°C with 5°C/minute ramp for 8 minutes; (3) 110–300°C with 25 °C/minute ramp for 7.6 minutes; and (4) 300°C hold for 3.4 minutes.
PerkinElmer XL Autosystem GC with a built-in FID detector, autosampler, and Totalchrom software (PerkinElmer, Waltham, MA, USA) was used in this study.
All compounds from GC analysis were identified by GC-mass spectrometry and retention times were compared with authentic compounds. All authentic compounds were available from Flavor Materials International (Avenel).
2.3. Equipment and HPLC conditions
Among the several HPLC columns that were evaluated and compared, the Phenomenex Luna 3 μm PFP(2), liquid chromatography column 150 mm × 4.6 mm w/guard column (Phenomenex) was chosen. A guard column was applied in this study to prevent terpene accumulation onto the hydrophobic stationary phase, which might lead to pressure buildup.
A binary (methanol and water) solvent system was optimized for citrus essential oil analysis as: (1) 75–80% methanol in 10 minutes; (2) 80–95% methanol in 12 minutes; (3) 95–100% methanol in 1 minute; and (4) hold for 2 minutes. The flow rate was kept constant at 1.0 mL/minutes and the column temperature was kept at ambient 25°C.
Dionex Ultimate 3000 HPLC with autosampler and UV detector (Dionex, Sunnyvale, CA, USA) was used in this study. UV absorbance was recorded at 315 nm.
A stock solution of 0.1% (w/w) coumarin (internal standard) in ethyl alcohol was prepared. Before HPLC-UV injection, 100 mg of oil (approximately 118 μL) and 100 μL of coumarin stock solution was accurately measured and diluted in 800 μL of ethyl alcohol. The injection volume was 10 μL.
2.4. Solid phase extraction fractionation conditions
All citrus oil samples were subjected to a solid phase extraction (SPE) column prior to mass spectrometric detection for terpenic fraction removal. A Varian C-18 3 mL SPE column (Varian, Cranford, NJ, USA) was applied in this study. For each fractionation batch, 20 μL of citrus oil was loaded onto the pre-equilibrated SPE column. A mixture of water and methanol at 1:9 was chosen as eluent. For each fraction, 1 mL of eluent was collected under gravity. The majority of the nonvolatile compounds were found in fractions 2 and 3, and they were combined for HPLC-MS analysis.
2.5. Equipment and mass spectrometry conditions
An Agilent 1100 series liquid chromatography/mass detector (LC/MSD) System (Agilent Technologies, Waldbronn, Germany) was applied in this study. The LC/MSD system was equipped with an autosampler, a quaternary pump system, a Diode Array Detector (DAD) detector, a degasser, an MSD trap with an electrospray ion source (ESI), HP Chemstation software, Bruker Daltonics 4.2 and Data Analysis 4.2 (Hewlett-Packard GmbH, Waldbronn, Germany). The flow rate was set at 1.0 mL/minute with a 1:10 splitter after the output of the UV detector leading ~100 μL/minute to the ESI-MS. The samples were scanned from m/z 100 to 800. ESI was conducted by using needle voltages of 3.5 kV. High-purity nitrogen (99.999%) was used as dry gas at a flow rate of 8 L/minute, and the capillary temperature was 350°C. Nitrogen was used as a nebulizer at 40 psi and helium as the collision gas. HPLC conditions in the HPLC-MS study were the same as previously described in section C HPLC parameters.
3. Results and discussion
3.1. HPLC profile comparison
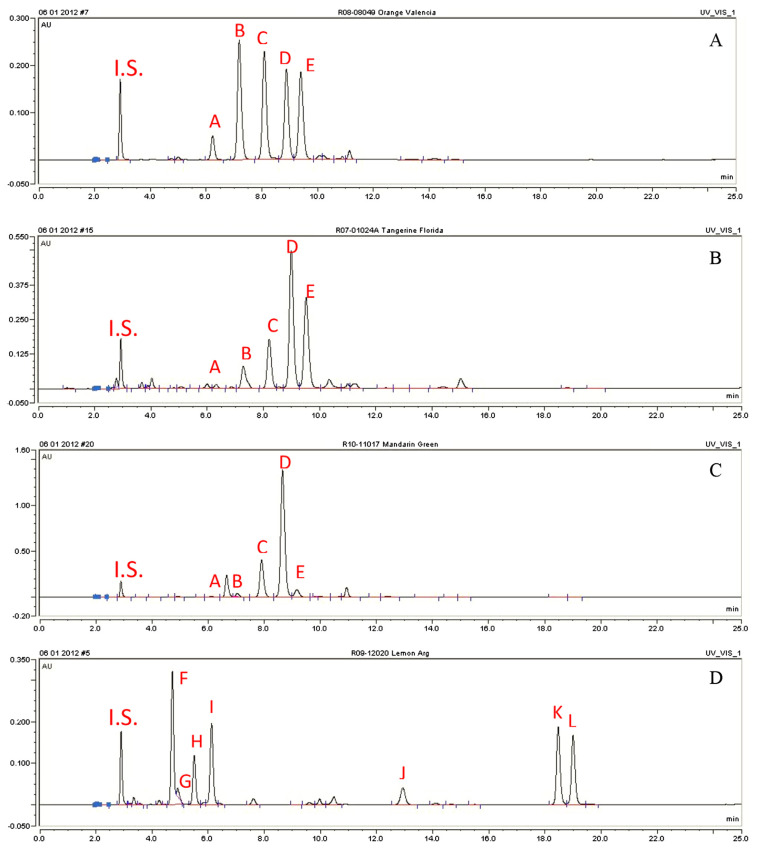
The HPLC profiles of typical oils extracted from four citrus species (orange, tangerine, mandarin, and lemon) are shown in Fig. 3. It can be concluded that the percentage of total oxygenated heterocyclic compounds varies greatly among citrus species. It was also obvious that orange oil, mandarin oil, and tangerine oil contain totally different peaks compared to lemon oil: PMFs can only be found in orange, tangerine, and mandarin oils, while furocoumarins and coumarins can only be found in lemon oil. Identifications of major peaks in these four citrus oils can be found in Table 1. All PMFs were identified by comparing their LC-MS characteristics with those of authentic compounds. Authentic compounds were previous isolated and identified in our laboratory at Rutgers University [13]. Furocoumarins and coumarins were characterized by LC-MS only, and therefore can only be considered tentative identification.
Fig. 3.
High performance liquid chromatography (HPLC) profiles of citrus oils from four species: (A) orange oil; (B) tangerine oil; (C) mandarin oil; (D) lemon oil.
Table 1.
ID of major nonvolatile components in citrus essential oils.
| Compound | Structure | |
|---|---|---|
| A | Sinensetin |
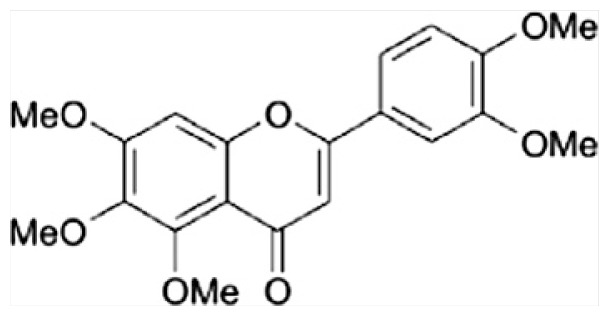
|
| B | 5,6,7, 4′-Tetramethoxyflavone |
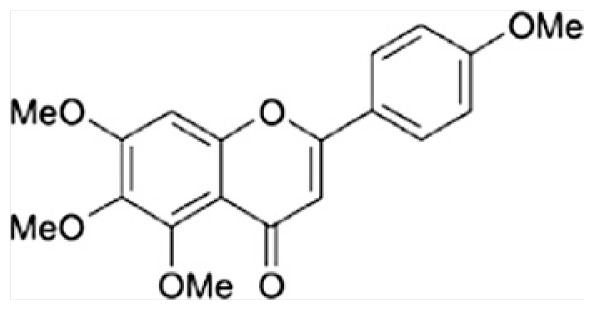
|
| C | Nobiletin |
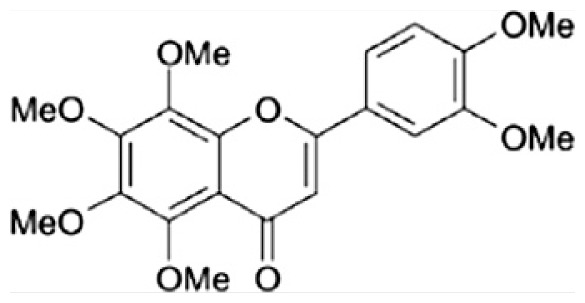
|
| D | Tangeretin |
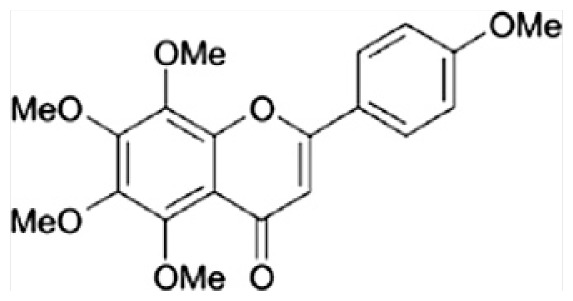
|
| E | 3,5,6,7,8,3′,4′-Heptamethoxyflavone |
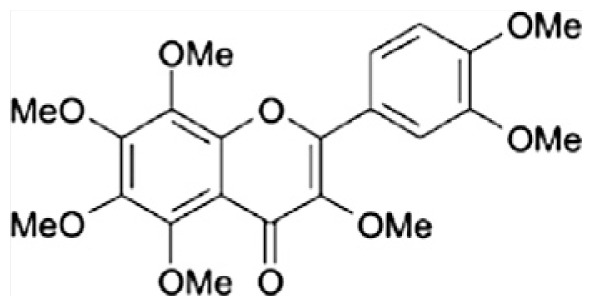
|
| F | Citropten |
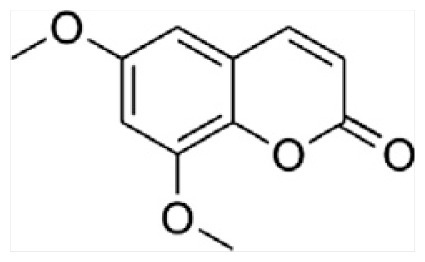
|
| G | Byakangelicin |
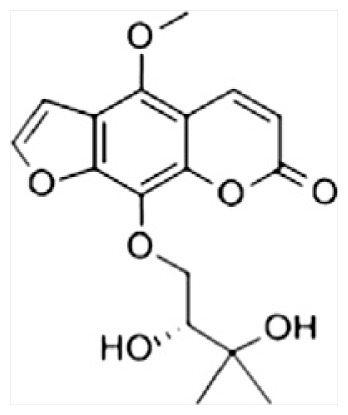
|
| H | Byakangelicol |
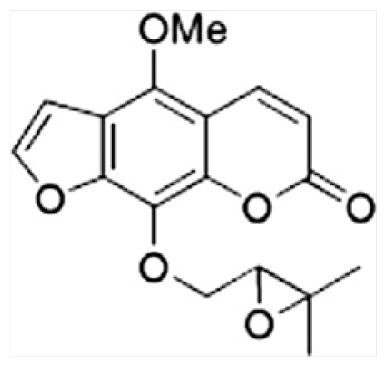
|
| I | Oxypeucedanin |
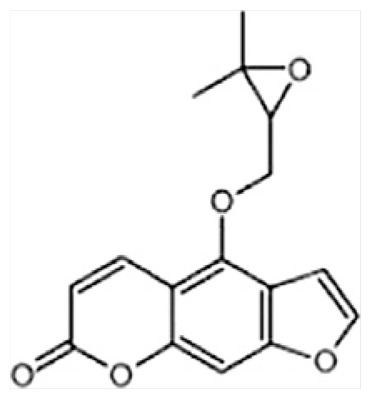
|
| J | 8-Geranyloxypsoralen |
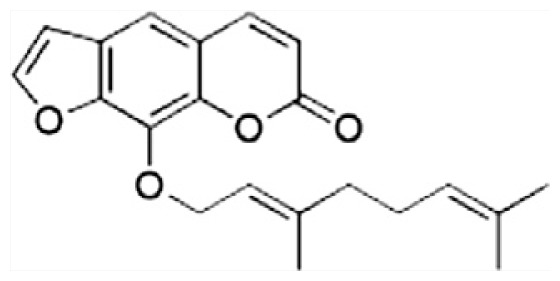
|
| K | Bergamottin |
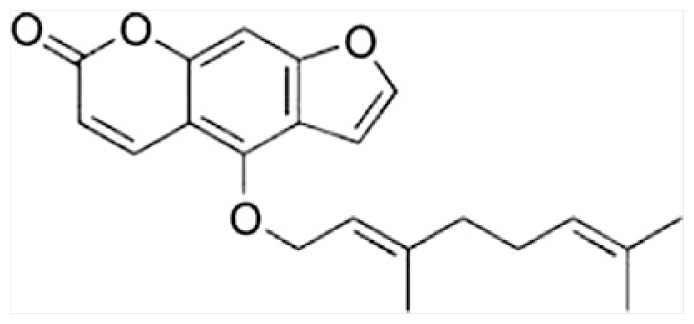
|
| L | 5-Geranyloxy-7-methoxycoumarin |
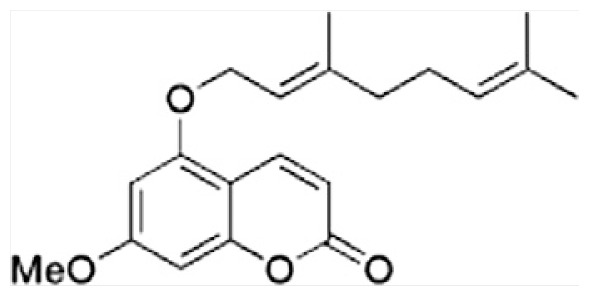
|
3.2. Adulteration case studies
Among all the citrus essential oils that were analyzed, the following three cases were chosen and presented in this section to illustrate the superiority of HPLC analysis over GC analysis against certain adulteration practices.
3.2.1. Case I – orange oil
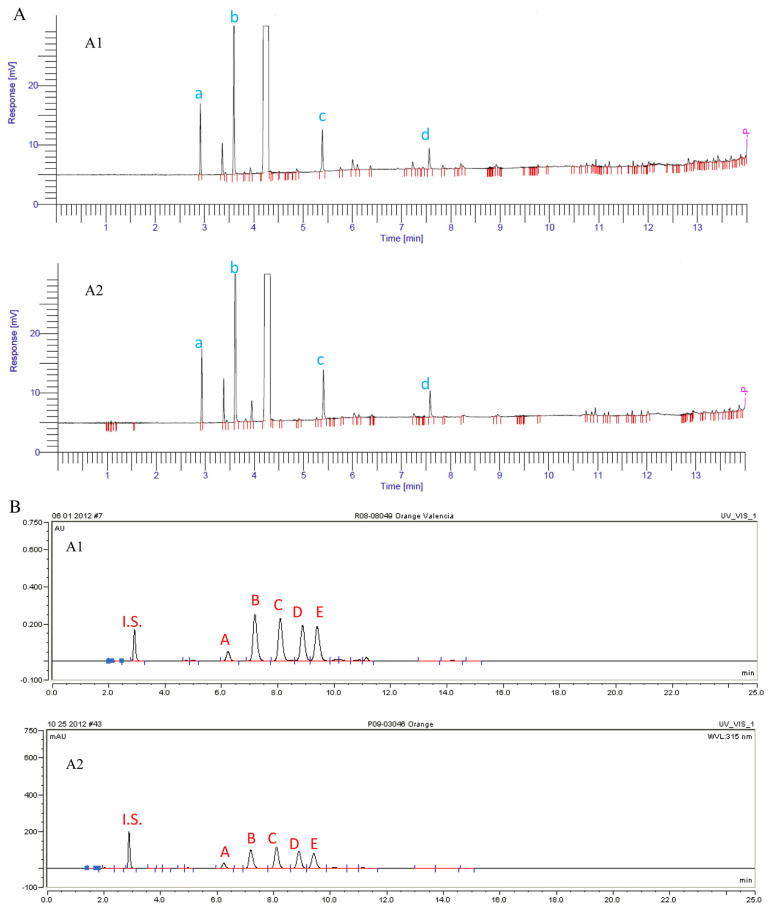
Orange oil sample O was purchased directly from a trusted grower, therefore its authenticity can be guaranteed. Orange oil sample X was purchased from a vendor and its authenticity was yet to be confirmed. Both GC and HPLC analyses were carried out on both samples. The GC profiles of the two samples were strikingly similar (Fig. 4A). In addition to the major compound, d-limonene, the levels of key compounds α-pinene (a), myrcene (b), linalool (c), and decanal (d) were very close between these two samples (Table 2A).
Fig. 4.
(A) GC profiles of target orange oil O (4A1) and doubtful orange oil X (4A2) in Case I. (B) HPLC profiles of target orange oil O (4A1) and doubtful orange oil X (4A2) in Case I.
Table 2A.
Gas chromatographic peak ID and relative area percentage – Case I.
| Peak ID | Sample O | Sample X | |
|---|---|---|---|
| a | α-Pinene | 0.53% | 0.48% |
| b | Myrcene | 1.85% | 1.95% |
| c | Linalool | 0.45% | 0.46% |
| d | Decanal | 0.23% | 0.27% |
However, HPLC results had suggested otherwise. For all five major PMFs that were found in orange oil (Table 1), their levels in sample X were only approximately 50% of those in target sample O (Fig. 4B). Natural variation itself cannot account for such a huge difference between nonvolatile fractions of the two samples. It is very likely that sample X has been adulterated with terpene fraction of orange oil which lacks PMF components, and then was reconstituted by added important aroma chemicals such as linalool and decanal in order to give an acceptable GC profile.
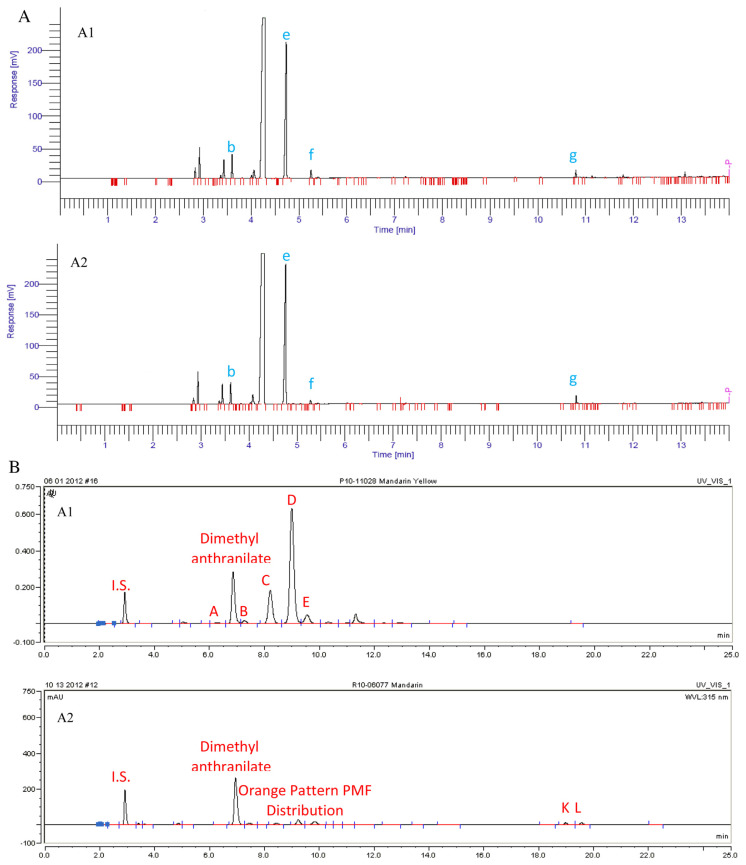
3.2.2. Case II – mandarin oil
Mandarin oil sample M was from a trusted grower, thus there no question about authenticity. Mandarin oil sample Y was from a vendor and its authenticity was yet to be confirmed. GC and HPLC analyses were carried out like before. The GC profiles of sample M and Y look very similar (Fig. 5A and Table 2B).
Fig. 5.
(A) GC profiles of target mandarin oil M (A:5A1) and doubtful mandarin oil Y (A:5A1) in Case II. (B) HPLC profiles of target mandarin oil M (B:5A1) and doubtful mandarin oil Y (B:5A2) in Case II.
Table 2B.
Gas chromatographic peak ID and relative area percentage – Case II.
| Peak ID | Sample M | Sample Y | |
|---|---|---|---|
| b | Myrcene | 1.82% | 1.56% |
| e | γ-Terpinene | 16.46% | 17.02% |
| f | Terpinolene | 0.69% | 0.34% |
| g | Dimethyl anthranilate | 0.46% | 0.47% |
The two small peaks at the end of the HPLC chromatogram of sample Y were confirmed by MS data to be bergamottin and 5-geranyloxy-7-methoxycoumarin (Fig. 5B), which belong to the furocoumarin family and are not supposed to be present in orange oil. Bergamottin and 5-geranyloxy-7-methoxycoumarin are found in significant amounts in both lemon and lime essential oils and their presence in orange oil clearly suggested interspecies adulteration.
By contrast, the peak areas of PMFs in sample Y were too evenly distributed (Fig. 5B), which was not typically seen in mandarin oil where tangeretin should always dominate. The relative ratio between the major PMFs in sample Y suggested an orange origin rather than a mandarin origin. Putting all the findings together, sample Y might have been heavily adulterated with or even made from orange fractions (considering the low PMF levels), then blended with a lemon oil fraction, and finally its volatile fraction was reconstituted by adjusting chemical levels (manifested by the level of dimethyl anthranilate) to achieve an unsuspicious GC profile.
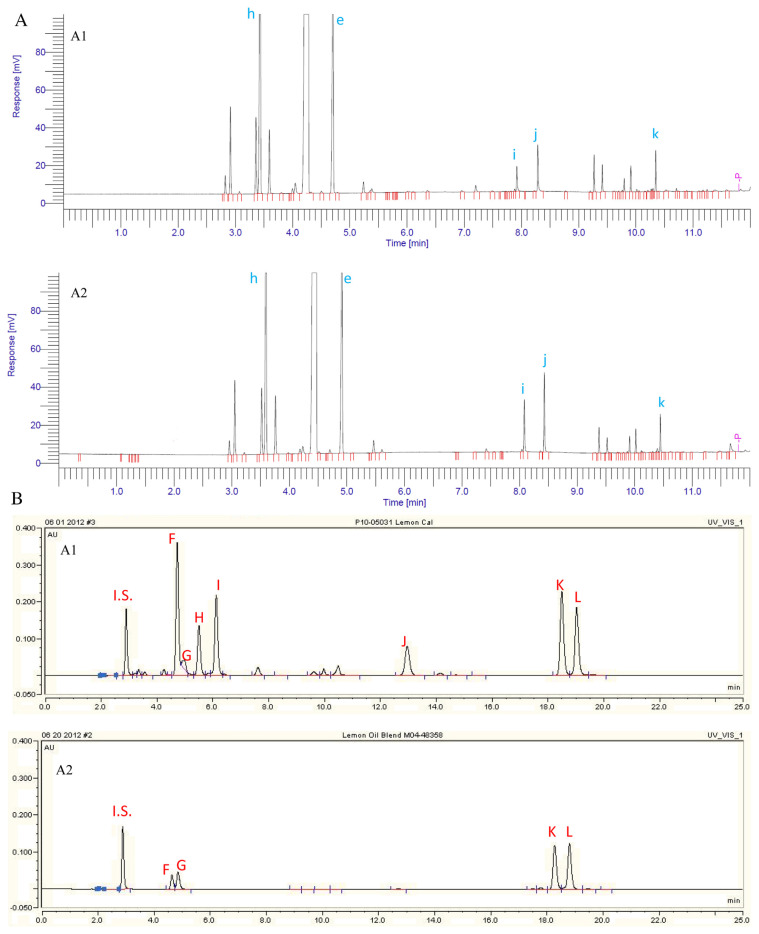
3.2.3. Case III – lemon oil
Lemon oil sample L was regarded as authentic oil free of adulteration. Lemon oil sample Z was under investigation. From their GC profiles (Fig. 6A and Table 2C), sample Z appeared to be superior to sample L due to its high citral (neral and geranial) content. Sample Z also exhibited very similar organoleptic properties to sample L.
Fig. 6.
(A) GC profiles of target lemon oil L (A:6A1) and doubtful lemon oil Z (A:6A2) in Case III. (B) HPLC profiles of target lemon oil L (B:6A1) and doubtful lemon oil Z (B:6A2) in Case III.
Table 2C.
Gas chromatographic peak ID and relative area percentage – Case III.
| Peak ID | Sample L | Sample Z | |
|---|---|---|---|
| h | β-Pinene | 11.82% | 11.20% |
| e | γ-Terpinene | 7.73% | 8.03% |
| i | Neral | 0.55% | 1.10% |
| j | Geranial | 0.95% | 1.58% |
| k | β-Bisabolene | 0.61% | 0.59% |
Fig. 6B shows the HPLC profiles of the two lemon oil samples. Most of the peaks from sample L were reduced or even eliminated in sample Z. No PMFs were found in the profile of sample Z, indicating it was free of orange fractions. All of these facts suggested that lemon sample Z is a reconstituted oil blended from a light lemon fraction in which most of the nonvolatile compounds were stripped off. This light lemon fraction could be either lemon distilled oil or lemon terpenes. Aromatic chemicals like citral were added in order to bring up their percentage to pass GC screening.
From the previous discussion, it is obvious that HPLC analysis has advantages over GC analysis in citrus oil screening, due to the nature of citrus oils and the demand of the essential oil industry. It is common that sample evaluators discover doubtful citrus oils on a regular basis. With the right knowledge and analytical approaches, capital losses caused by adulteration should be minimized.
4. Conclusion
This study focused on the adulteration practices that have been plaguing the essential oil industry for years. Due to its low odor contribution, diversified pattern, high stability, and limited accessibility, the nonvolatile fraction (oxygenated heterocyclic components in the sense of citrus oil) is normally left unattended during most adulteration practices. Therefore, by studying the nonvolatile fraction, researchers are able to reveal the adulterations that have been imposed on the oil samples. By contrast, the volatile fraction of citrus oils is much easier to manipulate, thus inappropriate for citrus oil finger-printing. In conclusion, HPLC analysis has its intrinsic advantages over GC analysis in an authenticity study of citrus oils.
The samples (orange oil, mandarin oil, and lemon oil) that have been tested in this study are industrial oil that appeared in the United States in the past 5 years. The authors believe the data presented in this study can properly represent citrus oils that are currently circulating in the US market. The authors wish that this investigation could help the industry protect itself from poor grade oils.
Acknowledgments
This work was supported in part by Flavor Materials International, Avenel, NJ, USA. All citrus essential oil samples in this study were obtained from Flavor Materials International, Avenel, NJ, USA.
Funding Statement
This work was supported in part by Flavor Materials International, Avenel, NJ, USA.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1.Reineccius G. Flavor chemistry and technology. 2nd ed. New York, NY: Taylor & Francis; 2006. [Google Scholar]
- 2.Wright J. Flavor Creation. 2nd ed. Carol Stream, IL: Alluredbooks; 2011. [Google Scholar]
- 3. Dugo G, Stagno d'Alcontres I, Dugo P. On the genuineness of citrus essential oils. part XXXVI. Detection of added reconstituted lemon oil in genuine cold-pressed lemon essential oil by high resolution gas chromatography with chiral capillary columns. J Essent Oil Res. 1993;5:21–6. [Google Scholar]
- 4. Schipilliti L, Russo M, Mondello L. Genuineness assessment of mandarin essential oils employing gas chromatography-combustion-isotope ratio mS (GC-C-IRMS) J Sep Sci. 2010;33:617–25. doi: 10.1002/jssc.200900504. [DOI] [PubMed] [Google Scholar]
- 5. Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8:135–53. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 6.Murakami A, Ohigashi H. Polymethylated flavonoids: cancer preventive and therapeutic potentials derived from anti-inflammatory and drug metabolism-modifying properties. In: Bao Y, Fenwick R, editors. Oxidative stress and disease. New York: Marcel Dekker; 2004. pp. 187–211. [Google Scholar]
- 7. Li S, Pan MH, Ho CT. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J Funct Foods. 2009;1:2–12. [Google Scholar]
- 8. Ho CT, Pan MH, Li S. Polymethoxyflavones as food factors for the management of inflammatory diseases. J Food Drug Anal. 2012;20(Suppl 1):337–41. [Google Scholar]
- 9.Dugo G, Giacomo A. Citrus – the Genus Citrus. New York, NY: Taylor & Francis; 2002. p. 642. [Google Scholar]
- 10. Chen HC, Peng LW, Sheu MJ, Lin LY, Chiang HM, Wu CT, Wu CS, Chen YC. Effects of hot water treatment on the essential oil of calamondin. J Food Drug Anal. 2013;21:363–8. [Google Scholar]
- 11. Dugo P, Piperno A, Mondello L. Determination of oxygen heterocyclic components in citrus products by HPLC with UV detection. J Agric Food Chem. 2009;57:6543–51. doi: 10.1021/jf901209r. [DOI] [PubMed] [Google Scholar]
- 12. Bonaccorsi IL, McNair HM, Dugo G. Fast HPLC for the analysis of oxygen heterocyclic compounds of citrus essential oil. J Agric Food Chem. 1999;47:4237–9. doi: 10.1021/jf990417s. [DOI] [PubMed] [Google Scholar]
- 13. Li S, Lo CY, Ho CT. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J Agric Food Chem. 2006;54:4176–85. doi: 10.1021/jf060234n. [DOI] [PubMed] [Google Scholar]