Abstract
This study aimed to research the preparation techniques of total flavones from loquat flower (TFLF), its anti-oxidation capacity, and its protective effect on hepatic injury. The best extraction parameters by orthogonal experimentation were water at 100°C, extraction time 2.5 hours, solid/liquid ratio 1:20, and three decoctions. The chromogenic reaction to the flavones showed that loquat flowers mainly contained flavone, flavonol, and flavanone compounds combining orthophenolic hydroxyl group structure in the 10–30% ethanol fraction. The anti-oxidant capacity of was 26.09% and of OH−· was 83.01% by salicylic acid and pyrogallol auto-oxidation. Compared with the model group, TFLF lowered the levels of alanine aminotransferase, aspartate aminotransferase, triglyceride, and malondialdehyde and liver index significantly, and upregulated the expression of adipose triglyceride lipase and Heine oxygenase-1 mRNA. The present findings suggest that TFLF has protective effect on acute alcoholinduced liver injury in mice and may be related to its antioxidant and free-radical scavenging activity.
Keywords: antioxidant, extraction, hepatoprotection, identification, loquat flower, total flavones
1. Introduction
Loquat flower is the dry flower of Eriobotrya japonica (Thunb.) Lindl, which belongs to Eriobotrya, Rosaceae. In traditional Chinese medicine, loquat flower is tasteless, tepid, into the lung meridian. Loquat flower has been mainly used to treat common cold, cough, rhinorrhea with clear discharge, immune deficiency, phlegm with blood, and so on. In the countryside, it is commonly used for children who cough with lung heat and patients with persistent cough [1]. China is abundant in loquat, and holds about 70% of this resource worldwide. Flavonoids are a large group of compounds that exist in many plants. They are the aromatic secondary plant metabolites, and are considered important due to their physiological and pharmacological roles and their health effects [2]. It also has been identified that flavonoids are the main chemical components in the loquat flower. Most of them are connected with carbohydrate to form glycosides. However, some exist in the free state (such as aglycone). Flavonoids not only play an important role in the growth, blooming, and fruiting of the plants, but also have a strong effects on antioxidant, antiviral, and anti-inflammatory activities [3], improve bone properties [4], and prevent cardiovascular and cerebrovascular diseases [5].
In this study, the flavonoids of the loquat flower were isolated, and their protective effect on acute alcohol-induced liver injury in mice was examined. The study provides the scientific basis for the research and exploitation of loquat flower resources.
2. Materials and methods
2.1. Plant material
Loquat flowers, which were provided by Shanghai Lian-Yi Loquat Ecological Park, were authenticated to be the dry alabastrum of white loquat belonging to Eriobotrya of Rosaceae by Professor Huiming Wang, Department of Pharmacognosy, Zhejiang Chinese Medical University, China.
2.2. Animals
Animals used in this study were male Kun Ming mice, weighing 19 ± 1 g provided by the Department of Laboratory Animal Science, Medical Center of Fudan University, Shanghai, China. Animals were fed in a standard animal house at an ambient temperature of 22 ± 2°C and 40–60% relative humidity. Animals were allowed free access to food and water.
2.3. Chemicals
DM301 macroporous adsorptive resin was purchased from Cangzhou Baoen Adsorbing Material Technology (10 Beihai road, Cangzhou, China). Bifendate Pills was purchased from Zhejiang medicine corporation (268 Dengyun road, Hangzhou, China). Triglyceride (TG), alanine aminotransferase (ALT), aspartate amino transferase (AST), and malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (258-27 Central road, Nanjing, China). All other chemicals used were of analytical grade.
2.4. Determination of the content of total flavonoids
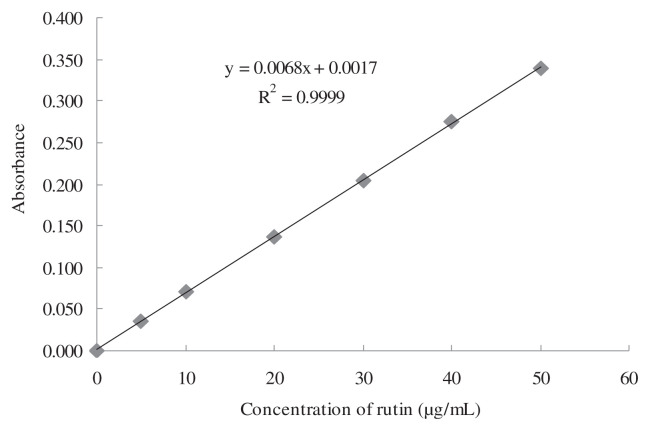
The content of total flavonoids in loquat flowers (TFLF) was detected using the Al(NO3)3–NaNO2–NaOH colorimetric method [6]. Briefly, rutin was used as standard substance, and was dissolved in methanol to get a concentration of 1 mg/mL. The solution was diluted with distilled water to get the 0.05 mg/mL rutin standard solution. Aliquots of 0.0 mL, 0.4 mL, 0.8 mL, 1.6 mL, 2.4 mL, 3.2 mL, and 4.0 mL of the rutin standard solution were diluted with distilled water to 4.0 mL, and 0.2 mL of 5% NaNO2 was added. After 6 minutes, 0.2 mL of 10% Al(NO3)3 was added, and then 1.6 mL of 4% NaOH was added and mixed. The absorbance value was measured at 510 nm after 15 minutes. The standard curve was drawn with the abscissa of rutin concentration (0–0.05 mg/mL), and the ordinate of absorbance value. The regression equation was A = 0.0068C + 0.0017, R2 = 0.9999. The concentration of rutin (C) and absorbance (A) were in good linear relationship within 0.00–0.05 mg/mL (Fig. 1). Flavonoids content was determined according to the above method.
Fig. 1.
Standard curve for total flavonoids detection.
2.5. Extraction of TFLF
TFLF was extracted according to the procedure of traditional boiling optimized by orthogonal experiment, and macroporous resin chromatography.
The optimal conditions of solid–liquid ratio, extraction temperature, and decocting time were investigated through single factor tests. According to the results of single factor experiments, the identified optimum extracting procedure was optimized with L9(34) orthogonal experimentation. A 60 g sample of crude total flavonoids extracted from loquat flowers using optimal methods was dissolved with distilled water, and loaded on to the DM301 macroporous adsorption resin column (6 cm × 50 cm) at the flow rate of 15 mL/min. Ethanol at 0% (water), 10%, 30%, are 50% was used to elute the column at 25 mL/min. Each fraction of 200 mL was collected and the total volume of stepwise elution was 4000 mL; the concentration of total flavonoids in each fraction was detected, then the concentration of fraction above 80% was collected, and named as TFLF.
2.6. Components analysis of TFLF
2.6.1. HCl–Zn reaction
A 1-mL aliquot of 10% sample ethanol solution and 30 mg Zn dust were added into a test tube and mixed; 100 μL of 10M HCl was added and the color change of the solution observed for several minutes. Most flavone, flavonol, flavanone, and flavanonol compounds will become red to prunosus, and some will become blue or green. The color will become deeper, especially when the benzene ring is replaced by auxochrome. Meanwhile the same sample without Zn dust but with 100 μL of 10M HCI was taken as the blank control to avoid the false positive reactions. If the solution becomes red, this indicates that the sample contains pigments or some aurones or chalcone.
2.6.2. KBH4 reduction reaction
A 100-μL aliquot of 10% sample ethanol solution was dropped on a silica gel plate, sprayed with 1% KBH4-isopropanol solution, and fumigated with 10M HCl after 5 minutes. Flavonone and flavanonol were reduced to become red to prunosus. The color will be deeper if A and B annulations are substituted by auxochrome. The other flavones give a negative reaction.
2.6.3. AlCl3 reaction
A 100-μL aliquot of 10% sample ethanol solution was dropped on a filter paper, then 2% AlCl3 ethanol solution was sprayed and observed in UV light. If yellow fluorescence is observed, this suggests that it contains 5-OH flavonoids. If sky blue fluorescence is observed, this demonstrates that it contains 4′-OH flavonol or 7,4′-OH flavonol compounds.
2.6.4. FeCl3 reaction
A 100-μL aliquot of FeCl3 solution was added to 2 mL of 10% sample ethanol solution. A positive chromogenic reaction showed the existence of phenol hydroxyl.
2.6.5. SrCl2 reaction
A 2-mL aliquot of 10% sample ethanol solution was taken into a test tube; 100 μL of 0.01 mM SrCl2 methanol solution and 100 μL of methanol solution saturated by ammonia vapor were added. If any green to brown or even black precipitation is generated, this suggests that adjacent two phenolic hydroxyl groups are present.
2.6.6. PbAc2 reaction
A 1-mL aliquot of 10% sample solution was taken into a test tube, and then 1 mL of 1% PbAc2 aqueous solution in distilled water was added; its color change and the presence of precipitation after shaking was observed. A positive reaction denoted that the sample contained an orthodiphenolic hydroxyl group or combined with 3-OH, 4-ketone group or 5-OH,4-ketone group of the structure.
2.6.7. Na2CO3 reaction
A 1-mL aliquot of 10% sample solution was taken into a test tube and 1 mL 1% Na2CO3 solution added. Its color change and the presence of precipitation after shaking were observed, with a positive reaction indicating that the sample contained flavonol compounds [7].
2.7. Detection of antioxidant capacity of TFLF
2.7.1. Clearance test of superoxide radical anion
The test was carried out using the method of Wang et al [8] with some modifications. Briefly, 0.05M Tris-HCl (pH 8.2) and 10 mM pyrogallol were preheated at 25°C for 20 minutes; 2 mL 0.05M Tris-HCl buffer was mixed with 100 μL distilled water and 50 μL 10mM pyrogallol. Tris-HCl buffer (0.05M) was used as zero setting, with absorbance (A0) measured at 325 nm/30 seconds. The linear regression was done with abscissa T (time), ordinate A (absorbance), and the slope was recorded as V0. Then another 2 mL 0.05M Tris-HCl buffer was taken and the distilled water replaced with 100 μL 2 mg/mL TFLF and 2 mL VC (positive control) to determine the absorbance (A1) with the same methods. Its slope was V1. The capacity of superoxide radical anion clearance was calculated with the following formula:
| (1) |
2.7.2. Clearance test of hydroxyl radical
The test was carried out according to the Fenton reaction by the procedures of Luo et al [9] with some modifications. Briefly, 2 mL 2 mg/mL TFLF, 1 mL 9 mM salicylic acid–ethanol solution, 1 mL 9mM FeSO4, and 8.8mM H2O2 were added into a test tube with 37°C water heating for 30 minutes. Then the absorbance (A1) at 510 nm was measured with double distilled water used as zero setting. Meanwhile, the absorbance (A0) was determined with 2 mg/mL of VC as the positive control and equal-volume distilled water as the blank control. Because the TFLF itself had a little absorbance, H2O2 was replaced by an equal volume of distilled water as the background control to determine the absorbance (A2). The clearance capacity was calculated using the following formula:
| (2) |
2.8. Detection of protective effect of TFLF on acute alcohol-induced liver injury in mice
2.8.1. Animal treatment and biochemical analysis
Fifty-six male Kunming mice were randomly assigned to seven groups of eight mice each: control group; model group; 200 mg/kg TFLF group; 400 mg/kg TFLF group; 800 mg/kg TFLF group; 150 mg/kg bifendate group; and 800 mg/kg aqueous extract of loquat flower group. The preparations were administrated intragastrically at a dose of 0.10 mL/10 g once per day for 10 days; 0.12 mL/10 g body weight of 50% (V/V) dehydrated alcohol was administrated intragastrically at 2 hours after the last administration of the preparations except the normal group. Then the animals were fasted for 16 hours.
The animals were sacrificed, and 0.5 mL of venous blood was collected from the eye socket vein. The liver, spleen, thymus, and kidney were dissected and weighed. 100 mg of hepatic tissue was dissected and homogenated. The concentrations of ALT, AST, and TG in serum and MDA in liver homogenate were determined according to the manufacturer’s instructions.
2.8.2. Detection of RNA expression of adipose triglyceride lipase and Heine oxygenase-1 in liver
Total RNA from liver tissue was isolated using Trizol reagent according to the manufacturer’s instructions (TaKaRa Biotechnology (Dalian) Co., Ltd, 19 Dongbei 2nd street, Development Zone, Dalian, China). The gene expression of adipose triglyceride lipase (ATGL) and Heine oxygenase-1 (HO-1) was measured by real-time quantitative polymerase chain reaction (PCR), using a model Chromo 4 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Relative mRNA levels were calculated by the 2−ΔΔCt method and normalized against β-actin. Primers used in real-time PCR are listed in Table 1.
Table 1.
Primer sequences for real-time polymerase chain reaction of adipose triglyceride lipase (ATGLI) and Heine oxygenase-1 (HO-1).
| GenBank no. | Gene | Sense primer (5′–3′) | Antisense primer (5′–3′) | Product length (bp) |
|---|---|---|---|---|
| NM_001163689.1 | ATGL | GGTGCCAACATTATTGAGGTG | TGAAACACGAGTCAGGGAGAT | 171 |
| NM_010442.2 | HO-1 | AGACACCGCTCCTCCAGT | CAGGTATCTCCCTCCATTCC | 242 |
| NM_007393.3 | β-actin | TGCTGTCCCTGTATGCCTCT | CTTTGATGTCACGCACGATT | 225 |
2.9. Statistical analysis
The results are expressed as mean ± standard deviation. Statistical analyses were done by one-way analysis of variance examined by SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Values of p < 0.05 were considered as significantly different, and p < 0.01 as extremely different.
3. Results
3.1. The optimal extraction procedure of crude flavonoids of loquat flower
The crude flavonoids of loquat flower were extracted by traditional boiling. The optimal conditions of solid–liquid ratio, extraction temperature, decocting time, and number of decoctions were optimized with L9(34) orthogonal experimentation. Factors and levels are shown in Table 2. As shown in Table 3, the optimal procedure to extract crude flavonoids was: temperature 100°C, decocting time 2.5 hours, solid–liquid ratio 1:20, and decocting three times. The results show that the rank of each factor for extraction from best to least was frequency, temperature, solid–liquid ratio, and extraction time.
Table 2.
Factors and levels for orthogonal design of total flavonoids extracted from loquat flowers.
| Level | Factor | |||
|---|---|---|---|---|
|
| ||||
| Temperature (°C) | Extraction time (h) | Solid–liquid ratio | Frequency | |
| 1 | 80 | 1.5 | 1:15 | 1 |
| 2 | 90 | 2.0 | 1:20 | 2 |
| 3 | 100 | 2.5 | 1:25 | 3 |
Table 3.
L9(34) orthogonal experiment.
| Number | Factors | Content of flavonoids (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Temperature | Time | Solid–liquid ratio | Frequency | ||
| 1 | 1 | 1 | 1 | 1 | 2.79 |
| 2 | 1 | 2 | 2 | 2 | 4.94 |
| 3 | 1 | 3 | 3 | 3 | 5.98 |
| 4 | 2 | 1 | 2 | 3 | 6.37 |
| 5 | 2 | 2 | 3 | 1 | 3.88 |
| 6 | 2 | 3 | 1 | 2 | 5.34 |
| 7 | 3 | 1 | 3 | 2 | 6.78 |
| 8 | 3 | 2 | 1 | 3 | 7.64 |
| 9 | 3 | 3 | 2 | 1 | 5.46 |
| K1 | 4.57 | 5.313 | 5.257 | 4.043 | |
| K2 | 5.197 | 5.487 | 5.590 | 5.687 | |
| K3 | 6.627 | 5.593 | 5.547 | 6.663 | |
| R | 2.057 | 0.280 | 0.333 | 2.620 | |
3.2. Preparation of TFLF with macroporous resin chromatography
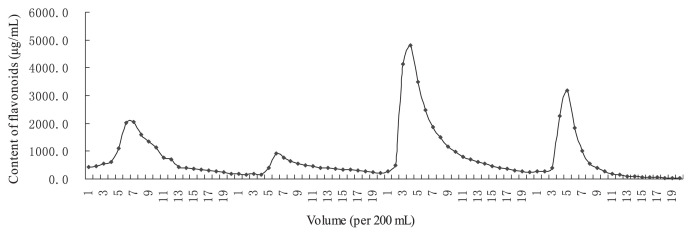
The eluted curve of TFLF from macroporous resin chromatography is shown in Fig. 2. Twenty fractions were collected with volumes of 200 mL. The content of flavonoids in each fraction was measured. The flavonoid content of the four peak fractions collected was 56.06%, 23.62%, 83.69%, and 23.62%. The 30% ethanol fraction had the highest concentration of flavonoids (> 80%); this was collected, vacuum dried, and used as TFLF.
Fig. 2.
Preparation curve of total flavones from loquat flower.
3.3. Component analysis of the total flavonoids
The chromogenic reaction to the flavonoids showed that the water eluent mainly contained phenolic hydroxyl group, 4′-OH flavonol or 7, 4′-OH flavonol. The 10% ethanol eluent mainly contained flavanone and flavanonol. The 30% ethanol eluent mainly contained flavones, flavonol, flavanone, and flavanonol with orthophenolic hydroxyl group. The 50% ethanol eluent mainly contained the same type of flavonoid, but the specific varieties and contents are different. All of the fractions contained 3-OH, 4-ketone group, or 5-OH, 4-ketone group, but none of them contained anthocyan or chalcone (Table 4).
Table 4.
Chromogenic reaction results of the flavones.
| Condition | Water elution | 10%EtOH elution | 30%EtOH elution | 50%EtOH elution |
|---|---|---|---|---|
| HCl | — | — | — | — |
| HCl-Zn | — | — | Light red | red |
| KBH4 | — | Red | Purple | Red |
| AlCl3 | Sky bluea | Light bluea | Gray–yellowa | Bright yellowa |
| FeCl3 | Clay bank precipitation | Light brown | Brown precipitation | Brown precipitation |
| SrCl2 | Light green | Light green | Brown precipitation | Flavor green precipitation |
| PbAc2 | Light green precipitation | Earthy yellow | Sandy beige precipitation | Yellow precipitation |
| Na2CO3 | Light yellow | Light mignonette | Sepia precipitation | Yellow precipitation |
— = no obvious change.
Represents fluorescence.
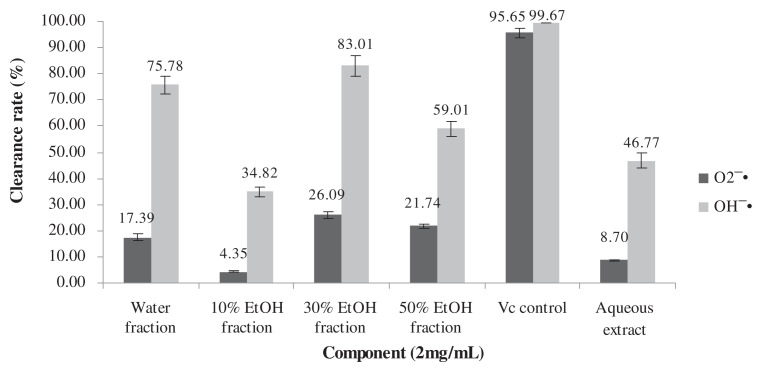
3.4. The capacity to clear free radicals of the total flavonoids
The ability of the four eluents, the aqueous extract, and the VC control to clear the and OH−· is shown in Fig. 3. The clearance of 30% ethanol eluent was 26.09% and 83.01%, respectively, at the dose of 2 mg/mL.
Fig. 3.
The capacity of each fraction to clear free radicals.
3.5. The effect of TFLF on the levels of ALT, AST, TG, and MDA and organ index
The concentrations of ALT, AST, and TG in serum and MDA in liver homogenate are shown in Table 5. Compared with the normal group, the contents of ALT, AST, TG, and MDA of model groups were increased significantly (p < 0.05, p < 0.05, p < 0.01, and p < 0.01, respectively), which suggests that the model was successfully made. Comparing with the model group, ALT, TG, and MDA in all of the TFLF treated groups decreased significantly (p < 0.05), and AST in the 200 mg/kg and 400 mg/kg TFLF treated groups decreased significantly (p < 0.01 and p < 0.05, respectively). The effect of TFLF on the organ index is shown in Table 6. Thymus, spleen, and kidney index had no significant change in all groups (p > 0.05). Liver index in the model group was increased significantly when compared with the normal control group (p < 0.01), and it was decreased significantly in 400 mg/kg and 800 mg/kg TFLF treated groups when compared with the model group (p < 0.05). These results suggest that TFLF has advantages of decreasing the levels of AST, ALT, TG, and MDA.
Table 5.
The influence of total flavones from loquat flower (TFLF) on alanine aminotransferase (ALT), aspartate amino transferase (AST), triglyceride (TG), and malondialdehyde (MDA) in acute alcoholic liver injury mice.
| Group | ALT | AST | TG | MDA |
|---|---|---|---|---|
|
|
|
|
|
|
| (U/L) | (U/L) | (mM) | (nmol/mg) | |
| Normal | 30.61 ± 3.73 | 70.11 ± 7.07 | 2.49 ± 0.38 | 1.15 ± 0.33 |
| Model | 42.39 ± 10.28* | 94.46 ± 22.80* | 3.49 ± 0.72** | 1.69 ± 0.20** |
| 150 mg/kg Bifendate | 31.87 ± 7.47*** | 66.44 ± 13.32*** | 2.91 ± 0.46 | 1.27 ± 0.22**** |
| 200 mg/kg TFLF-Low | 31.90 ± 6.48*** | 60.91 ± 13.08**** | 2.60 ± 0.68*** | 1.45 ± 0.30 |
| 400 mg/kg TFLF-Mid | 31.49 ± 7.81*** | 73.25 ± 11.66*** | 2.37 ± 0.84*** | 1.44 ± 0.21*** |
| 800 mg/kg TFLF-High | 31.63 ± 4.47*** | 81.58 ± 14.72 | 2.66 ± 0.79*** | 1.34 ± 0.35*** |
| 800 mg/kg Aqueous extract | 32.54 ± 5.31*** | 60.38 ± 10.42**** | 2.94 ± 0.46 | 1.38 ± 0.25*** |
Data are presented as mean ± standard deviation (n = 8).
p < 0.05,
p < 0.01, compared with normal group.
p < 0.05,
p < 0.01, compared with model group.
Table 6.
The effects of total flavones from loquat flower (TFLF) on organ index in acute alcoholic liver injury mice.
| Group | Liver index (mg/g) | Thymus index (mg/g) | Spleen index (mg/g) | Kidney index (mg/g) |
|---|---|---|---|---|
| Normal | 29.38 ± 2.83 | 3.73 ± 1.13 | 2.40 ± 0.34 | 13.81 ± 1.26 |
| Model | 45.48 ± 2.23** | 3.32 ± 0.74 | 2.10 ± 0.48 | 14.01 ± 1.24 |
| 150 mg/kg Bifendate | 43.73 ± 4.30 | 3.94 ± 0.97 | 2.31 ± 0.48 | 13.38 ± 1.56 |
| 200 mg/kg TFLF-Low | 42.84 ± 2.65 | 3.81 ± 0.67 | 2.05 ± 0.48 | 14.05 ± 1.61 |
| 400 mg/kg TFLF-Mid | 40.93 ± 4.14*** | 3.66 ± 0.70 | 2.02 ± 0.76 | 13.96 ± 2.48 |
| 800 mg/kg TFLF-High | 42.10 ± 2.48*** | 3.56 ± 0.80 | 2.00 ± 0.79 | 14.22 ± 1.59 |
| 800 mg/kg Aqueous extract | 45.15 ± 4.61 | 3.90 ± 1.09 | 2.31 ± 0.48 | 14.52 ± 0.90 |
Data are presented as mean ± standard deviation (n = 8).
p < 0.01, compared with normal group.
p < 0.05, compared with model group.
3.6. The effect of TFLF on the expression of hepatic ATGL and HO-1 genes in acute alcoholic liver injury mice
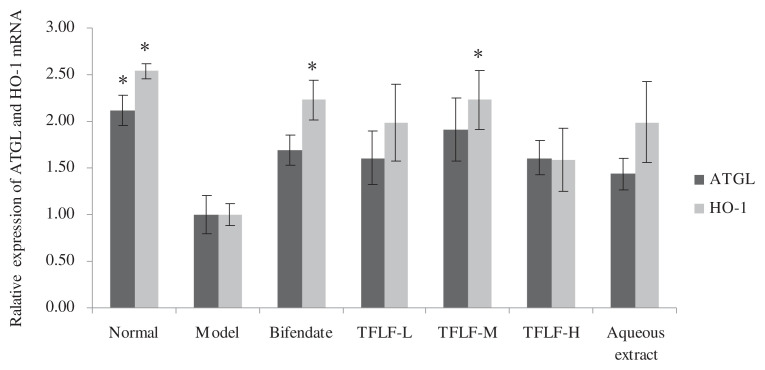
As shown in Fig. 4, compared with the normal group, the mRNA expression of ATGL and HO-1 genes in the model group was decreased 2.1 times and 2.5 times respectively. Compared with the model group, the ATGL gene expression increased 1.6 times, 1.9 times, and 1.6 times respectively in the 200 mg/kg, 400 mg/kg, and 800 mg/kg TFLF treated groups, and the HO-1 gene expression increased 1.9 times, 2.3 times, and 1.6 times, respectively, in the three TFLF treated groups.
Fig. 4.
The effect of TFLF on the relative expression of ATGL and HO-1 mRNA. * Compared with the model group, there is a significant difference if the expression quantity of ATGL or HO-1 is more than 2 times.
4. Discussion
Loquat flower has a complex chemical composition, in which flavonoids and triterpenes hold a large part. Other active components, such as hemiterpene, polyphenols, glucoside, and aetherolea, are also major active components. Flavonoids are a series of compounds with aromatic ring A and B connected through three central carbon atoms, which can be discriminated by colorable reactions depending on the phenolic hydroxyl group and pyrone ring of the structure. Colorations result indicate that, with the increase of alcohol concentration, the polarity of flavonoids degrades, and the categories also change, illustrating that macroporous adsorptive resins have separated and abstracted the loquat flower flavonoids to a certain degree.
Macroporous adsorptive resins are man-made organic high molecular polymers with a porosity stere-framework. Active components can be abstracted and purified using the principles of aperture, surf-electric charge, and hydrogen bond adsorption. Component selectivity varies under the different polarities and aperture of resins. Generally speaking, semi-polarity macroporous resins contain ester groups that have hydrophilic and hydrophobic parts on the surface, which can absorb nonpolar compounds in polar solvent and polar compounds in nonpolar solvent. They are especially good at separating and purifying organic compounds with pole and low-pole. DM301 is a semi-polarity copolymer of benzene vinyl type, which is suitable for the abstraction of aqueous extract with high polarity. Therefore, through screening four resins (D101, AB-8, DM301, and S-8), we selected DM301 as the optimization resin to do the further study on abstraction and purification of the loquat flower flavones based on the optimization aqua-extract technology.
A free radical is a group that contains an unpaired electron in the external electron orbit and can be produced intracell, in biolast, endocytoplasmic reticulum, cytoblast and cytochyma, et al. Oxygen inside the cell converts into a superoxide anion free radical when it absorbs the electron, and generates various kinds of free radicals, such as peroxy radical, hydroxyl radical, and alcoxyl radical, which are very active and easy to attack soma, glandular organs, cells (cell envelope), and bio-macromolecules, leading to oxidative damage [10]. In the aspect of antioxidation mechanism, flavonoids are considered to turn to semiquinone free radicals, which have a more stable structure after the reaction between phenolic hydroxyl groups and free radicals, and the chain reaction is thus terminated. Generally speaking, phenolic hydroxyl groups of ring B have the highest antioxygen activity, ring B ranks the second, and ring A is the weakest [11]. In recent years, antioxidant active component studies on loquat flowers mainly focus on aqueous or alcohol extract. Yan et al [12] determined the antioxidant activity of the phenolics of loquat flowers by measuring DPPH (1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), FRAP (ferric reducing antioxidant power), and F-C (Friedel-Crafts acylation). The entire test indicated that the antioxidant activity of aqueous and methanolic extract is higher than that of ethanol. Zhou et al [13] confirmed that flavonoids and polyphenols have a better antioxidant activity. We used macroporous adsorptive resins to get further abstraction of the flavonoids based on the aqueous extract of loquat flowers, adopting measures of ortho-oxybenzoic acid and pyrogallic autoxidation to determine the antioxygen activity. The result indicates that after ethanol gradient elution, each composition had a marked change on eliminating superoxide radical anion and hydroxy radical compared to aqueous extract, the composition of 30% ethanol elution has the highest activity of antioxygen activity.
There are 22 kinds of amino acids participating in transamination reactions in the human body and each demands a distinct aminopherase. ALT and AST are the two important aminopherases in liver, and they mostly reside in endochylema and mitochondrion. After heavy drinking, the alcohol and its metabolic products may have a direct toxic effect on the hepatic cell, which would cause a rise of the permeability of the cell membrane, leading to the emission (as high as 65%) of aminopherase, and thus increase the content of the two enzymes in blood serum. Therefore, the content of aminopherase in blood serum is one of the most direct and sensitive indexes to reflect the cellular damage of hepatocyte [14]. In addition, excess H+ emerging during the metabolic process of alcohol in liver could promote increased synthesis of α-glycerol-phosphoric acid and fatty acid, and decreased fatty acid catabolism, leading to faster TG synthesis, which is then deposited in the liver and results in fatty substance metabolism abnormality. The deposition of TG in the liver will assemble into very low-density lipoprotein, which is then delivered into the blood and will thus increase the level of TG.
As the end product of lipid preoxidation, MDA may reflect the preoxidative degree of the free radicals in vivo. Therefore, changes in MDA content can indicate the damage of cells indirectly [15]. ATGL, the key enzyme of the first step in adipose decomposition, is mainly responsible for converting TG to diglyceride, while it has little effect on lipolysis of other lipids. HO-1 also called heat shock protein 32, is a highly inducible, stress-responsive protein. It catalyzes the first and rate-limiting step in heme degradation. Heme is a potent oxidant, and the induction of HO-1 can provide cytoprotection against oxidative stress. The results show that loquat flower aqueous extract and its flavonoids have effect of reducing the levels of ALT, AST, TG, MDA on the acute alcoholic liver injury mice, and the mechanism is associated with the upregulation of ATGL and HO-1 expression.
Acknowledgments
This study was supported by the PLA grants (No.AS11J003-11). Thanks for the helpless from Qing-Rong Wang, Ding-Wen Jiang, Deng-Yong Hou, Yu-Ming Liu, Ke-Xian Li, Wei-Chen, and Qiong-Liu in Naval Medical Research Institute.
Funding Statement
This study was supported by the PLA grants (No.AS11J003-11).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1.The Chinese materia medica Editorial Committee of State Administration of Traditional Chinese Medicine Chinese materia medica (4) Shanghai Science and Technology Press; 1999. p. 145. [Google Scholar]
- 2. Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of citrus juices. Molecules. 2007;12:1641–73. doi: 10.3390/12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharififar F, Khazaeli P, Alli N, Talebian E, Zarehshahi R, Amiri S. Study of antinociceptive and anti-inflammatory activities of certain Iranian medicinal plants. J Intercult Ethnopharmacol. 2012;1:19–24. [Google Scholar]
- 4. Pang WY, Wang XL, Wong KC, Leung PC, Yao XS, Wong MS. Total flavonoid fraction of rhizoma drynaria improves bone properties in ovariectomized mice and exerts estrogen-like activities in rat osteoblast-like (UMR-106) cells. J Food Drug Anal. 2012;20:265–9. [Google Scholar]
- 5. Gross M. Flavonoids and cardiovascular disease. Arch Physiol Biochem. 2004;42:21–35. [Google Scholar]
- 6. Hu J, Zhang H, Liu G, Zhong H. Studies on extraction technology of total lavonoids from flower of Eriobotrya japonica. Zhog Yao Cai. 2008;31(11):1724–7. [In Chinese] [PubMed] [Google Scholar]
- 7.Kuang HX, Dong XP, Shi RB, Wang D, Wang XL, Feng WS, Liu JQ, He SM, Li X, Zhang ZR, Chen JZ, Chen SH, Rao GX, Guo M, Cui J, Pei MR. Chemistry of Chinese Materia medica. China Press of Traditional Chinese Medicine; 2003. pp. 144–8. [Google Scholar]
- 8. Wang R, He M, Yuan XC, Jiang B, Xue M. Antioxidant activity of total flavonoids from Oroxylum indicum. Chin J Exp Trad Med Form. 2012;18:102–5. [Google Scholar]
- 9. Luo CZ, Kong YQ, Zhang H, Zheng H, Zhang JY, Guo YH. The radical scavenging activity of flavonoids from Buddleja officinalis Maxim. Chem Ind Forest Prod. 2012;32:97–101. [Google Scholar]
- 10.Xiong ZY. The biological research of free radical in exercise. Science Press; 2010. pp. 1–5. [Google Scholar]
- 11. Jiang ST, Nie CM, Shi CY, Lin YW. Theoretical study on the anti-oxidant activity of flavonoids. J Univ South China (Sci Technol) 2010;24:101–7. [Google Scholar]
- 12. Yan YF, Sun J, Meng TZ, Yi XQ. Antioxidant capacities of loquat flower extracts using different solvents. Sci Technol Food Ind. 2012;33:122–4. [Google Scholar]
- 13. Zhou CH, Sun CD, Chen KS, Li X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int J Mol Sci. 2011;12:2935–45. doi: 10.3390/ijms12052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dolganiuc A, Szabo G. In vitro and vivo models of acute alcohol exposure. World J Gastroenterol. 2009;15:1168–77. doi: 10.3748/wjg.15.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao M, Du YQ, Yuan L, Wang NN. protective effect of puerarin on acute alcoholic liver injury. Am J Chin Med. 2010;38:241–9. doi: 10.1142/S0192415X10007816. [DOI] [PubMed] [Google Scholar]