Abstract
Acetylcholinesterase (AChE) inhibition enhances learning and cognitive ability for treatment of Alzheimer’s disease. Polysaccharide–peptide complexes were identified in Cordyceps militaris (CPSPs) and characterized for their AChE inhibitory properties. Three polymers (CPSP-F1, -F2, and -F3) were extracted and separated by ultrasound-assisted extraction and dieth-ylaminoethanol (DEAE)–Sepharose CL-6B column chromatography. Polysaccharide–peptide complexes were identified by DEAE–Sepharose CL-6B column chromatography and high-performance gel-filtration chromatography, Fourier transform infrared spectra, amino sugar composition analysis, and β-elimination reaction to identify polysaccharide–peptide bond categories. Separation of CPSP can increase AChE inhibitory activity from the crude poly-saccharide of C. militaris. CPSP-F1 and CPSP-F2 exhibited half maximal inhibitory concentrations of 32.2 ± 0.2 mg/mL and 5.3 ± 0.0 mg/mL. Thus, we identified polysaccharide–peptide complexes from C. militaris and suggest CPSP has great potential in AChE inhibition bioassay.
Keywords: acetylcholinesterase, Cordyceps militaris, Fourier transform infrared spectra, high-performance gel-filtration, chromatography, polysaccharide-peptide complexes
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that impairs memory and behavior in elderly people [1,2]. Reduced acetylcholine in the brain is the most remarkable biochemical change in AD patients [3]. Acetylcholinesterase (AChE) terminates nerve impulse transmission by rapid hydrolysis of acetylcholine; thus, AChE inhibition serves as a mechanism for the treatment of AD [4]. AChE inhibitors such as tacrine, donepezil, rivastigmine, and galanthamine have been used for clinical AD therapy [5].
Several screening methods for AChE inhibitory activity in natural sources have been reported based on Ellman’s reactions [4]; a rapid method of colorimetric determination of AChE inhibitory activity [6]. In this inhibitory mechanism, the inhibitor binds to the same active site as the enzyme substrate, and this is suggested to be a nonmetabolizable reaction [7]. Polysaccharide (PS) have been extracted or modified and assessed for their ability to inhibit AChE. Flammulina velutipes polysaccharides were extracted by ultrasonic extraction and reported to be the main AChE-inhibiting component of this organism; 18.51% AChE inhibition was obtained at 0.6 mg/mL Flammulina velutipes polysaccharide [8]. Gallic acid-grafted chitosans produced at different grafting ratios exhibited potent AChE inhibition with half maximal inhibitory concentration (IC50) of 138.5 ± 0.25 μg/mL to 397.6 ± 5.2 μg/mL [9]. Cordyceps militaris possesses antioxidant, immunopotentiation, antitumor, anti-inflammatory, hypoglycemic and hypocholesterolemic activities [10–12]. In our continued search for bioactive materials, we explored AChE inhibitory activity in C. militaris.
Polysaccharides are present in several mushrooms as polysaccharide–peptides (PSPs) and polysaccharide–triterpenoids [13]. Several studies have reported PSP complexes in mushrooms such as Tricholoma lobayense, Coriolus versicolor, Lentinus edodes, and Grifola frondosa [14–16]. Many C. militaris polysaccharides have been structurally characterized and analyzed for bioactivity [10,17], but PSP complexeshavenot beenreported in C. militaris. In previous studies of Cordyceps sp., three different PSP complexes of Cordyceps sphecocephala were fractionated and their anticancer activities were investigated in human hepatocarcinoma (HepG2) and neuroblastoma (SK-N-SH) cells [18,19]. A protein-bound polysaccharide HS002-II was produced from Hirsutella sinensis Liu, Guo, Yu & Zeng, which is the anamorph of Cordyceps sinensis, and its immunomodulatory activity was investigated in murine macrophage cell line RAW264.7 cells [20]. The protein-bound polysaccharides were extracted from Cordyceps ophioglossoides and their anticancer activities were investigated in mice [21]. We explored C. militaris for the presence of PSP complexes. C. militaris polysaccharide–peptide complexes (CPSPs) were fractionated and identified by column chromatography and high-performance gel-filtration chromatography (HPGFC), Fourier transform infrared (FTIR) spectra, amino sugar composition analysis, and β-elimination reaction to identifypolysaccharide–peptide bond categories. We also studied the AChE inhibitory activity of CPSPs to understand their potential utility as AChE inhibitors.
2. Materials and methods
2.1. Materials
C. militaris fruiting bodies (BCRC 32219, deposited at the Bio-resource Collection and Research Centre, Food Industry Research and Development Institute, Hsinchu, Taiwan) were provided by Xin-Run Biotechnology Co. (Changhua, Taiwan). Glucose and glucosamine hydrochloride standards were purchased from Sigma-Aldrich (St Louis, MO, USA). 5,5′-dithio-bis-(2-nitrobenzoic) acid, acetylthiocholine iodide, AChE type VI-S, bovine serum albumin (BSA), and galanthamine hydrobromide were obtained from Sigma-Aldrich. All reagents and organic solvents were analytical grade. Absorbance was recorded with a UV-Vis Spectrophotometer V-530 (Jasco, Tokyo, Japan).
2.2. Extraction and preparation of crude PS
Crude PS was extracted by ultrasound-assisted extraction (UAE) (Model: MS-600, Min Shin Machinery Co., Changhua, Taiwan) from C. militaris fruiting bodies. Powdered C. militaris fruiting bodies (3.0 g) were extracted in 45 mL reverse osmosis (RO) water. Extractions were performed at 100°C for 30 minutes at 300 W ultrasonic power. After extraction, 45 mL RO water was added to the residual powder for a second extraction to maximize yield. The extract was collected and concentrated under reduced pressure. The concentrated extracts were mixed with three volumes 95% ethanol at 4°C overnight. The crude PS was precipitated by centrifugation at 6000 rpm (3864g) for 20 minutes.
2.3. Chemical analysis
Total carbohydrate content was determined by the phenol sulfuric acid method [22]. Reducing sugars were measured by the dinitrosalicylate method [23]. Glucose was used for the standard curve. The crude PS content was calculated as the difference between the contents of total carbohydrate and reducing sugars [24]. Total protein content was determined by the Lowry assay [25] with BSA for the standard curve.
Total amino sugar content was estimated by the method of Rondle and Morgan [26]. In this study, we referred to the method of Chen and Johnson [27] to estimate total amino sugar of CPSP with a glucosamine hydrochloride standard. Prior to analysis, samples were hydrolyzed by heating in a sealed tube with 4N HCl at 100°C for 10 hours. After hydrolysis, excess acid was removed by adjusting to neutral pH with a minimum quantity of 1N NaOH and the final volume was adjusted to 10.0 mL with RO water.
2.4. Chromatographic fractionations of CPSP
The concentrated crude PS solution was filtered through a 0.45-μm nylon membrane. The concentrated filtrate (212.5 mg/mL in 10.0 mL) was loaded onto a DEAE–Sepharose CL-6B anion-exchange column (2.5 cm × 10.0 cm; GE Health-care, Uppsala, Sweden). Fractions were eluted with a stepped NaCl gradient (from 0.1M to 0.5M to 1.0M) at 0.6 mL/minute (0.7 rpm, Chrom Tech TP-T100T peristaltic pump; Taipei, Taiwan). The eluate was collected in 6.0 mL fractions (Chrom Tech FC-100A). Eluates were analyzed by measuring the absorbance at 490 nm for carbohydrates after using the phenol sulfuric acid method and at 280 nm for proteins [18]. Each CPSP fraction was concentrated and desalted using a dialysis membrane (Molecular Weight Cut-Off (MWCO) 12–16 kDa) with distilled/deionized water.
2.5. Infrared spectral analysis of CPSP
The IR spectrum of CPSP was recorded with an FTIR spectrometer (FTIR-8400S; Shimadzu, Kyoto, Japan) equipped with Shimadzu IR solution 1.30 software. The CPSP sample was ground with KBr powder and then pressed into pellets for FTIR measurement in the frequency range of 4000/cm to 400/cm.
2.6. Molecular characteristics of CPSP
The molecular characteristics of CPSP were determined by an HPGFC system (Jasco) consisting of a PU-2080 Plus pump, UV-2075 Plus UV detector at 280 nm, RI-2031 Plus RI detector, and a PolySep-GFC-P5000 column (300 mm × 7.80 mm; Phenomenex, Torrance, CA, USA). The mobile phase was distilled/deionized water and the flow rate was 0.8 mL/minute at room temperature. The sample injection amount was 20 μL.
2.7. Characterization of monosaccharide and amino acid compositions
Monosaccharides and amino acids in each CPSP fraction were identified and quantified by gas chromatography/mass spectrometry (Agilent 6890; Wilmington, DE, USA) [28] with a capillary column (30 m × 0.32 mm; Restek Rtx-225; Bellefonte, PA, USA) and a capillary column (30 m × 0.25 mm; HP-5MS; Agilent), respectively, which was commissioned at the testing and analysis center for food and cosmetics at Hung-kuang University (Taichung, Taiwan).
2.8. Analysis of glycan–peptide bond
CPSP linkages were analyzed by β-elimination reaction [29,30]. The CPSP sample was incubated with 0.2 mol/L NaOH containing 1.0 mol/L NaBH4 for 30 minutes at 45°C, scanned from 210 nm to 270 nm on a UV-Vis spectrophotometer, and compared to the non-alkali-treated sample [31].
2.9. AChE inhibitory assay
AChE inhibitory activity was assessed as described by Sacan and Yanardag [5] with modifications. Sample aliquots of varying concentrations (100 μL), 325 μL Tris–HCl buffer (50mM, pH 8.0), and 25 μL 0.28 U/mL AChE in 50mM Tris–HCl buffer containing 0.1% (w/v) BSA were incubated for 15 minutes at room temperature. Then, 75 μL of 15mM acetylthiocholine iodide in water and 475 μL of 3mM 5,5′-dithio-bis-(2-nitrobenzoic) acid in Tris–HCl buffer (50mM, pH 8.0) were added, and the final mixture was incubated for 30 minutes at room temperature. Absorbance was measured at 405 nm. Galanthamine hydrobromide was used as a positive control.
3. Results and discussion
3.1. Extraction and preparation of crude PS
Crude PS extracts were prepared. To maximize yield, 3.0 g powdered C. militaris fruiting bodies was extracted five times by continuous ultrasound-assisted extraction. The maximum yield of crude PS was achieved by extracting three times, for a total yield of 27.3 ± 0.3% (w/w) (Table 1). The accumulated extraction rate was over 95% after the second extraction; therefore, the C. militaris powder was extracted twice for every crude PS extract preparation.
Table 1.
The five continuous ultrasound-assisted extractions for polysaccharide.
| Extraction times | Extraction yield (%) | Accumulated extraction rate (%) |
|---|---|---|
| No. 1 | 23.2 ± 0.2 | 84.8 |
| No. 2 | 3.4 ± 0.0 | 97.3 |
| No. 3 | 0.8 ± 0.0 | 100 |
| No. 4 | N.D. | |
| No. 5 | N.D. | |
| Total extraction yield (%) | 27.3 ± 0.3 |
All experimental data are expressed as mean ± SD (n ≥ 3).
N.D. = not detected.
3.2. Chromatographic fractionations of CPSP
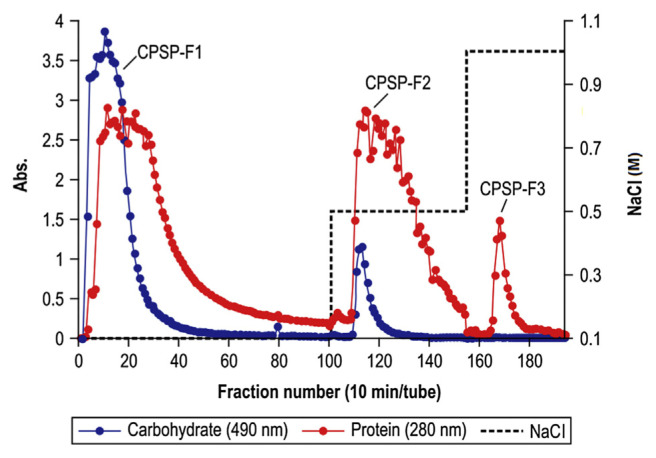
CPSP was separated from crude PS on a DEAE–Sepharose CL-6B anion-exchange column to yield fractions CPSP-F1, CPSP-F2, and CPSP-F3 (Fig. 1). The recovery of each fraction was 88.6%, 3.7% and 0.3%, relative to the total amount of crude PS. The recovery ratio was calculated from the following Equation (1). These fractions were eluted with an increasing NaCl gradient. Anion-exchange column chromatography revealed coincident peaks for carbohydrates and proteins, indicating that PS and protein were linked in CPSP-F1, CPSP-F2, and CPSP-F3 [32]. Then, CPSP-F1, CPSP-F2 and CPSP-F3 were collected, concentrated, characterized, and evaluated for AChE inhibitory activity.
Fig. 1.
Elution profiles of crude polysaccharide in DEAE–Sepharose CL-6B anion-exchange column chromatography. The column was eluted with a step gradient of NaCl (0.1–0.5–1.0M) at a flow rate of 0.6 mL/minute. Eluates were analyzed by measuring the absorbance at 490 nm for carbohydrates after using the phenol sulfuric acid method and at 280 nm for proteins.
| (1) |
3.3. Characterization of CPSP-F1, CPSP-F2 and CPSP-F3
The carbohydrate and protein components of the concentrated CPSP-F1, CPSP-F2, and CPSP-F3 solutions are listed in Table 2. CPSP-F1 contained a larger amount of carbohydrate than protein, but CPSP-F2 and CPSP-F3 contained more protein than carbohydrate. After hydrolysis, amino sugars in the CPSP-F1, CPSP-F2, and CPSP-F3 hydrolytes were measured by colorimetric analysis. All fractions contained amino sugar, with all reactions yielding a light red color [26]. Table 2 shows CPSP-F1 and CPSP-F2 contained 5–6‰ (w/w) amino sugars, but the absorption of CPSP-F3 was too low to be calculated; thus, amino sugar residues were present in all three CPSP fractions.
Table 2.
Total carbohydrate, total protein, and total amino sugar contents of concentrated CPSP-F1, CPSP-F2, and CPSP-F3.
| Polymer | Total carbohydrate content (%, w/w) | Total protein content (%, w/w) | Total amino sugar content (‰, w/w) |
|---|---|---|---|
| CPSP-F1 | 72.7 ± 1.4 | 36.9 ± 3.5 | 5.7 ± 0.8 |
| CPSP-F2 | 4.9 ± 0.4 | 95.6 ± 1.1 | 5.0 ± 0.3 |
| CPSP-F3 | 3.0 ± 0.1 | 91.7 ± 0.1 | N.C. |
All experimental data are expressed as mean ± SD (n ≥ 3).
CPSP = C. militaris polysaccharide-peptide; N.C. = absorption was too small to be calculated.
3.4. FTIR analysis of CPSP-F1, CPSP-F2, and CPSP-F3
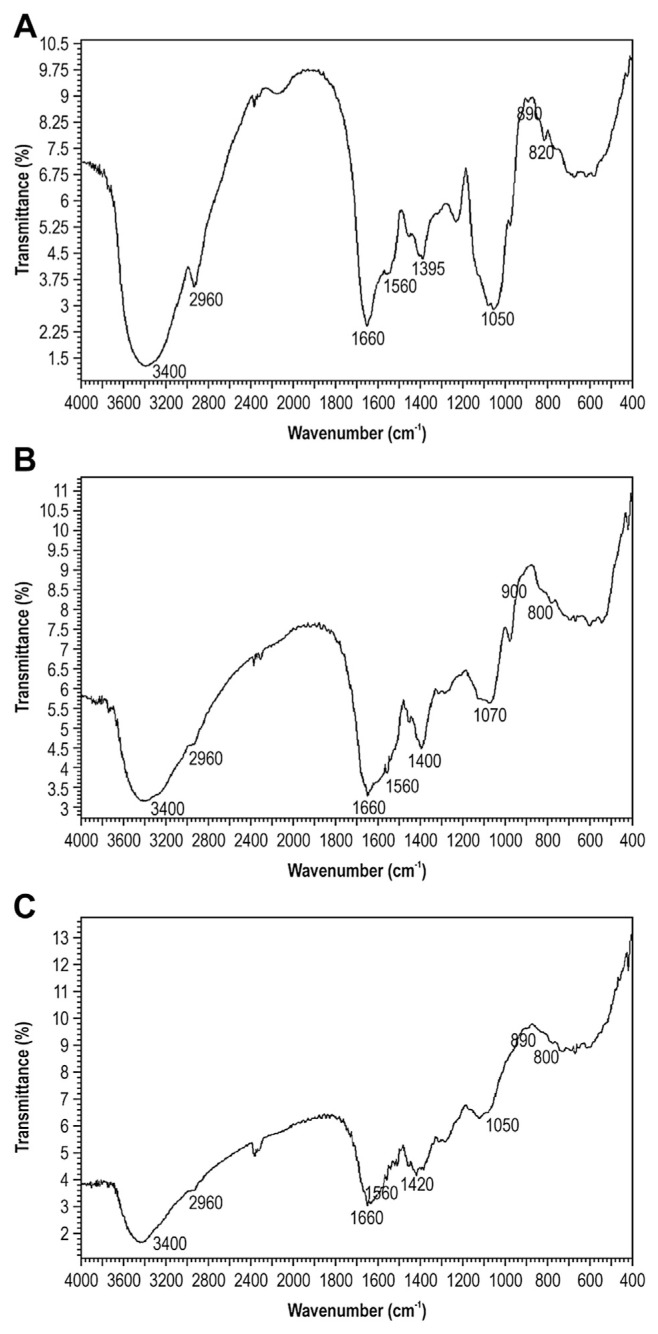
The functional groups of CPSP-F1, CPSP-F2, and CPSP-F3 were investigated by FTIR analysis at 4000–400/cm (Fig. 2). All samples displayed a broadly stretched, intense peak at around 3400/cm characteristic of a hydroxyl group, and a weak C–H stretching band near 2960/cm for constituent sugar residues [33,34]. The absorption band between 1440/cm and 1395/cm was associated with the stretching vibration of C–O bonds [35]. The absorption bands of CPSP-F1, CPSP-F2, and CPSP-F3 were at 1395/cm, 1400/cm, and 1420/cm, respectively. Near 1660/cm and 1560/cm, the absorption showed protein feathering, corresponding to the carbonyl bond of an amide group and bending vibration of the N–H bond [36]. The absorption peak at 825/cm indicated an α-glycosidic linkage [37]. The absorption at 893/cm suggested a β-glycosidic linkage, but the bands at 930/cm, 1038/cm, 1078/cm, and 1161/cm were also indicative of β (1→3) glycosidic bonds [38].
Fig. 2.
FTIR spectra of CPSP-F1, CPSP-F2, and CPSP-F3. CPSP samples were ground with KBr powder and pressed into pellets for FTIR measurement at 4000–400/cm. (A) CPSP-F1; (B) CPSP-F2; and (C) CPSP-F3. CPSP = C. militaris polysaccharide-peptide; FTIR = Fourier transform infrared.
The PSP was one form of PS linking peptide. Spectroscopy revealed characteristic functional groups of PS, peptide and glycosidic linkages in CPSP-F1, CPSP-F2, and CPSP-F3, suggesting they are PSP complexes.
3.5. Molecular characteristics of CPSP-F1, CPSP-F2, and CPSP-F3
In a previous study, size exclusion chromatography was used to separate PSP complexes of Pleurotus geesteranus, in which the UV peaks for protein had the same retention time as the polysaccharide peaks detected by Refractive Index (RI), suggesting the proteins were bound to the polysaccharide components [39]. The polysaccharopeptide of C. versicolor was separated by gel permeation HPLC and monitored with UV and RI detectors, which showed the protein and polysaccharide moieties were tightly linked, with coincident UV and RI peaks [32].
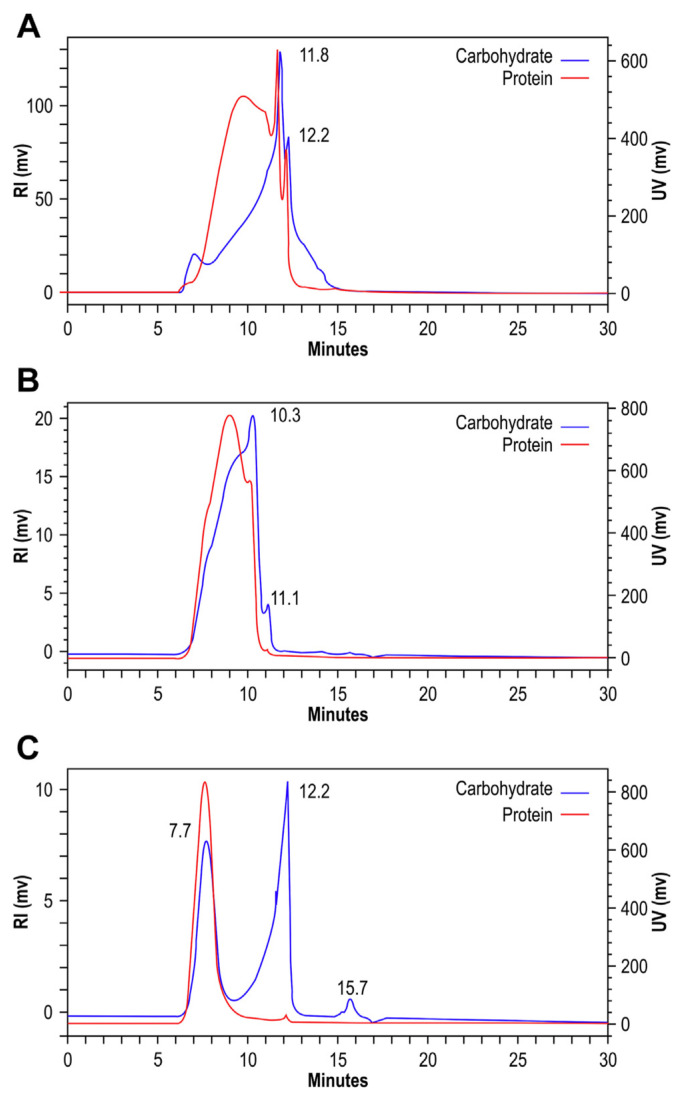
We used HPGFC to separate CPSP-F1, CPSP-F2, and CPSP-F3. The UV and RI superimposed chromatograms are shown in Fig. 3. As shown in the RI chromatogram for carbohydrates, CPSP-F1 contained two different polymer distributions at 11.8 minutes and 12.2 minutes with protein signals in the UV chromatogram (Fig. 3A); CPSP-F2 contained polymer distributions at 10.3 minutes and 11.1 minutes with protein signals in the UV chromatogram (Fig. 3B); CPSP-F3 included polymer distributions at 7.7 minutes, 12.2 minutes, and 15.7 minutes, but the protein signals in the UV chromatogram appeared only at 7.7 minutes and 12.2 minutes (Fig. 3C). We conclude that PSP complexes were present in CPSP-F1, CPSP-F2, and CPSP-F3.
Fig. 3.
HPGFC spectra of CPSP-F1, CPSP-F2, and CPSP-F3. The HPGFC system consisted of a UV detector at 280 nm, an Refractive Index (RI) detector, and a PolySep-GFC-P5000 column. The mobile phase was distilled/deionized water with flow at 0.8 mL/minute at room temperature. (A) CPSP-F1; (B) CPSP-F2; and (C) CPSP-F3. CPSP = C. militaris polysaccharide-peptide; HPGFC = high-performance gel-filtration chromatography.
3.6. Characterization of monosaccharide and amino acid compositions
The monosaccharide and amino acid compositions have been characterized in C. sphecocephala and H. sinensis [18,20]. In our studies, the CPSP fractions were composed of mainly mannose, galactose, and arabinose sugar moieties (Table 3). The polymers consisted of acidic and nonpolar amino acids such as glutamic acid and proline. Furthermore, nonpolar amino acids alanine, glycine, and leucine were higher in CPSP-F1, while basic and nonpolar lysine and methionine were higher in CPSP-F2 and CPSP-F3 (Table 4).
Table 3.
Monosaccharide compositions of CPSP-F1, CPSP-F2, and CPSP-F3.a
| Polymer | Monosaccharide contents (molar ratio) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mannose | Galactose | Glucose | Arabinose | Fucose | Xylose | |
| CPSP-F1 | 2.2 | 1.4 | 0.2 | 3.0 | 0.2 | 1.2 |
| CPSP-F2 | 2.1 | 2.2 | 0.1 | 1.9 | 0.9 | 1.0 |
| CPSP-F3 | 2.3 | 1.7 | 0.1 | 3.0 | 0.1 | 1.0 |
CPSP = C. militaris polysaccharide-peptide.
Monosaccharide compositions were analyzed by gas chromatography/mass spectrometry.
Table 4.
Amino acid compositions of CPSP-F1, CPSP-F2, and CPSP-F3.a
| Amino acid | Composition (%, w/w) | ||
|---|---|---|---|
|
| |||
| CPSP-F1 | CPSP-F2 | CPSP-F3 | |
| Alanine | 12.5 | 0.6 | 0.5 |
| Glycine | 10.4 | 0.8 | 0.2 |
| Valine | 7.3 | 2.5 | 0.3 |
| Leucine | 9.3 | 2.4 | 1.2 |
| Isoleucine | 6.0 | 1.6 | 0.6 |
| Proline | 23.4 | 12.4 | 13.0 |
| Glutamic acid | 7.2 | 19.8 | 7.5 |
| Methionine | 1.2 | 8.8 | 8.3 |
| Asprtic acid | 3.9 | 2.5 | 2.0 |
| Hydroxyproline | 4.4 | 5.1 | 1.3 |
| Phenylalanine | 2.1 | 0.8 | 0.7 |
| Cysteine | 2.2 | 0.6 | 0.5 |
| Lysine | 7.6 | 39.0 | 61.4 |
| Histidine | 0.4 | 0.3 | 1.8 |
| Tyrosine | 2.0 | 2.8 | 0.8 |
CPSP = C. militaris polysaccharide-peptide.
Amino acid compositions were analyzed by gas chromatography/mass spectrometry.
3.7. Glycan–peptide linkage analysis
Glycan–peptide linkages are classified by their stability under alkaline, such as O-glycosidic and N-glycosidic conditions. The alkali-sensitive O-glycosidic linkages are readily cleaved under relatively mild conditions by a β-elimination mechanism that releases the carbohydrate moiety. This method has been widely used to analyze glycoprotein linkages [40].
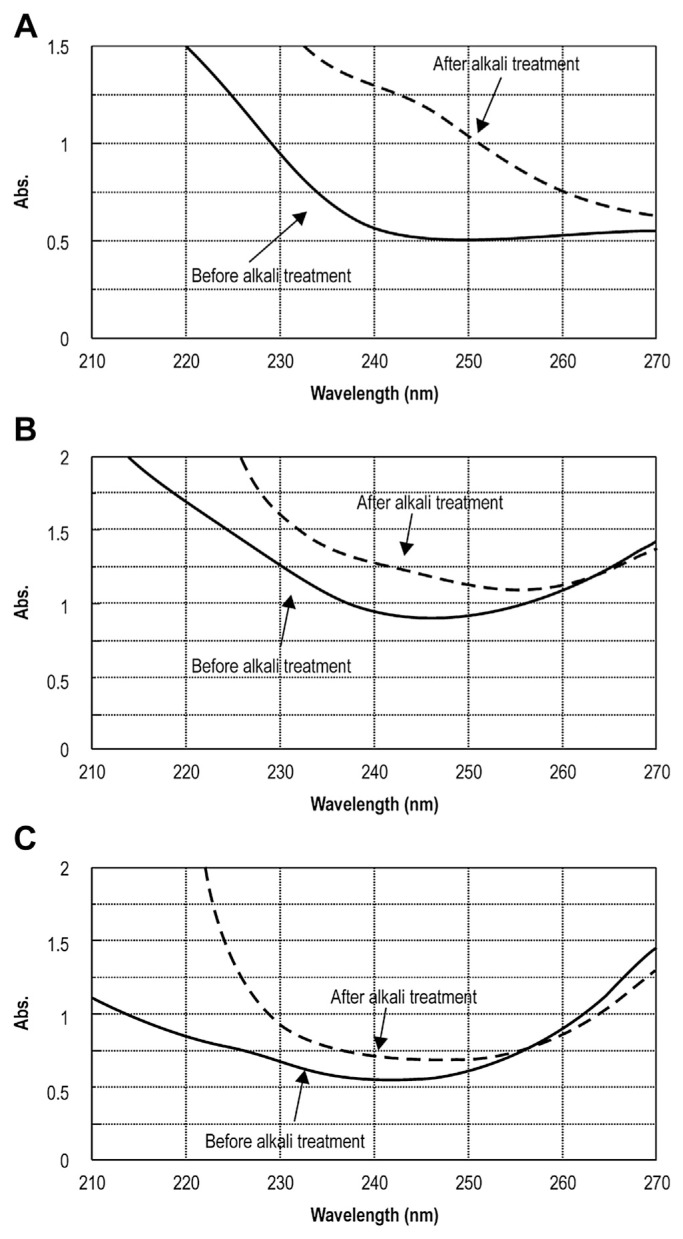
To analyze the linkages in our PSP complexes, UV scanning spectra of CPSP-F1, CPSP-F2, and CPSP-F3 with and without alkali treatment were obtained (Fig. 4). Alkali-treated samples had distinctly higher absorbance at 240 nm, showing that the β-elimination reaction had taken place, and that CPSP-F1 contained O-glycosidic linkages but CPSP-F2 and CPSP-F3 did not. Thus, CPSP-F2 and CPSP-F3 have only N-glycosidic linkages.
Fig. 4.
UV spectra of β-elimination before and after alkali treatment for CPSP. The sample was incubated with 0.2 mol/L NaOH containing 1.0 mol/L NaBH4 for 30 minutes at 45°C, scanned the UV spectrum from 210 nm to 270 nm, and compared to the non-alkali-treated sample. (A) CPSP-F1; (B) CPSP-F2; and (C) CPSP-F3. CPSP = C. militaris polysaccharide-peptide.
3.8. AChE inhibitory activity
AChE inhibition by CPSP-F1, CPSP-F2, and CPSP-F3 and crude PS extracts of C. militaris was measured; the IC50 values are shown in Table 5. Although the active content of CPSP-F3 was too low to reach 50% AChE inhibition, crude PS, CPSP-F1, and CPSP-F2 exhibited significant AChE inhibitory activity. IC50 values of crude PS, CPSP-F1 and CPSP-F2 were 48.9 ± 0.2 mg/mL, 32.2 ± 0.2 mg/mL, and 5.3 ± 0.0 mg/mL, respectively. Further, the AChE inhibitory activity of CPSP can be increased by separation from crude PS. The most potent inhibition appeared to be present in CPSP-F2. We are believed to be the first to report in vitro inhibition of AChE by CPSP and our results suggest CPSP-F2 may have great potential to provide a chemical framework for the development of new AChE inhibitors.
Table 5.
AChE inhibitory activity of different CPSPs.
| Processes/sample name | IC50 valued Solid (mg/mL) |
|---|---|
| Precipitationa | |
| Crude PS | 48.9 ± 0.2 |
| DEAE–Sepharose CL-6Bb | |
| CPSP-F1 | 32.2 ± 0.2 |
| CPSP-F2 | 5.3 ± 0.0 |
| CPSP-F3 | Not activee |
| Positive control | |
| Galanthamine hydrobromidec | 0.0187 ± 0.0004 |
AChE = acetylcholinesterase; CPSP = C. militaris polysaccharide-peptide; DEAE = diethylaminoethanol.
AChE inhibition = (A0 – A1)/A0 × 100%. A0 is the absorbance of the blank. A1 is the absorbance of sample – absorbance of sample color.
Crude polysaccharides were precipitated by 3 volumes of 95% ethanol.
CPSP-F1, CPSP-F2 and CPSP-F3 were NaCl-eluted fractions separated by DEAE–Sepharose CL-6B column chromatography.
Purity of galanthamine hydrobromide was ≥ 94%.
IC50 was defined as the concentration that yielded 50% AChE inhibition, and the results are means ± standard deviation (n ≥ 3).
Sample content was too small to reach 50% AChE inhibition.
4. Conclusion
PSP complexes were identified in CPSP-F1, CPSP-F2, and CPSP-F3 from C. militaris. The PSP complexes produced coincident PS and protein peaks by DEAE–Sepharose CL-6B column chromatography and HPGFC. FTIR spectra revealed characteristic functional groups for PS, peptide, and glycosidic linkages. The polymers also contained amino sugar residues. β-Elimination showed O-glycosidic linkages in CPSP-F1 and N-glycosidic linkages in CPSP-F2 and CPSP-F3. We analyzed the monosaccharide and amino acid compositions as well as the total carbohydrate and protein contents.
Inhibition of AChE, the key enzyme in the breakdown of ACh, is considered a promising method for the treatment of neurological disorders such as AD and senile dementia. Here, we found that CPSP is a strong inhibitor of AChE and significant activity was observed in the crude PS, CPSP-F1, and CPSP-F2. Activity was enhanced by separation and CPSP-F2 displayed the best AChE inhibitory activity. CPSP from C. militaris fruiting bodies are a potential source of AChE inhibitors. In the future, CPSP may be used as a nutraceutical product for preparation of functional foods.
Acknowledgments
We thank Xin-Run Biotechnology Co. for providing the C. militaris fruiting bodies for our research.
REFERENCES
- 1. Karmakar A, Zhang QL, Zhang YB. Neurotoxicity of nanoscale materials. J Food Drug Anal. 2014;22:147–60. doi: 10.1016/j.jfda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ingkaninan K, Temkitthawon P, Chuenchom K, et al. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–4. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3. Syad AN, Shunmugiah KP, Kasi PD. Assessment of anticholinesterase activity of Gelidiella acerosa: Implications for its therapeutic potential against Alzheimer’s disease. Evid Based Complement Alternat Med. 2012;2012:497242. doi: 10.1155/2012/497242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mukherjee PK, Kumar V, Mal M, et al. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5. Sacan O, Yanardag R. Antioxidant and antiacetylcholinesterase activities of chard (Beta vulgaris L. var. cicla) Food Chem Toxicol. 2010;48:1275–80. doi: 10.1016/j.fct.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 6. Ellman GL, Courtney KD, Andres V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 7. Yoon NY, Ngo DN, Kim SK. Acetylcholinesterase inhibitory activity of novel chitooligosaccharide derivatives. Carbohydr Polym. 2009;78:869–72. [Google Scholar]
- 8. Yang WJ, Fang Y, Liang J, et al. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Food Res Int. 2011;44:1269–75. [Google Scholar]
- 9. Cho YS, Kim SK, Ahn CB, et al. Inhibition of acetylcholinesterase by gallic acid-grafted-chitosans. Carbohydr Polym. 2011;84:690–3. [Google Scholar]
- 10. Yan H, Zhu DG, Xu DB, et al. A study on Cordyceps militaris polysaccharide purification, composition and activity analysis. Afr J Biotechnol. 2008;7:4004–9. [Google Scholar]
- 11. Park SE, Kim J, Lee YW, et al. Antitumor activity of water extracts from Cordyceps militaris in NCI-H460 cell xenografted nude mice. J. Acupuncture Meridian Studies. 2009;2:294–300. doi: 10.1016/S2005-2901(09)60071-6. [DOI] [PubMed] [Google Scholar]
- 12. Yu RM, Yin Y, Yang W, et al. Structural elucidation and biological activity of a novel polysaccharide by alkaline extraction from cultured Cordyceps militaris. Carbohydr Polym. 2009;75:166–71. [Google Scholar]
- 13. Hsu WK, Hsu TH, Lin FY, et al. Separation, purification, and α-glucosidase inhibition of polysaccharides from Coriolus versicolor LH1 mycelia. Carbohydr Polym. 2013;92:297–306. doi: 10.1016/j.carbpol.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 14. Wang HX, Liu WK, Ng TB, et al. Immunomodulatory and antitumor activities of a polysaccharide-peptide complex from a mycelia culture of Tricholoma sp., a local edible mushroom. Life Sci. 1995;57:269–81. doi: 10.1016/0024-3205(95)00270-g. [DOI] [PubMed] [Google Scholar]
- 15. Liu MQ, Li JZ, Kong FZ, et al. Induction of immunomodulating cytokines by a new polysaccharide-peptide complex from culture mycelia of Lentinus edodes. Immunopharmacol. 1998;40:187–98. doi: 10.1016/s0162-3109(98)00043-5. [DOI] [PubMed] [Google Scholar]
- 16. Cui FJ, Li Y, Xu YY, et al. Induction of apoptosis in SGC-7901 cells by polysaccharide-peptide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Toxicol In Vitro. 2007;21:417–27. doi: 10.1016/j.tiv.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17. Yu RM, Yang W, Song LY, et al. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris. Carbohydr Polym. 2007;70:430–6. [Google Scholar]
- 18. Oh JY, Cho EJ, Nam SH, et al. Production of polysaccharide-peptide complexes by submerged mycelia culture of an entomopathogenic fungus Cordyceps sphecocephala. Process Biochem. 2007;42:352–62. [Google Scholar]
- 19. Oh JY, Baek YM, Kim SW, et al. Apoptosis of human hepatocarcinoma (HepG2) and neuroblastoma (SKN-SH) cells induced by polysaccharides-peptide complexes produced by submerged mycelial culture of an entomopathogenic fungus Cordyceps sphecocephala. J Microbiol Biotechnol. 2008;18:512–9. [PubMed] [Google Scholar]
- 20. He L, Ji PF, Cheng JW, et al. Structural characterization and immunostimulatory activity of a novel protein-bound polysaccharide produced by Hirsutella sinensis Liu, Guo, Yu & Zeng. Food Chem. 2013;141:946–53. doi: 10.1016/j.foodchem.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 21. Khan MA, Tania M, Zhang DZ, et al. Cordyceps mushroom: a potent anticancer nutraceutical. Open Nutraceuticals J. 2010;3:179–83. [Google Scholar]
- 22. Jin H, Zhang YJ, Jiang JX, et al. Studies on the extraction of pumpkin components and their biological effects on blood glucose of diabetic mice. J Food Drug Anal. 2013;21:184–9. [Google Scholar]
- 23. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–8. [Google Scholar]
- 24. Lee CL, Yang X, Wan Jennifer MF. The culture duration affects the immunomodulatory and anticancer effect of polysaccharopeptide derived from Coriolus versicolor. Enzyme Microb Technol. 2006;38:14–21. [Google Scholar]
- 25. Winters AL, Minchin FR. Modification of the Lowry assay to measure proteins and phenols in covalently bound complexes. Anal Biochem. 2005;346:43–8. doi: 10.1016/j.ab.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 26. Rondle CJM, Morgan WTJ. The determination of glucosamine and galactosamine. Biochem J. 1955;61:586–9. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen GC, Johnson BR. Improved colorimetric determination of cell wall chitin in wood decay fungi. Appl Environ Microbiol. 1983;46:13–6. doi: 10.1128/aem.46.1.13-16.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ker YB, Peng CH, Chyau CC, et al. Soluble polysaccharide composition and myo-inositol content help differentiate the antioxidative and hypolipidemic capacity of peeled apples. J Agric Food Chem. 2010;58:4660–5. doi: 10.1021/jf903495h. [DOI] [PubMed] [Google Scholar]
- 29. Chen Y, Xie MY, Nie SP, et al. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107:231–41. [Google Scholar]
- 30. Zhu KX, Zhou HM. Purification and characterization of a novel glycoprotein from wheat germ water-soluble extracts. Process Biochem. 2005;40:1469–74. [Google Scholar]
- 31. Ge SG, Yang SJ, Zhang SZ, et al. Bonding between sugar and peptide chain in glucoamylase from Monascus rubiginosus. Acta Microbiologica Sinica. 1983;23:265–9. [Google Scholar]
- 32. Yang XT, Mi K, Feng HQ. The chromatographic discrimination of polysaccharide-peptide conjugation in the polysaccharopeptide of Coriolus versicolor (PSP) Mycosystema. 2005;24(Suppl):312–4. [Google Scholar]
- 33. Shi Y, Sheng JC, Yang FM, et al. Purification and identification of polysaccharide derived from Chlorella pyrenoidosa. Food Chem. 2007;103:101–5. [Google Scholar]
- 34. Yang B, Wang JS, Zhao MM, et al. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res. 2006;341:634–8. doi: 10.1016/j.carres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 35. Yang JPW, Hsu TH, Lin FY, et al. Potential antidiabetic activity of extracellular polysaccharides in submerged fermentation culture of Coriolus versicolor LH1. Carbohydr Polym. 2012;90:174–80. doi: 10.1016/j.carbpol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 36. Thetsrimuang C, Khammuang S, Sarnthima R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. Int J Pharmacol. 2011;7:58–65. [Google Scholar]
- 37. Wang ZM, Peng X, Lee Daniel KL, et al. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011;125:637–43. [Google Scholar]
- 38. Lin FY, Lai YK, Yu HC, et al. Effects of Lycium barbarum extract on production and immunomodulatory activity of the extracellular polysaccharopeptides from submerged fermentation culture of Coriolus versicolor. Food Chem. 2008;110:446–53. doi: 10.1016/j.foodchem.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 39. Zhang M, Zhu L, Cui Steve W, et al. Fractionation, partial characterization and bioactivity of water-soluble polysaccharides and polysaccharide-protein complexes from Pleurotus geesteranus. Int J Biol Macromol. 2011;48:5–12. doi: 10.1016/j.ijbiomac.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Elizabeth FH. Glycoanalysis protocols. Totowa, NJ: Humana Press; 1998. Analytical methods for the study of O-GlcNAc glycoproteins and glycopeptides; pp. 19–33. [DOI] [PubMed] [Google Scholar]