Abstract
Of the various derivatives of caffeic acid, caffeic acid phenethyl ester (CAPE) is a hydrophobic, bioactive polyphenolic ester obtained from propolis extract. The objective in writing this review article was to summarize all published studies on therapeutics of CAPE in inflammation and cancer to extract direction for future research. The possible molecular targets for the action of CAPE, include various transcription factors such as nuclear factor-κB, tissue necrosis factor-α, interleukin-6, cyclooxygenase-2, Nrf2, inducible nitric oxide synthase, nuclear factor of activated T cells, hypoxia-inducible factor-1α, and signal transducers and activators of transcription. Based on the valuable data on its therapeutics in inflammation and cancer, clinical studies of CAPE should also be conducted to explore its toxicities, if any.
Keywords: caffeic acid phenethyl ester, cancer, chemotherapy, inflammation, molecular targets
1. Introduction
Due to the lethal side effects of synthetic chemical-based drugs, enthusiastic efforts are currently being applied to explore natural therapeutic agents with minimum toxicity. In this context, plant or herbal origin compounds are being studied to investigate the bioactivities of their natural active compounds. Polyphenols represent one of the most intensively studied groups of natural compounds.
Caffeic acid has been proposed to act as a multipurpose active polyphenol and its derivatives have also been subjected to considerable study. One of the derivatives of caffeic acid is caffeic acid phenethyl ester (CAPE), which possesses promising therapeutic potential against various pathologies such as inflammation, cancer, infection, and neurodegeneration [1–5]. This naturally bioactive, hydrophobic polyphenolic ester occurs in numerous plants [6–9] and propolis [10] and can also be prepared by reacting caffeic acid with phenethyl alcohols [1–3]. The molecular formula of CAPE is C17H16O4 and is chemically recognized as 2-phenylethyl (2E)-3-(3,4-dihydroxyphenyl)acrylate (commonly termed as phenylethyl caffeate or phenethyl caffeate) [4].
To achieve biological effects, CAPE should be administered at a therapeutic concentration so that prolonged maintenance of blood CAPE-concentration at a particular level could be achieved. Thus pharmacokinetic and bioavailability study of CAPE is crucial for determining its route of administration. Fig. 1 depicts the chemical structure of CAPE consisting of a catechol ring and two hydroxyl groups; the former is considered to be responsible for its therapeutic features [5]. It has been proposed that metabolism of CAPE is a saturable process because an increase in the area under the plasma concentration–time curve for CAPE was observed in a proportion higher than the increase in its dose. Moreover, volume of distribution and total body clearance values for CAPE were found to be in the ranges of 1555–5209 mL/kg and 42–172 mL/minute/kg, respectively, proposing that these values are in an inverse relationship with the dose of CAPE. Additionally, no relationship was observed between the values of elimination half-life (21.24–26.71 minutes) of CAPE and its dose. Pharmacokinetic study of CAPE showed its high values of volume of distribution and short elimination half-life, revealing its extensive distribution and swift elimination from the body after intravenous administration [11]. Another pharmaco-kinetic study of CAPE showed comparable results [12]. Furthermore, pharmacokinetic analysis of CAPE and its metabolites should also be carried out after its oral administration. Another study has revealed that CAPE can efficiently cross the blood–brain barrier in rats [13]. Besides, although CAPE is stable for 6 hours in rat plasma, after which it hydrolyzes to caffeic acid, CAPE hydrolysis does not occur in human plasma showing its stability, possibly owing to the absence of carboxylesterase in this biofluid [14,15].
Fig. 1.
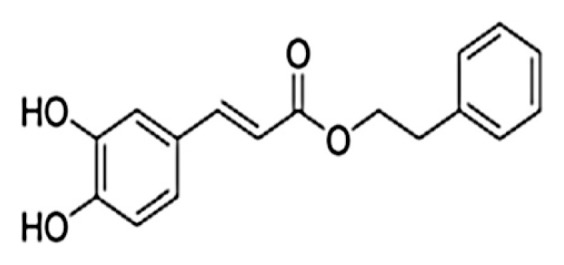
Chemical structure of caffeic acid phenethyl ester [9].
After an extensive search, no data were found about toxicity study of CAPE. Rather, slight toxicity of propolis was seen in a range of 2000–7300 g of propolis/kg in mice that is an origin of CAPE [16,17]. At a dose of approximately 80 μM, CAPE generally inhibits the activated nuclear factor-κB (NF-κB) and other transcription factors via suppressing their binding with DNA [15].
The objective in writing this review article was to summarize various published studies on the therapeutics of CAPE in inflammation and cancer, especially focusing on their molecular targets that are responsible for therapeutic effect of CAPE.
2. Literature search methodology
An extensive literature search in English was conducted, using various electronic databases including Medline (1966–2014) and EMBASE (1980–2014). An initial search was made using terms caffeic acid phenethyl ester and activity jointly. Then, other terms such as inflammation, cancer, and molecular targets were combined with caffeic acid phenethyl ester and activity for an advanced search. The literature investigation was done by assessing the bibliography of the selected publications showing original research to make a quality review article.
3. Results and discussion
There are many studies in the literature that elaborate the anti-inflammatory activity of CAPE [18,19]. Moreover, CAPE-induced inhibition of normal cell transformation to the neoplastic cell has also been reported [20,21]. Table 1 [20,22–32] elaborates the dose (μM) or concentration causing 50% growth inhibition (μM) of CAPE effective in different cancer cell-lines. In addition, CAPE selectively destroys the cancerous cells leaving noncancer cells unaffected as observed in human immortal lung fibroblast WI-38 cells [29].
Table 1.
The dose or concentration causing 50% growth inhibition (IC50) of CAPE effective in different cancer cell-lines.
| No. | Types of cancer and their cell lines | Dose (μM) | IC50 (μM) | Refs |
|---|---|---|---|---|
| 1 | U973 myeloid leukemia cells | 0.4–53 | — | [22] |
| 2 | GNM neck metastasis of Gingiva carcinoma | 25–200 | — | [23] |
| 3 | TSCC tongue squamous carcinoma cells | 25–200 | — | [23] |
| 4 | Daoy medulloblastoma cells | 1–100 | — | Lee et al 2005 |
| 5 | SW480 colon cancer cells | 9–18 | — | Wang et al 2005 |
| 6 | HCT116 colon cancer cells | 9–182 | — | [24] |
| 7 | PC-3 prostate cancer cells | 88 | — | [25] |
| 8 | HL-60 leukemia cells | 21 | — | [26], Chen et al 2001b |
| 9 | MCF-7 breast cancer cells | 10–100 | — | [27] |
| 10 | Meng 1 oral epidermal carcinoma cells | 50–200 | — | [20] |
| 11 | H1299 lung cancer cells | — | 21.2 | Lin et al 2011 |
| 12 | Nalm6 lymphoma cells | — | 3.1 | [28] |
| 13 | Farage lymphoma cells | — | 2.0 | [28] |
| 14 | Pfeiffer lymphoma cells | — | 1.2 | [28] |
| 15 | Ramos lymphoma cells | — | 4.0 | [28] |
| 16 | HDMAR lymphoma cells | — | 2.1 | [28] |
| 17 | HT-1080 fibrosarcoma cells | — | 9.5 | [29] |
| 18 | HeLa cervical cancer cells | — | 2.4 | [29] |
| 19 | CT26 colon cancer cells | — | 35.0 | [30] |
| 20 | A549 lung cancer cells | — | 20.9 | [29,31] |
These studies hypothesize that CAPE inhibits the release of arachidonic acid from the cell membrane, and moreover, suppresses the gene responsible for cyclooxygenase-2 (COX-2) expression [33–36]. Moreover, CAPE suppresses NF-κB activity by limiting the formation of NF-κB DNA and nuclear factor of activated T cells (NFAT)-DNA complexes and thus retarding NF-κB-dependent transcription in Jurkat cells [37–42]. In 2005, Abdel-Latif et al presented anticancer and anti-inflammatory activities of CAPE in a gastric epithelial cell line, claiming that CAPE inhibits the production of tissue necrosis factor-α (TNF-α) and interleukin (IL)-8; it eventually retards the expression of NF-κB, AP-1, and COX-2 [43]. It is noteworthy to mention here that CAPE does not influence other tissues of body, and thus the usage of this natural anticancer agent is free of side effects with effective chemopreventive feature [44–47]. This outcome elaborates the nutritional importance of CAPE, particularly for patients whose tumors express gradually elevated levels of above given activated transcription factors.
Lipopolysaccharide-mediated inflammation in human neutrophils has also been combated using CAPE which suppresses the synthesis of TNF-α and IL-6 [48]. The same authors also found that CAPE attenuates the phosphorylation of extracellular signal-regulated kinase 1/2 and c-JunN-terminal kinase [48]. Raso et al [49] found that CAPE has potential for reducing inflammation through inhibiting IL-2 gene in activated T-cells that are normally the source of inflammation [34].
Biological studies have also revealed the activity of CAPE against angiogenesis, tumor invasion, metastasis, proliferation, and apoptosis in different cancers such as human pancreatic and colon cancer [23,35,44,50–55]. The improvement in the viability of colon adenocarcinoma cells (CT26) has been noted in a dose-dependent manner when these cells are treated with CAPE [30]. This cytotoxic effect of CAPE has been attributed to the reduced expression of matrix metalloproteinase and synthesis of vascular endothelial growth factor under the effect of CAPE. In this way, this chemical activity obstructs the angiogenesis and metastasis [56–62].
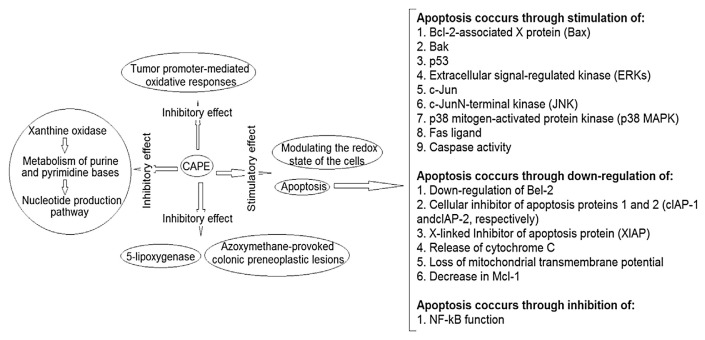
CAPE can suppress apoptosis via inhibiting the activated NF-kB [26], Bak [63], Bcl-2-associated X protein (Bax) [31,63–65], p53 [25,27,63], extracellular signal-regulated kinase [63], c-Jun and p21ap [27], c-JunN-terminal kinase and Fas ligand [65], p38 mitogen-activated protein kinase (p38 MAPK) [25,63], and caspase activity [27,31,63,64]. Moreover, upregulation of Bel-2 [29,66], the cellular inhibitor of apoptosis proteins 1 and 2, and X-linked inhibitor of apoptosis protein [26,31], release of cytochrome C [63,64], loss of mitochondrial transmembrane potential [27], and decrease in Mcl-1 [21,27] by CAPE are also responsible for its antiapoptotic effect.
In many cancer cells, CAPE-mediated-cell cycle arrest has been reported through the suppression of various factors including cyclin B1 [28,29]. CAPE-induced necrosis has also been described [22]. In addition, suppression of Akt phosphorylation is also induced by CAPE, resulting in the inhibition of cancer cell invasiveness [24].
The literature also contains many animal studies that reveal the inhibitory role of CAPE on tumor growth and metastasis. For example, at a dietary level of 0.15% CAPE, C57BL/6J-Min/+mice having a germ-line mutation exhibit 63% suppression in tumor growth through increased apoptosis and cell proliferation [67]. At a dose of 50 mg/kg, CAPE-treated rats showed the emergence of colon–rectal carcinoma provoked by azoxymethane [44]. In addition, mice with C6 glioma xenografts have exhibited dose-dependent inhibition in tumor metastasis at 1–10 mg intraperitoneal dose of CAPE/kg/ day [21]. As far as mechanisms of anticancer activity of CAPE are concerned, CAPE is capable of affecting various processes [32,42,46,60,68–73] as summarized in Fig. 2.
Fig. 2.
Various modes of anticancer activities of caffeic acid phenethyl ester (CAPE).
Through numerous experimental studies, the therapeutic potentials of CAPE against various cancers have been explored. The findings of those studies are summarized below and the possible target sites of CAPE action are also described.
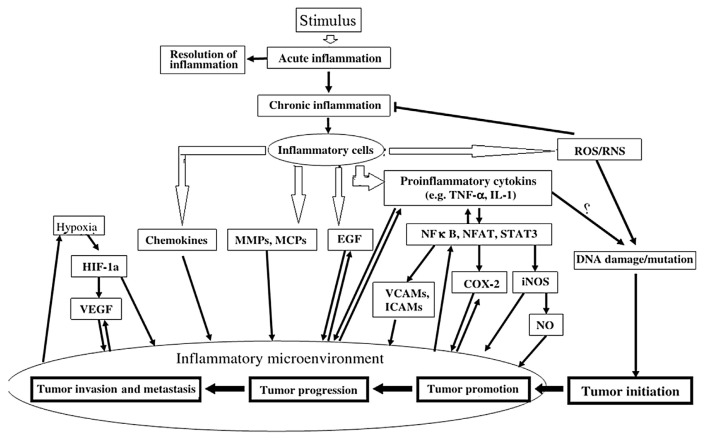
In response to a stimulus such as tissue damage, inflammation develops. It is a physiologic phenomenon that may contribute to cancer development through various intermediates (Fig. 3) [74]. Key modulators, which drive inflammation to cancer, include various transcription factors such as NF-κB, TNF-α, IL-6, COX-2, Nrf2, inducible nitric oxide synthase (iNOS), NFAT, hypoxia-inducible factor-1α, and signal transducers and activators of transcription [74].
Fig. 3.
A summary of linkage between inflammation and cancer development. Tissue necrosis factor-α (TNF-α), interleukin (IL)-1, hypoxia-inducible factor-1a (HIF-1a), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), modified citrus pectins (MCPs), endothelial growth factor (EGF), nuclear factor-κB (NF-κB), nuclear factor of activated T cells (NFAT), signal transducer and activator of transcription 3 (STAT3), vascular cell adhesion molecules (VCAMs), intercellular adhesion molecules (ICAMs), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), nitric oxide (NO).
The cytoplasm of all nondiseased B cells contains inactive NF-κB factor [75]. NF-κB is a collective term referring to proinflammatory heterotrimer transcription factors, a family of five proteins having Rel-domain. These proteins, including NF-κB1 (or p50), NF-κB2 (or p52), Rel A (or p65), Rel B, and c-Rel, remain inactive under the influence of IκBα, IκBβ, IκBγ, IκBαɛ, bcl-3, p105, and p100 proteins that have the anchoring domain [76].
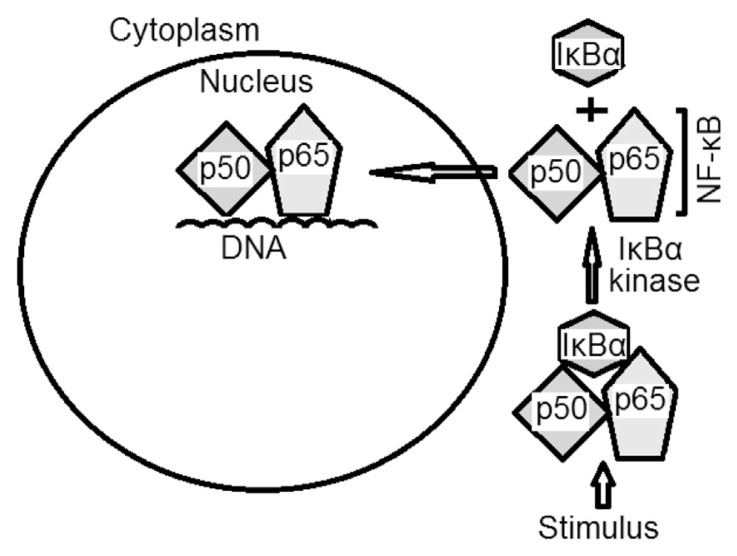
In tumor cells, as well as proliferating thymocytes, monocytes, astrocytes, T cells, and B cells, NF-κB is reported to be activated after phosphorylation and removal of IκBα by IκBα kinase (IKK; Fig. 4). The IKK family consists of three enzymes (IKKα, IKKβ, and IKKγ), of which, IKKβ is proposed to be involved in NF-κB activation by cytokines (tissue necrosis factor, IL-6, growth factors, and differentiation factors) and many other carcinogens and tumor promoters [76–78]. Shishodia et al [79] demonstrated tissue necrosis factor as the strongest NF-κB activator, under the influence of which tumor cells proliferate, invade, metastasize, and suppress apoptosis. Matrix metalloproteinases, urokinase type of plasminogen activator, and IL-8 are examples of NF-κB-regulated gene products that regulate the invasion of tumor cells [79–82]. Metastasis of tumor cells is regulated by NF-κB and is mediated through the expression of different adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1, endothelium leukocyte adhesion molecule-1, and iNOS [83,84].
Fig. 4.
An illustrative summary of the nuclear factor (NF)-κB activation route.
Immediately after activation, translocation of NF-κB occurs from the cytoplasm to the nucleus of the cell followed by the binding of NF-κB to its particular harmonized site consisting of 10 base pairs, GGGPuNNPyPyCC [85,86]. The active NF-κB, in normal physiology, controls the expression of many genes that regulate the immune, growth, and inflammation features of cell.
By contrast, the excessive and improper activation of NF-κB can intervene inflammation and tumorigenesis. In addition, NF-κB acts as a linkage between inflammation and cancer remembering that cancer is a proinflammatory disease [45,87]. Thus, activation of NF-κB by any inflammatory agents can produce inflammation that is mediated through adhesion molecules, such as intercellular adhesion molecule-1 [88]. Similarly, suppression of NF-κB by any anti-inflammatory agents can reduce inflammation and proliferation causing cell cycle arrest, eventually leading to apoptosis [45]. The microenvironment of tumors, as well as different inflammatory agents, carcinogens, and tumor promoters may activate the NF-κB [76]. There are some members of the NF-κB group that are oncogenic in nature and can intervene their effects by activating NF-κB [76,89].
Various stimuli, such as lipopolysaccharide, proin-flammatory cytokines (e.g., IL-1 and tissue necrosis factor), and growth factors (e.g., epidermal growth factor), have been found to be involved in expression of COX-2, which acts on arachidonic acids and produces prostaglandins, the crucial mediators of inflammation. Moreover, COX-2 is overexpressed in cancer [74,90,91]. Various antioxidant genes are regulated by Nrf2's role in inflammation; this effect of Nrf2 can be attributed to the involvement of prostaglandins and/or NO leading to the diminished susceptibility to apoptotic factors including TNF-α. Nrf2 is reported to exhibit protection against DNA damage and carcinogenesis [92,93]. Likewise, iNOS, an enzyme that catalyzes the production of NO, is also over-expressed in inflammation and cancer [29,94,95]. In addition, NFAT is proposed to play a crucial role in inflammatory responses through the expression of various proinflammatory cytokines, including IL-2, IL-3, IL-4, IL-5, IL-13, and TNF-α. NFAT is involved in COX-2 expression induced by TNF-α [96,97]. By contrast, inflammation is always accompanied by hypoxia due to metabolic shifts during inflammation. In response to hypoxia, hypoxia-inducible factor-1α, a hetero-dimeric transcription factor, activates various molecules including erythropoietin, iNOS, vascular endothelial growth factor, and glucose transporter-1 [98–100]. Signal transducers and activators of transcription factors are also activated by various cytokines in inflammation and cancer [29].
4. Conclusion
This literature mining study revealed anti-inflammatory and anticancer activities of CAPE. The possible molecular targets for the action of CAPE in inflammation and cancer include various transcription factors such as NF-κB. Based on the valuable data about the above presented bioactivities, clinical studies of CAPE should be conducted to explore its toxicities, if any.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Chen HC, Chen JH, Chang C, et al. Optimization of ultrasound accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrason Sonochem. 2011;18:455–9. doi: 10.1016/j.ultsonch.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 2. Chen HC, Ju HY, Twu YK, et al. Optimized enzymatic synthesis of caffeic acid phenethyl ester by RSM. N Biotechnol. 2010;27:89–93. doi: 10.1016/j.nbt.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3. Kurata A, Kitamura Y, Irie S, et al. Enzymatic synthesis of caffeic acid phenethyl ester analogues in ionic liquid. J Biotechnol. 2010;148:133–8. doi: 10.1016/j.jbiotec.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4. Kumazawa S, Ahn MR, Fujimoto T, et al. Radical-scavenging activity and phenolic constituents of propolis from different regions of Argentina. Nat Prod Res. 2010;24:804–12. doi: 10.1080/14786410802615270. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Stavchansky S, Bowman PD, et al. Cytoprotective effect of caffeic acid phenethyl ester (CAPE) and catechol ring-fluorinated CAPE derivatives against menadione-induced oxidative stress in human endothelial cells. Bioorg Med Chem. 2006;14:4879–87. doi: 10.1016/j.bmc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6. Grunberger D, Banerjee R, Eisinger K, et al. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988;44:230–2. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- 7. Metzner J, Beckemeier H, Paintz M, et al. Zur antimikrobieller Wirksamkeit von Porpolis und Propolisinhaltstoffen. Pharmazie. 1979;34:97–102. [in German] [PubMed] [Google Scholar]
- 8. Barrientos L, Herrera CL, Montenegro G, et al. Chemical and botanical characterization of Chilean propolis and biological activity on cariogenic bacteria Streptococcus mutans and Streptococcus sobrinus. Braz J Microbiol. 2013;44:577–85. doi: 10.1590/S1517-83822013000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bankova V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J ApiProd ApiMed Sci. 2009;1:23–8. [Google Scholar]
- 10. Murtaza G, Karim S, Akram MR, et al. Caffeic acid phenethyl ester and therapeutic potentials. Biomed Res Int. 2014;2014:145342. doi: 10.1155/2014/145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Pang J, Maffucci JA, et al. Pharmacokinetics of caffeic acid phenethyl ester and its catechol-ring fluorinated derivative following intravenous administration to rats. Biopharm Drug Dispos. 2009;30:221–8. doi: 10.1002/bdd.657. [DOI] [PubMed] [Google Scholar]
- 12. Guo X, Shen L, Tong Y, et al. Antitumor activity of caffeic acid 3,4-dihydroxyphenethyl ester and its pharmacokinetic and metabolic properties. Phytomedicine. 2013;20:904–12. doi: 10.1016/j.phymed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 13. Barros Silva R, Santos NA, Martins NM, et al. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience. 2013;233:86–94. doi: 10.1016/j.neuroscience.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 14. Celli N, Dragani LK, Murzilli S, et al. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J Agric Food Chem. 2007;55:3398–407. doi: 10.1021/jf063477o. [DOI] [PubMed] [Google Scholar]
- 15. Tolba MF, Azab SS, Khalifa AE, et al. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: a review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life. 2013;65:699–709. doi: 10.1002/iub.1189. [DOI] [PubMed] [Google Scholar]
- 16. Akyol S, Ozturk G, Ginis Z, et al. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013;65:515–26. doi: 10.1080/01635581.2013.776693. [DOI] [PubMed] [Google Scholar]
- 17. Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–63. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 18. Jo SY, Lee N, Hong SM, et al. Caffeic acid phenethyl ester inhibits diesel exhaust particle–induced inflammation of human middle ear epithelial cells via NOX4 inhibition. Ann Otol Rhinol Laryngol. 2013;122:595–600. doi: 10.1177/000348941312200910. [DOI] [PubMed] [Google Scholar]
- 19. da Cunha FM, Duma D, Assreuy J, et al. Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radical Res. 2004;38:1241–53. doi: 10.1080/10715760400016139. [DOI] [PubMed] [Google Scholar]
- 20. Lee YJ, Liao PH, Chen WK, et al. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000;153:51–6. doi: 10.1016/s0304-3835(00)00389-x. [DOI] [PubMed] [Google Scholar]
- 21. Lin HP, Lin CY, Huo C, et al. Anticancer effect of caffeic acid phenethyl ester. Pharmacologia. 2012;3:26–30. [Google Scholar]
- 22. Su ZZ, Lin J, Grunberger D, et al. Growth suppression and toxicity induced by caffeic acid phenethyl ester (CAPE) in type 5 adenovirus- transformed rat embryo cells correlate directly with transformation progression. Cancer Res. 1994;54:1865–70. [PubMed] [Google Scholar]
- 23. Xiang D, Wang D, He Y, et al. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the β catenin/T cell factor signalling. Anticancer Drugs. 2006;17:753–62. doi: 10.1097/01.cad.0000224441.01082.bb. [DOI] [PubMed] [Google Scholar]
- 24. Berger N, Ben Bassat H, Klein BY, et al. Cytotoxicity of NF-kappaB inhibitors Bay 11-7085 and caffeic acid phenethyl ester to Ramos and other human B-lymphoma cell lines. Exp Hematol. 2007;35:1495–509. doi: 10.1016/j.exphem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25. Ozturk G, Ginis Z, Akyol S, et al. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci. 2012;16:2064–8. [PubMed] [Google Scholar]
- 26. McEleny K, Coffey R, Morrissey C, et al. Caffeic acid phenethyl ester- induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int. 2004;94:402–6. doi: 10.1111/j.1464-410X.2004.04936.x. [DOI] [PubMed] [Google Scholar]
- 27. Hung MW, Shiao MS, Tsai LC, et al. Apoptotic effect of caffeic acid phenethyl ester and its ester and amide analogues in human cervical cancer ME180 cells. Anticancer Res. 2003;23:4773–80. [PubMed] [Google Scholar]
- 28. Lin YH, Chiu JH, Tseng WS, et al. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother Pharmacol. 2006;57:525–32. doi: 10.1007/s00280-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 29. Chen MF, Wu CT, Chen YJ, et al. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiat Res. 2004;45:253–60. doi: 10.1269/jrr.45.253. [DOI] [PubMed] [Google Scholar]
- 30. Liao HF, Chen YY, Liu JJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51:7907–12. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 31. Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143–9. doi: 10.1097/00001813-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 32. Mahmoud NN, Carothers AM, Grunberger D, et al. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–7. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 33. Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–9. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 34. Lee KW, Chun KS, Lee JS, et al. Inhibition of cyclooxygenase-2 expression and restoration of gap junction intercellular communication in H-ras-transformed rat liver epithelial cells by caffeic acid phenethyl ester. Ann NY Acad Sci. 2004;1030:501–7. doi: 10.1196/annals.1329.062. [DOI] [PubMed] [Google Scholar]
- 35. Michaluart P, Masferrer JL, Carothers AM, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–52. [PubMed] [Google Scholar]
- 36. Orban Z, Mitsiades N, Burke TR, Jr, et al. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
- 37. Márquez N, Sancho R, Macho A, et al. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-κB transcription factors. J Pharmacol Exp Ther. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]
- 38. Zhao WX, Wang L, Yang JL, et al. Caffeic acid phenethyl ester attenuates pro-inflammatory and fibrogenic phenotypes of LPS-stimulated hepatic stellate cells through the inhibition of NF-κB signaling. Oncol Res. 2014;33:687–94. doi: 10.3892/ijmm.2013.1613. [DOI] [PubMed] [Google Scholar]
- 39. Akyol S, Acar M, Ünal ZN, et al. The effects of caffeic acid phenethyl ester (CAPE), royal jelly, and curcumin on gene expression of ADAMTS-1, -5, and -9 in OUMS-27 chondrosarcoma cells: a preliminary study. Ann Paediatr Rheum. 2013;2:27–37. [Google Scholar]
- 40. Lee HS, Lee SY, Park SH, et al. Antimicrobial medical sutures with caffeic acid phenethyl ester and their in vitro/in vivo biological assessment. Med Chem Commun. 2013;4:777–82. [Google Scholar]
- 41. Chuu CP, Lin HP, Ciaccio MF, et al. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev Res (Phila) 2012;5:788–97. doi: 10.1158/1940-6207.CAPR-12-0004-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sud'ina GF, Mirzoeva OK, Pushkareva MA, et al. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993;329:21–4. doi: 10.1016/0014-5793(93)80184-v. [DOI] [PubMed] [Google Scholar]
- 43. Abdel-Latif MM, Windle HJ, Homasany BS, et al. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br J Pharmacol. 2005;146:1139–47. doi: 10.1038/sj.bjp.0706421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Natarajan K, Singh S, Burke TR, Jr, et al. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NFkappa B. Proc Natl Acad Sci USA. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–8. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 46. Frenkel K, Wei H, Bhimani R, et al. Inhibition of tumor promoter-mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester. Cancer Res. 1993;53:1255–61. [PubMed] [Google Scholar]
- 47. Komericki P, Kranker B. Maculopapular exanthem from propolis: case report and review of systemic cutaneous and noncutaneous reactions. Contact Derm. 2009;61:353–5. doi: 10.1111/j.1600-0536.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 48. Sanghyum K, Seok-Jai K. Effect of caffeic acid phenethyl ester on phagocytosis of septic neutrophil Crit Care Med 2012. 40 1 328 23213646 [Google Scholar]
- 49. Raso GM, Meli R, Di Carlo G, et al. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–31. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 50. Zhang L, Zhang H, Zheng X, et al. Structural basis for the inhibition of akr1b10 by caffeic acid phenethyl ester (CAPE) Chem Med Chem. 2014;9:706–9. doi: 10.1002/cmdc.201300455. [DOI] [PubMed] [Google Scholar]
- 51. Ho HC, Chang HC, Ting CT, et al. Caffeic acid phenethyl ester inhibits proliferation and migration, and induces apoptosis in platelet-derived growth factor-BB-stimulated human coronary smooth muscle cells. J Vasc Res. 2012;49:24–32. doi: 10.1159/000329819. [DOI] [PubMed] [Google Scholar]
- 52. Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 53. Chen MJ, Chang WH, Lin CC, et al. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology. 2008;8:566–76. doi: 10.1159/000159843. [DOI] [PubMed] [Google Scholar]
- 54. Kuo HC, Kuo WH, Lee YJ, et al. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006;234:199–208. doi: 10.1016/j.canlet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 55. Su ZZ, Lin J, Prewett M, et al. Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells. Anticancer Res. 1995;15:1841–8. [PubMed] [Google Scholar]
- 56. Lotfy M. Biological activity of bee propolis in health and disease. Asian Pacific J Cancer Prevent. 2006;7:22–31. [PubMed] [Google Scholar]
- 57. Song YS, Park EH, Jung KJ, et al. Inhibition of angiogenesis by propolis. Arch Pharm Res. 2002;25:500–4. doi: 10.1007/BF02976609. [DOI] [PubMed] [Google Scholar]
- 58. Lin WL, Liang WH, Lee YJ, et al. Antitumor progression potential of caffeic acid phenethyl ester involving p75(NTR) in C6 glioma cells. Chem Biol Interact. 2010;188:607–15. doi: 10.1016/j.cbi.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 59. Oršolić N, Bašić I. Water-soluble derivative of propolis and its polyphenolic compounds enhance tumoricidal activity of macrophages. J Ethnopharmacol. 2005;102:37–45. doi: 10.1016/j.jep.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 60. Basini G, Baioni L, Bussolati S, et al. Antiangiogenic properties of an unusual benzo[k,l]xanthene lignan derived from CAPE (caffeic acid phenethyl ester) Invest New Drugs. 2012;30:186–90. doi: 10.1007/s10637-010-9550-z. [DOI] [PubMed] [Google Scholar]
- 61. Nomura M, Kaji A, Ma W, et al. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol Carcinog. 2001;31:83–9. doi: 10.1002/mc.1043. [DOI] [PubMed] [Google Scholar]
- 62. Amodio R, De Ruvo C, Sacchetti A, et al. Caffeic acid phenethyl ester blocks apoptosis induced by low potassium in cerebellar granule cells. Int J Dev Neurosci. 2003;21:379–89. doi: 10.1016/s0736-5748(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 63. Lee YJ, Kuo HC, Chu CY, et al. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66:2281–9. doi: 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 64. Jin UH, Song KH, Motomura M, et al. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol Cell Biochem. 2008;310:43–8. doi: 10.1007/s11010-007-9663-7. [DOI] [PubMed] [Google Scholar]
- 65. Watabe M, Hishikawa K, Takayanagi A, et al. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–26. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 66. Orsolić N, Knezević AH, Sver L, et al. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94:307–15. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 67. Shigeoka Y, Igishi T, Matsumoto S, et al. Sulindac sulfide and caffeic acid phenethyl ester suppress the motility of lung adenocarcinoma cells promoted by transforming growth factor-beta through Akt inhibition. J Cancer Res Clin Oncol. 2004;130:146–52. doi: 10.1007/s00432-003-0520-0. [DOI] [PubMed] [Google Scholar]
- 68. Eid HM, Vallerand D, Muhammad A, et al. Structural constraints and the importance of lipophilicity for the mitochondrial uncoupling activity of naturally occurring caffeic acid esters with potential for the treatment of insulin resistance. Biochem Pharmacol. 2010;79:444–54. doi: 10.1016/j.bcp.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 69. Wang T, Chen L, Wu W, et al. Potential cytoprotection: antioxidant defence by caffeic acid phenethyl ester against free radical-induced damage of lipids, DNA, and proteins. Can J Physiol Pharmacol. 2008;86:279–87. doi: 10.1139/y08-029. [DOI] [PubMed] [Google Scholar]
- 70. Bhimani RS, Troll W, Grunberger D, et al. Inhibition of oxidative stress in HeLa cells by chemopreventive agents. Cancer Res. 1993;53:4528–33. [PubMed] [Google Scholar]
- 71. Chiao C, Carothers AM, Grunberger D, et al. Apoptosis and altered redox state ınduced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–83. [PubMed] [Google Scholar]
- 72. Kudugunti SK, Thorsheim H, Yousef MS, et al. The metabolic bioactivation of caffeic acid phenethyl ester (CAPE) mediated by tyrosinase selectively inhibits glutathione S-transferase. Chem Biol Interact. 2011;192:243–56. doi: 10.1016/j.cbi.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kudugunti SK, Vad NM, Ekogbo E, et al. Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Invest New Drugs. 2011;29:52–62. doi: 10.1007/s10637-009-9334-5. [DOI] [PubMed] [Google Scholar]
- 74. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Cancer. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 75. Sen R, Baltimore D. Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell. 1986;47:921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 76. Aggarwal BB. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 77. Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–54. [PubMed] [Google Scholar]
- 78. Anto RJ, Mukhopadhyay A, Shishodia S, et al. Cigarette smoke condensate activates nuclear transcription factor-κB. through phosphorylation and degradation of IκBα: Correlation with induction of cyclooxygenase-2. Carcinogenesis. 2002;23:1511–8. doi: 10.1093/carcin/23.9.1511. [DOI] [PubMed] [Google Scholar]
- 79. Shishodia S, Majumdar S, Banerjee S, et al. Ursolic acid inhibits nuclear factor-κB activation induced by carcinogenic agents through suppression of IκBαkinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–83. [PubMed] [Google Scholar]
- 80. Farina AR, Tacconelli A, Vacca A, et al. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box. and nuclear factor κB. elements. Cell Growth Differ. 1999;10:353–67. [PubMed] [Google Scholar]
- 81. Bond M, Fabunmi RP, Baker AH, et al. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-κB. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 82. Novak U, Cocks BG, Hamilton JA. A labile repressor acts through the NFkB-like binding sites of the human urokinase gene. Nucleic Acids Res. 1991;19:3389–93. doi: 10.1093/nar/19.12.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van de Stolpe A, Caldenhoven E, Stade BG, et al. 12-Otetradecanoylphorbol-13-acetate- and tumor necrosis factor α-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269:6185–92. [PubMed] [Google Scholar]
- 84. Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–18. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 85. Griffin JD. Leukemia stem cells and constitutive activation of NF-κB. Blood. 2001;98:2291–8. doi: 10.1182/blood.v98.8.2291a. [DOI] [PubMed] [Google Scholar]
- 86. Baron F, Turhan AG, Giron-Michel J, et al. Leukemic target susceptibility to natural killer cytotoxicity: relationship with BCR-ABL expression. Blood. 2002;99:2107–13. doi: 10.1182/blood.v99.6.2107. [DOI] [PubMed] [Google Scholar]
- 87. Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 88. Taketomi A, Takenaka K, Matsumata T, et al. Circulating intercellular adhesion molecule-1 in patients with hepatocellular carcinoma before and after hepatic resection. Hepatogastroenterology. 1997;44:477–83. [PubMed] [Google Scholar]
- 89. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 90. Steele VE, Hawk ET, Viner JL, et al. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat Res. 2003;523–524:137–44. doi: 10.1016/s0027-5107(02)00329-9. [DOI] [PubMed] [Google Scholar]
- 91. Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 92. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 93. Cho HY, Reddy SP, Yamamoto M, et al. The transcription factor NRF2 protects pulmonary fibrosis. FASEB J. 2004;18:1258–60. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 94. Chen T, Nines RG, Peschke SM, et al. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–7. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 95. Kim YH, Woo KJ, Lim JH, et al. 8-Hydroxyquinoline inhibits iNOS expression and nitric oxide production by down-regulating LPS-induced activity of NF-kappaB and C/EBPhin Raw 264.7 cells. Biochem Biophys Res Commun. 2005;329:591–7. doi: 10.1016/j.bbrc.2005.01.159. [DOI] [PubMed] [Google Scholar]
- 96. Duque J, Fresno M, Iñiguez MA. Expression and function of the nuclear factor of activated T cells in colon carcinoma cells. J Biol Chem. 2005;289:8686–93. doi: 10.1074/jbc.M413076200. [DOI] [PubMed] [Google Scholar]
- 97. Jimenez JL, Iñiguez MA, Muñoz-Fernández MA, et al. Effect of phosphodiesterase 4 inhibitors on NFAT-dependent cyclooxygenase-2 expression in human T lymphocytes. Cell Signal. 2004;16:1363–73. doi: 10.1016/j.cellsig.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 98. Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–7. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 100. Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]