Abstract
Twenty-nine commercial essential oil (EO) products that were purchased from the Taiwan market, including three different company-made Melissa officinalis essential oils, were assayed on their glucose consumption activity and lipid accumulation activity on 3T3-L1 adipocytes. The EOs of M. officinalis were significantly active in both model assays. By contrast, EOs of peppermint, lavender, bergamot, cypress, niaouli nerolidol, geranium-rose, and revensara did not increase glucose consumption activity from media, but displayed inhibited lipid accumulation activity (65–90% of lipid accumulation vs. the control 100%). Because of the promising activity of M. officinalis EOs, three different products were collected and compared for their gas chromatography chemical profiles and bioactivity. The Western blot data suggest that the key factors of the adenosine monophosphate-activated protein kinase/acetyl-CoA carboxylase pathway can be mediated by M. officinalis EOs. Together with biodata, gas chromatography–mass spectrometry profiles suggested mixtures of citrals and minor compounds of M. officinalis EOs may play an important role on effect of antidiabetes.
Keywords: antidiabetes, essential oils, Melissa officinalis
1. Introduction
Obesity and metabolic syndromes are life-threatening events that lead to growing health issues worldwide. Obesity is the central and causal component in metabolic syndromes, resulting from an imbalance between energy intake and energy expenditure that can cause impaired glucose tolerance, insulin resistance, and type 2 diabetes [1,2,3,4,5]. However, prevention strategies involving diet control and physical activity are not easy for obese and diabetic patients [6,7].
Adipose tissues are major sites for postprandial glucose uptake. Several clinical drugs are used in decreasing insulin resistance in tissues, increasing usage of blood glucose and balancing levels of body glucose [8]. For example, thiazolidinediones are a class of medications used in the treatment of type 2 diabetes mellitus that act by activating peroxisome proliferator-activated receptors (PPARs) to decrease insulin resistance and modify adipocyte differentiation. Another choice is metformin, which is an oral antidiabetic drug in the biguanide class. It is the first-line drug of choice for the treatment of type 2 diabetes in overweight and obese people and those with normal kidney function. Metformin is a well-known adenosine monophosphate-activated protein kinase (AMPK) activator [9]. Many studies have suggested that activation of AMPK plays an important role in insulin signaling, whole body energy balance, and metabolism of glucose and fats. This was also shown to be required for metformin’s inhibitory effect on the production of glucose by liver cells. Recent experiments have found that metformin also increases AMPK phosphorylation level in adipose and muscle tissues to increase blood glucose consumption and decrease insulin resistance. Moreover, AMPK and PPAR pathway both show an important role in regulating body glucose balance and decreasing insulin resistance in adipocyte tissue [10]. Furthermore, the use of an in vitro adipocyte model, which is a useful and well-established antidiabetic agent screening model, can enable discovery of new AMPK activators or PPAR ligands [11].
Folk medicine, such as traditional medicine, alternative medicine, indigenous medicine, complementary medicine, and natural medicine, has become popular in recent decades. Essential oils from plants or herbs have found applications in folk medicine, food flavoring and preservation, as well as in fragrance industries [12]. The EOs commonly found in fruits, vegetables, herbs, and various plants have been shown to possess possible health benefits with antioxidative, antimicrobial, antitumor, anticarcinogenic, anti-inflammatory, atherosclerosis, antimutagenic, antiplatelet aggregation, and angiogenesis inhibitory activities [13,14,15,16]. Our previous study has found that nonpolar Toona sinensis extract prepared by supercritical-CO2 fluid extraction method show antidiabetic properties [17]. Therefore, we believe that the natural nonpolar compounds or components, such as those derived from supercritical-CO2 fluid extracts or essential oils, exist as potential antidiabetic agents. Here, we test the bioactivity of 29 essential oils from the Taiwanese market, in 3T3L1 adipocytes, and analyze the glucose consumption activity and lipid drop accumulation activity, to find potential antiobesity or antidiabetic agents. Furthermore, mechanisms of action and gas chromatography–mass spectrometry (GC-MS) chemical profiles of active EOs were also investigated.
2. Materials and methods
2.1. Materials
The HPLC-grade solvents were purchased from Echo Chemical Co. Ltd., Miaoli, Taiwan and Merck, Darmstadt, Germany. All chemicals were purchased from Sigma–Aldrich (St Louis, MO, USA). Volatile oils were obtained from the companies from the Taiwanese market with Certified Ophthalmic Assistant® (COA) certificates, including the product name, botanical name, origin, Chemical Abstract No., European Inventory of Existing Commercial chemical Substances (EINECS) no., Flavor and Extract Manufactures Association (FEMA), No., Food and Drug Administration (FDA) No., Product No., Lot No., date, appearance, color, odor, special gravity at 20°C, optical rotation at 20°C, requirements for stability and storage etc., individually, and the detail was noticed in Table 1 [14].
Table 1.
Data of glucose consumption and lipid drop accumulation of treatment with control, insulin, and 29 essential oils on 3T3-L1 adipocytes.
| No. | Name | Scientific name | Family | Glucose consumption, %a | Oil drop accumulation, %b |
|---|---|---|---|---|---|
| 1 | Lemon balm-A | Melissa officinalis | Lamiaceae | 63.64 ± 11.46** | 66.48 ± 0.33# |
| 2 | Lemon balm-B | Melissa officinalis | Lamiaceae | 59.96 ± 3.65** | 83.75 ± 7.54# |
| 3 | Lemon balm-C | Melissa officinalis | Lamiaceae | 65.63 ± 9.76*** | 53.78 ± 9.73# |
| 4 | Spanish-Sage | Salvia lavandulifolia | Lamiaceae | 34.30 ± 8.79 | 130.46 ± 7.20# |
| 5 | Rosemary | Rosmarinus officinalis | Lamiaceae | 32.76 ± 7.54 | 130.46 ± 4.14# |
| 6 | Marjoram | Origanom marjorana | Lamiaceae | 32.94 ± 7.84 | 152.11 ± 2.32# |
| 7 | Peppermint | Mentha piperita | Lamiaceae | 42.92 ± 4.58 | 66.09 ± 0.57# |
| 8 | Lavender | Lavandula angustifolia | Lamiaceae | 35.67 ± 7.57 | 83.91 ± 1.72# |
| 9 | Thyme | Thymus vulgaris | Lamiaceae | 41.72 ± 10.47 | 96.17 ± 3.17 |
| 10 | Basil | Ocimum basilicum L. | Lamiaceae | 41.12 ± 7.08 | 106.84 ± 5.95 |
| 11 | Orange | Citrus sinensis | Rutaceae | 39.39 ± 8.43 | 122.03 ± 2.89# |
| 12 | Bergamot | Citrus bergamia | Rutaceae | 36.60 ± 7.35 | 90.42 ± 1.20 |
| 13 | Lemon | Citrus limonum | Rutaceae | 38.01 ± 8.00 | 96.55 ± 2.30 |
| 14 | Mandarin | Citrus reticulate | Rutaceae | 42.94 ± 10.88 | 109.96 ± 2.02 |
| 15 | Grapefruit | citrus paradise | Rutaceae | 38.78 ± 6.22 | 105.94 ± 2.18 |
| 16 | Tea tree | Melaleuca alternifolia | Myrtaceae | 32.94 ± 5.29 | 97.70 ± 1.52 |
| 17 | Niaouli nerolidol | Melaleuca quinquenervia | Myrtaceae | 42.89 ± 8.73 | 91.57 ± 1.20 |
| 18 | Eucalyptus | Eucalyptus globulus | Myrtaceae | 32.94 ± 7.53 | 110.34 ± 2.51 |
| 19 | Cypress | Cupressus sempervirens | Cupressaceae | 32.94 ± 6.55 | 89.66 ± 1.99 |
| 20 | Cedarwood | Juniperus virginiana | Cupressaceae | 34.18 ± 8.10 | 134.10 ± 2.32# |
| 21 | Juniper-Berry | Juniperus communis | Cupressaceae | 38.42 ± 7.64 | 107.28 ± 1.85 |
| 22 | Black pepper | Piper nigrum | Piperaceae | 37.13 ± 6.56 | 131.61 ± 3.20# |
| 23 | Frankincense | Boswellia carterii | Burseraceae | 35.07 ± 6.91 | 106.51 ± 2.59 |
| 24 | Ginger | Zingibar officinale | Zingiberaceae | 38.99 ± 7.84 | 166.67 ± 3.04# |
| 25 | Geranium-rose | Pelargonium x aspermum | Geraniaceae | 42.54 ± 6.74 | 76.05 ± 1.45# |
| 26 | Fennel | Fonneculum vulgare | Apiaceae | 34.81 ± 7.17 | 93.49 ± 0.88 |
| 27 | Chamomile-Roman | Anthemis nobilis | Asteraceae | 35.87 ± 8.49 | 100.77 ± 1.76 |
| 28 | Pine | Abies balsamea | Pinaceae | 44.95 ± 8.22 | 92.15 ± 1.66 |
| 29 | Revensara | Cinnamomum camphora | Lauraceae | 42.73 ± 6.48 | 86.08 ± 4.96# |
| 30 | Insulin | 50.00 ± 4.85* | 135.82 ± 3.74# | ||
| 31 | Control | 27.46 ± 0.93 | 100.00 ± 0.91 |
p < 0.001.
Glucose consumption% = medium glucose concentration/450 mg/dL, result compared with control,
p < 0.001,
p < 0.01,
p < 0.05.
Oil drop accumulation % = (Sample Oil-red O stain result OD value/Control Oil-red O stain result OD value), result compared with control.
2.2. GC-MS
The components of Melisa officinalis EOs were analyzed by using a GC-MS system (Trace GC ultra, DSQ II-Mass Spectrometer, MS 2205862; Thermo Fisher Scientific, Inc., Waltham, MA, USA) equipped with an HP-5MS capillary column (5% phenyl methyl siloxane, length = 30 m, i.d. = 0.25 mm, film thickness = 0.25 μm). Electron impact ionization was carried out at energy of 70 eV. Helium was used as carrier gas at a flow rate of 1.0 mL/minute. An aliquot of 10 μL EO dissolved in 10 mL ethyl acetate, respectively, and adjusted to 250 ppm, 500 ppm, and 1000 ppm. The injection volumes of EOs were 1 μL, individually. The GC oven program used was: 60°C held for 11 minutes, 2°C/minute to 120°C held for 2 minutes, 35°C/minute to 250°C held for 2 minutes; for the operating conditions refer to our previous study [16]. The components were identified by comparing with the NIST/EPA/ NIH Mass spectral Library database (Version 2.0 d, build April 26, 2005). The area percentages of major compounds in 3 Mellisa EOs were quantified on the basis of the peak area integrated by the Thermo Xcalibur data analysis program.
2.3. 3T3-L1 adipocyte culture and glucose consumption assay
Equal amounts (5 × 105 cells) of 3T3-L1 pre-adipocytes were seeded and cultured in normal D-glucose (100 mg/dL) Dulbecco’s modified Eagle medium with 10% fetal bovine serum, 1% penicillin–streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C. When the cells reached 100% confluence, 3T3-L1 preadipocytes were induced to be differentiated by treating the culture with 450 mg/dL D-glucose, 0.32 μM insulin, 0.5 mM 3-isobutyl-1-methylxanthine and 1 μM dexamethasone for 2 days. Then, the culture medium of the differentiated adipocytes was changed to Dulbecco’s modified Eagle medium containing 450 mg/dL D-glucose with or without the administration of the tested compounds. After 24 hours, the glucose consumption activity was determined by measuring the medium glucose concentration. The coefficient of variation of the analyzer was 0.62–0.92% within-run and 1.1–1.2% between days. To confirm whether our in vitro model was sufficient to measure the glucose-lowering effect, insulin was used as the positive control. The insulin powder was dissolved in 0.01 M acetic acid (pH 3.0) to provide a 10−2 M stock solution and then diluted in distilled water. EO samples were dissolved in dimethyl sulfoxide (DMSO) to make 60 μL/ mL stock solutions and then diluted in DMSO; the final concentration of DMSO in the medium was 0.1% [17,18]. All samples test their bioactivity in 60 μ/L concentration.
2.4. Oil-Red O staining
Differentiated 3T3-L1 adipocytes were treated with the tested compounds for 6 days. On Day 6, the cells were washed twice with phosphate-buffered saline (PBS) and were fixed with 10% paraformaldehyde for 30 minutes at room temperature and finally washed with PBS. The fixed cells were stained for 1 hour with 0.5% Oil-Red O, which was diluted with propylene glycol and then washed with PBS. Lipid droplets were stained and photographed by light microscopy. Stained oil droplets were dissolved in isopropanol and quantified by spectrophotometric analysis at 490 nm [19].
2.5. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting
The expression of target proteins was analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting. Briefly, the cells were lysed in M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with Complete Protease Inhibitor Cocktail (Roche Applied Science, Penzberg, Germany). Protein concentration was measured using the Quant-iT Protein assay kit (Invitrogen, Carlsbad, CA, USA). For analysis of each specific protein, the cell lysate was loaded and separated on 10% sodium dodecyl sulfate–polyacrylamide gels. After transfer to polyvinylidene fluoride membranes, proteins of interest were detected using the appropriate antibodies.
2.6. Statistical analysis
The data are presented as the mean ± standard deviation. Differences between the means of the individual groups were analyzed using the analysis of variance procedure of Graph-Pad Prism software (Intuitive Software for Science, San Diego, CA, USA). The significance of differences was defined at the p < 0.05, p < 0.01, and p < 0.001 levels.
3. Results and discussion
3.1. Glucose consumption activity assay
We have developed an in vitro antidiabetic screening model based on measuring glucose consumption after 24 hours in culture medium of 3T3-L1 adipocytes. In our preliminary screening (Table 1), 27 EOs, including the Melissa product A (lemon balm-A), and insulin (the positive control) were tested for glucose consumption effects. Our results show that the adipocytes of the control group without insulin only consumed approximate 27% of glucose from the medium in 24 hours. Melissa product A (lemon balm-A) and the positive control (insulin) group significantly increased glucose consumption ca. 63.64 ± 11.46% and 50 ± 4.85%, respectively. Melissa is the only hit among the assays. Consequently, we tested two further Melissa products (lemon balm-B and C) from another company and they also increased the glucose consumption of the cells by 59 ± 3.65% and 65.64 ± 9.76%, respectively. The other 26 EO products did not show any significant change in glucose consumption activity in this in vitro model.
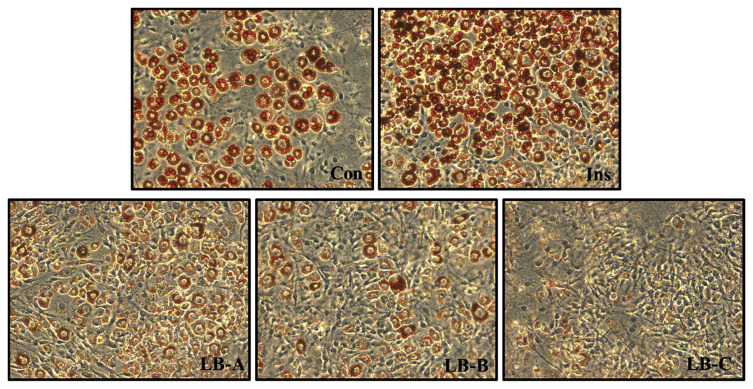
3.2. Lipid accumulation effect by Oil-Red O stain assay
The 3T3-L1 adipocytes in vitro assay can be used to test for materials that stimulated cell glucose consumption ability, and to evaluate the effects of lipid metabolism on cells. The effect of EO products on lipid metabolism was evaluated by detecting lipid accumulation in differentiated 3T3-L1 adipocytes using Oil Red O stain. After 6 days of treatment, the data indicated that the three Melissa products not only promoted cellular consumption of glucose from the medium, but also inhibited lipid accumulation into cells (Table 1). This data shows that lemon balm-A, lemon balm-B, and lemon balm-C only accumulated 66 ± 0.33%, 83 ± 7.54%, and 53 ± 9.73%, of lipids, respectively, suggesting that these essential oils significantly inhibited lipid accumulation in 3T3-L1 adipocytes (Fig. 1). In the assay, another seven essential oils also inhibited the lipid accumulation—peppermint, lavender, bergamot, cypress, niaouli nerolidol, geranium-rose, and revensara—where only 65–90% of lipid accumulation was found compared with the control (100%). Interestingly, Spanish sage, rosemary, marjoram, orange, eucalyptus, cedarwood, black pepper, ginger, and insulin increased lipid accumulation (110–167%). The other 11 essential oils—thyme, lemon, tea-tree, fennel, chamomile-roman, pine, basil, mandarin, grapefruit, juniper-berry, and frankincense—did not have any effect on lipid metabolism (90–110%). Although part of the mechanism of glucose consumption may affect and enhance lipid accumulation, the pathways of glucose consumption and lipid accumulation are not the same. It is an interesting finding in the current study that some EOs can modulate lipid accumulation without or with just a minor effect to glucose consumption.
Fig. 1.
Oil-Red O staining results of the lipid drop accumulation assay for control, insulin and three Melissa essential oils treatments on 3T3-L1 adipocytes.
3.3. Effect of Melissa EOs on the expression of glucose consumption activity and adipogenic-specific proteins
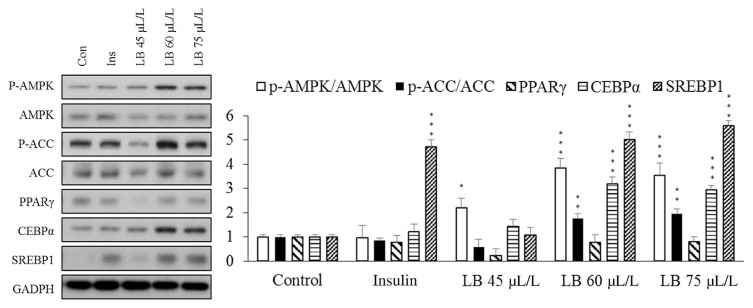
AMPK is a fuel-sensing enzyme that is activated by decreasing the cell’s energy state as reflected by an increased AMP/ATP ratio. When activated, it initiates metabolic and genetic events that restore ATP levels by stimulating processes that generate ATP and inhibiting others that consume energy but are not acutely required for survival. Another function of AMPK is to mediate lipid metabolism via regulation of acetyl-CoA carboxylase (ACC). The phosphorylated ACC is the inactivate form. AMPK phosphorylation increases in the level of phosphorylated ACC, which results in a decrease in fatty acid synthesis and elongation [9,20,21]. The phosphorylated ACC is the inactivate form, and the AMPK is the main kinase regulator of ACC that phosphorylate a number of serine residues on both isoforms of ACC. So, the p-AMPK should inhibit ACC activity by phosphorylated ACC. Here, we show data that suggest that Melissa essential oils significantly activate AMPK/ ACC pathway in dose dependent studies. Activation of AMPK causes cells to uptake more glucose from media and down-regulates ACC, thus inhibiting lipid accumulation into adipocytes. The effect of the Melissa EOs on the expression of key transcription factors, such as C/EBPR, SREBP1, and PPARγ, was evaluated using Western blotting analysis. In comparison to adipocytes without sample treatment, the presence of Melissa EO during adipogenic differentiation resulted in increased expression levels of SREBP1 and C/EBPR but not PPARγ. Although increase expression levels of SREBP1 and C/EBPR should increase lipid accumulation into cells, activated ACC proteins can inhibit lipid synthesis. In the final result, the Western blot data support and explain why Melissa EOs cause adipocytes to use more glucose but inhibit lipid accumulation (Fig. 2).
Fig. 2.
Effect of control, insulin and each dose of the lemon balm-A essential oil on the regulation of glucose and adipogenic transcription factors, such as p-AMPK, AMPK, p-ACC, ACC, PPAR, CEBPα, and SREBP1, determined by Western blot analysis. ***p < 0.001, **p < 0.01, *p < 0.05.
3.4. Chemical composition
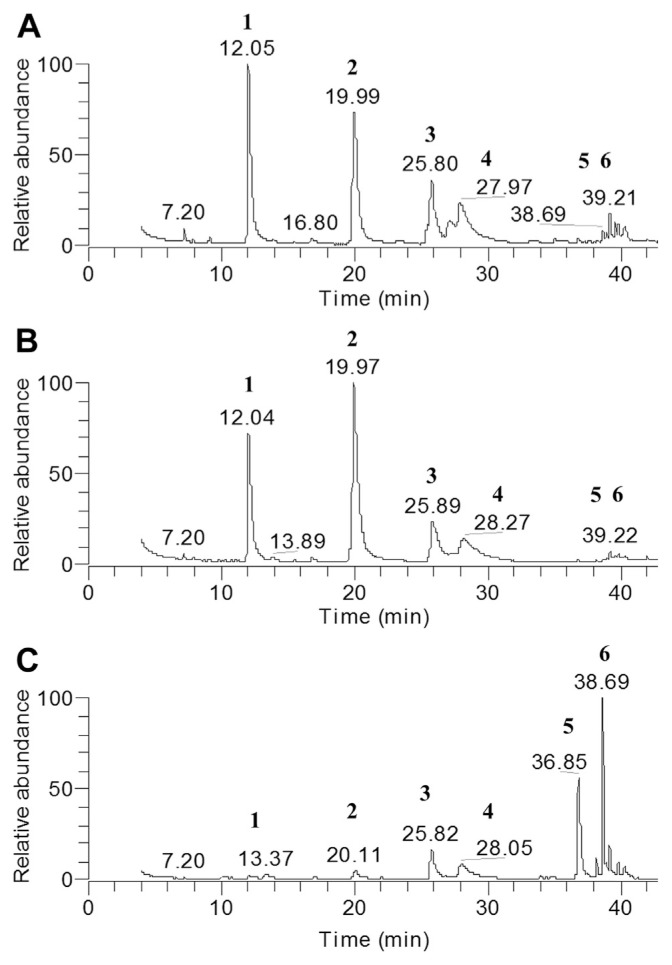
We used GC-MS to analyze chemical compositions of these three different Melissa products made by different companies and found that they show similar bioactivity in promoting glucose consumption activity and inhibiting lipid accumulation in adipocytes. These results show that these three Melissa products have similar major chemical components, including limonene, β-citronellal, β-citral, α-citral, caryophyllene, and germacrene D, which are standard compounds in Melissa essential oils (Fig. 3). We observed that lemon balm-A and B show similar patterns in GC-MS fingerprints, profiles having higher concentrations of limonene (24–26%) and β-citronellal (29–45%) but lower concentration of caryophyllene (0.3–0.7%) and germacrene D (1.1–2.2%). By contrast, lemon balm-C has less limonene (2.3%) and β-citronellal (4.5%) and high percentages of caryophyllene (31.2%) and cermacrene D (15.5%). However, both β-citral (12–15%) and α-citral (5–10%) are stable in percentage in the three Melissa EO products (Table 2). Compared with data of glucose consumption activity, the compositions of four compounds, limonene, β-citronellal, caryophyllene, and germacrene D, seem not to cause the different results, which suggests that these four compounds may not correlate with these bioactivities. By contrast, citrals and minor compounds in Melissa EOs can be crucial to the antidiabetes activity.
Fig. 3.
Gas chromatography–mass spectrometry total ion chromatograms for different lemon balm essential oils: (A) lemon balm-A, (B) lemon balm-B, and (C) lemon balm-C.
Table 2.
Gas chromatography–mass spectrometry analytic data for six major compounds of three Melissa essential oils products.
| Peak | Compound name | RT | LB-Aa | LB-Ba | LB-Ca |
|---|---|---|---|---|---|
| 1 | Limonene | 12.04 | 26.26 | 24.47 | 2.29 |
| 2 | β-Citronellal | 20.01 | 29.26 | 45.85 | 4.51 |
| 3 | β-Citral | 25.87 | 14.47 | 12.7 | 12.92 |
| 4 | α-Citral | 28.06 | 8.46 | 5.86 | 10.25 |
| 5 | Caryophyllene | 36.92 | 0.65 | 0.37 | 31.24 |
| 6 | Germacrene D | 39.21 | 2.23 | 1.16 | 15.55 |
RT = retention time, minutes.
Relative contribution (%) of component from each lemon balm essential oils product.
According to the study by Singh et al [22], EOs extracted from fresh or decaying leaves will lead the changes in the limonene, β-citronellal, caryophyllene, and germacrene D. Our study suggests that limonene, β-citronellal, caryophyllene, and germacrene D are not involved in bioactivity, indicating that the fresh and decaying leaves used to make lemon balm EO did not cause the observed bioactivity changes.
The antidiabetic effects of lemon balm (M. officinalis) essential oil have been demonstrated in an animal study [23]. In that paper, the GC-MS analysis data showed a very high concentration of citrals (the sum of peak area over 89%) in the EO sample, which displayed antidiabetic activities. Moreover, the Melissa EO showed activity in promoting PPARγ [23], which is completely different from the current study. In our study, citrals are also confirmed and may serve as active compounds toward 3T3-L1 adipocytes. The mechanism of action for antidiabetes in adipocytes was proven and correlated to not only expression levels of SREBP1 and C/EBPR but also effects on the AMPK/ACC pathway.
Are the citrals the major bioactive components in Melissa EOs? In the EO list of this study, several EOs origin from citrus species, such as orange and lemon, which are well-known to possess abundant amounts of citrals. However, they are inactive. Therefore, the compositions of other minor compounds with or without citrals in the Melissa EOs may be involved in their bioactivity. The secret still remains undefined.
In summary, 29 commercial EOs (including three Melissa EOs) were tested for their glucose consumption activity and lipid drop accumulation activity on 3T3-L1 adipocytes. The EOs of M. officinalis are excellent candidates for antidiabetes agents and worthy of further investigation.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 2. Aballay LR, Eynard AR, Diaz Mdel P, et al. Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev. 2013;71:168–79. doi: 10.1111/j.1753-4887.2012.00533.x. [DOI] [PubMed] [Google Scholar]
- 3. Nikolopoulou A, Kadoglou NP. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther. 2012;10:933–9. doi: 10.1586/erc.12.74. [DOI] [PubMed] [Google Scholar]
- 4. Sakurai T, Iimuro S, Araki A, et al. Age-associated increase in abdominal obesity and insulin resistance, and usefulness of AHA/NHLBI definition of metabolic syndrome for predicting cardiovascular disease in Japanese elderly with type 2 diabetes mellitus. Gerontology. 2010;56:141–9. doi: 10.1159/000246970. [DOI] [PubMed] [Google Scholar]
- 5. Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4:113–9. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen KN, Peng WH, Hou CW, et al. Codonopsis javanica root extracts attenuates hyperinsulinemia and lipid peroxidation in fructose-fed insulin resistant rats. J Food Drug Anal. 2013;21:347–55. [Google Scholar]
- 7. Jin H, Zhang YJ, Jiang JX, et al. Studies on the extraction of pumpkin components and their biological effects on blood glucose of diabetic mice. J Food Drug Anal. 2013;21:184–9. [Google Scholar]
- 8. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russo GL, Russo M, Ungaro P. AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol. 2013;86:339–50. doi: 10.1016/j.bcp.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 10. Moller DE. Metabolic disease drug discovery-“hitting the target” is easier said than done. Cell Metab. 2012;15:19–24. doi: 10.1016/j.cmet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 11. Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–6S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 12. Ngo LT, Okogun JI, Folk WR. 21st century natural product research and drug development and traditional medicines. Nat Prod Rep. 2013;30:584–92. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bakkali F, Averbeck S, Averbeck D, et al. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 14. Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 15. Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21:308–23. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 16. Yen HF, Wu CC, Lin WU, et al. Cytotoxicity, anti-platelet aggregation assay and chemical components analysis of thirty-eight kinds of essential oils. J Food Drug Anal. 2012;20:478–83. [Google Scholar]
- 17. Hsieh TJ, Tsai YH, Liao MC, et al. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem Toxicol. 2011;50:779–89. doi: 10.1016/j.fct.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 18. Hsieh CT, Hsieh TJ, El-Shazly M, et al. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg Med Chem Lett. 2012;22:3912–5. doi: 10.1016/j.bmcl.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 19. Hsieh TJ, Lin T, Hsieh PC, et al. Suppression of glutamine:fructose-6-phosphate amidotransferase-1 inhibits adipogenesis in 3T3-L1 adipocytes. J Cell Physiol. 2012;227:108–15. doi: 10.1002/jcp.22707. [DOI] [PubMed] [Google Scholar]
- 20. Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–36. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Israili ZH. Advances in the treatment of type 2 diabetes mellitus. Am J Ther. 2011;18:117–52. doi: 10.1097/MJT.0b013e3181afbf51. [DOI] [PubMed] [Google Scholar]
- 22. Singh HP, Mittal S, Kaur S, et al. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem. 2009;57:6962–6. doi: 10.1021/jf9012407. [DOI] [PubMed] [Google Scholar]
- 23. Chung MJ, Cho SY, Bhuiyan MJ, et al. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr. 2010;104:180–8. doi: 10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]