Abstract
To evaluate the efficacy and safety of TNF-α blockers for ulcerative colitis. A systematic search for randomized controlled trials (RCTs) of TNF-α blockers for treatment of ulcerative colitis (UC) were performed in PubMed, Web of Science, Embase and cochrane clinical trial. We estimated Pooled estimates of the odds ratio (OR) and relevant 95% confidence interval (CI) using fixed effects model or random effects model as appropriate. Heterogeneity, publication bias, and subgroup analyses were conducted. Nine randomized controlled studies met the selection criteria with a total of 2518 patients. Five studies compared Infliximab with placebo. Two studies compared Infliximab to corticosteroids. Two studies compared Adalimumab to placebo. One study compared subcutaneous golimumab to placebo. Short-term response, short-term remission, long-term remission and mucosal healing were better in the TNF-α blocker group than in the control group (p < 0.05). TNF-α blockers decreased the colectomy rate and serious adverse reactions (p < 0.05). The TNF-α blockers were superior to controls in achieving short-term clinical response/remission, long-term remission and mucosal healing and decreased the colectomy rate and serious adverse reactions.
Keywords: ulcerative colitis, tumor necrosis factor-α blocker, meta-analysis, efficacy and safety
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory disorder of the gastrointestinal tract with an unknown etiology, and the incidence of UC has markedly increased in Eastern Europe, South America, and Asia [1]. The clinical course of UC is characterized by periods of remission and relapse, with acute inflammatory exacerbations of disease activity which, when severe, are potentially life-threatening. The standard initial management of these inflammatory exacerbations includes high dose intravenous glucocorticosteroids in the first instance; however, this strategy may be unsuccessful in up to 50% of patients. Immunomodulating drugs such as azathioprine, although effective in maintaining remission, act too slowly to be of use in the acute setting [2]. With the development of molecular biology and immunology, the UC pathogenesis has been further studied. It has been reported that there is high tumor necrosis factor-alpha (TNF-α) expression in blood, colonic tissue, and stool of patients with UC [3]. TNF-α, a proinflammatory cytokine, is already known to play an important role in the pathogenesis of Crohn's disease (CD), and anti-TNF therapy has been demonstrated to be useful in moderate to severe CD [4,5]. It can induce mucosal healing and has been shown to have a high steroid-sparing efficacy in active CD [6]. In UC, however, the results have been conflicting. There is insufficient evidence to advocate using anti-TNF as a first-line agent for UC patients with mild or moderate to severe disease. The efficacy of anti-TNF in UC has been investigated by a limited number of randomized controlled trials (RCTs) and results from these earlier studies were equivocal and ambiguous [7,8]. Two previous meta-analyses of RCTs have evaluated the efficacy of TNF-α blockers including adalimumab for induction or maintenance of clinical remission in UC [9,10]. However, two further trials have been reported since these meta-analysis [11,12], and one meta-analysis showed greater heterogeneity between TNF-α blocker groups [10]. In order to provide a comprehensive up-to-date therapeutic effects and safety of TNF-α blockers in the treatment of moderate and severe UC, RCTs published in recent years were meta-analyzed.
2. Methods
2.1. Literature search strategy
We conducted a systematic search for clinical trials in PubMed, Web of Science, Embase, and Cochrane Clinical Trials from 1990 to May 2013 using the following keywords: tumor necrosis factor, anti-TNF, TNF, infliximab, adalimumab, golimumab, certolizumab pegol, UC, randomized, random, randomly, and controlled trial. The reference lists of eligible studies and review articles were also checked manually to identify other relevant publications. The primary authors were contacted for missing data. All studies included in this meta-analysis were written in English.
2.2. Inclusion and exclusion criteria
Inclusion criteria were (1) RCTs; (2) a study design that included a TNF-α blocker group and a placebo, glucocorticoid, or other drug control group; and (3) assessment of therapeutic effects including one or more parameters such a short-term response, short-term remission, long-term relief, mucosal healing, colectomy rate, and serious adverse reactions. We excluded studies not accessible to full research data. Reviews, case reports, letters, and editorials were excluded. Articles about children or pregnant women were also excluded.
2.3. Data extraction
Data were abstracted by two independent investigators (Y.-N.S.. and P.Z.). Each article was comprehensively scrutinized to determine whether it met the predetermined inclusion and exclusion criteria. Each investigator abstracted the following data from each report: first author, year of publication, total number of patients, control group, duration, and total Jadad scores. If the results obtained from the articles were different, disagreements were resolved by analyzing the data.
2.4. Quality evaluation
The methodological quality of included trials was assessed using the Jadad score, which judges descriptions of randomization, blinding, and dropouts (withdrawals) in trials [13]. The quality scales ranged from 0 points to 5 points, with a low-quality report scoring ≤ 2 points and a high-quality report scoring at least 3 points [14].
2.5. Statistical analysis
Pooled estimates of the odds ratio (OR) and relevant 95% confidence interval (CI) were obtained by using a random-effects model or a fixed-effects model. Heterogeneity across trials was evaluated with the I2 statistic. The I2 values ranged from 0% to 100%: 0% suggested no observed heterogeneity, 25–49% suggested low, 50–74% moderate, and ≥75% high heterogeneity [15]. A p value < 0.1 was defined as a significant heterogeneity. If heterogeneity existed, a random-effects model was used to assess the overall estimate. Otherwise, a fixed-effects model was chosen. Subgroup analysis was performed to account for heterogeneity.
3. Results
3.1. Study selection
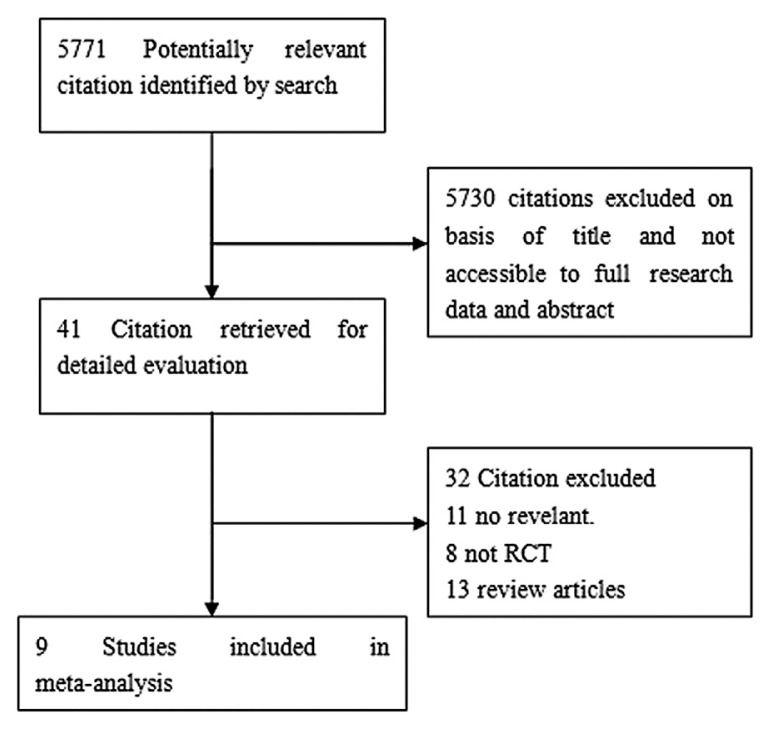
Our search strategy yielded 5771 citations (Fig. 1). We retrieved 41 citations for a detailed evaluation, of which 32 were excluded. Nine studies—four studies that compared infliximab to placebo, two studies that compared infliximab to corticosteroids (1 study to oral corticosteroids and the other study to intravenous corticosteroids), two studies that compared adalimumab to placebo, and one that compared golimumab to placebo—met the inclusion criteria [7,8,11,12,16–20].
Fig. 1.
Literature search strategy.
3.2. Characteristics of the studies
The basic characteristics of the studies are reported in Table 1. A total of 2518 patients were enrolled. Five studies that compared infliximab with placebo included 539 infliximab, and 288 placebo. Two studies compared infliximab to corticosteroids and two studies compared adalimumab to placebo to evaluate the efficacy of adalimumab in induction and maintenance of clinical remission in patients with moderate-to-severe UC. In Reinisch et al's study [16], there were two groups (ADA 160/80 and ADA 80/40). One study compared subcutaneous golimumab to placebo. Table 2 shows the main characteristics of the studies included in the meta-analysis.
Table 1.
Basic characteristics of studies included in meta-analysis.a
| First author | Published year | Cases (n) | Control group | Duration | TNF-α blocker group | Total Jadad scorea |
|---|---|---|---|---|---|---|
| Sands [7] | 2001 | 11 | placebo | 10 wk | Infliximab | 4 |
| Probert [8] | 2003 | 43 | Placebo | 6 wk | Infliximab | 5 |
| Rutgeerts (ACT1) [17] | 2005 | 364 | Placebo | 54 wk | Infliximab | 5 |
| Rutgeerts (ACT2) [17] | 2005 | 364 | Placebo | 30 wk | Infliximab | 5 |
| Jarnerot [18] | 2005 | 45 | Placebo | 6 mo | Infliximab | 5 |
| Armuzzi [20] | 2004 | 20 | Methylprednisolone | NR | Infliximab | 3 |
| Ochsenkuhn [19] | 2004 | 13 | Prednisolone | 13 wk | Infliximab | 3 |
| Reinisch [16] | 2011 | 390 | Placebo | 8 wk | Adalimumab | 5 |
| Sandborn [11] | 2012 | 494 | Placebo | 52 wk | Adalimumab | 5 |
| Sandborn [12] | 2013 | 774 n | Placebo | 6 wk | Golimumab | 5 |
NR = no report; SC = subcutaneous; TNF-α = tumor necrosis factor-alpha.
In Phase 2 of this study, the dose–response of SC golimumab induction therapy was assessed based on the change in Mayo score from baseline to Week 6. So the actual number of patients was 774.
Table 2.
Main characteristics of studies included in meta-analysis.
| First author | Year | Outcomes of different studies |
|---|---|---|
| Sands [7] | 2001 | Primary endpoint was clinical response defined as a modified Truelove and Witts score < 10 and a 5-point reduction compared with baseline at 2 wk. |
| Probert [8] | 2003 | Primary end points were clinical remission at Week 6: clinical remission defined as UCSS < 2 and sigmoidoscopic remission as a Baron score of 0. |
| Rutgeerts (ACT1 and ACT2) [17] | 2005 | The primary end point was a clinical response at Week 8. Clinical response was defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30 percent, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1. Clinical remission was defined as a total Mayo score of 2 points or lower, with no individual subscore exceeding 1 point. Mucosal healing was defined as an absolute subscore for endoscopy of 0 or 1. |
| Jarnerot [18] | 2005 | The primary end point was colectomy or death within 90 d after infusion. Secondary end points were clinical remission according to the Seo index and endoscopic remission 1 and 3 mo after the infliximab/placebo infusion. |
| Armuzzi [20] | 2004 | Primary outcome was remission defined as disease activity index (DAI) < 3 within 2 wk. |
| Ochsenkuhn [19] | 2004 | Therapy success was defined as clinical response in terms of a decrease of > 5 points from baseline score (modified Truelove and Witts activity score) and to < 10 points total after 3 wk as well as after 13 wk, and no need to start or increase high-dose prednisolone dosage or to perform colectomy. A secondary endpoint was the achievement of remission after 13 wk. |
| Reinisch [16] | 2011 | The primary efficacy endpoint was clinical remission (Mayo score ≤ 2 with no individual subscore > 1) at Week 8. |
| Sandborn [11] | 2012 | Primary end points were remission at Week 8 and Week 52. |
| Sandborn [12] | 2013 | The primary endpoint was clinical response at Week 6, and secondary endpoints were clinical remission, mucosal healing, and IBDQ change from baseline, all at Week 6. |
IBDQ = The Inflammatory Bowel Disease Questionnaire (IBDQ); UCSS = ulcerative colitis symptom score (UCSS).
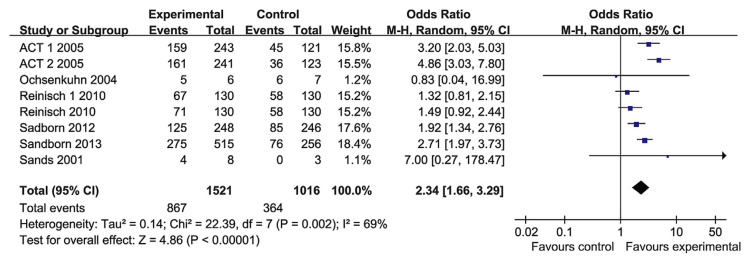
3.3. Short-term clinical response and remission
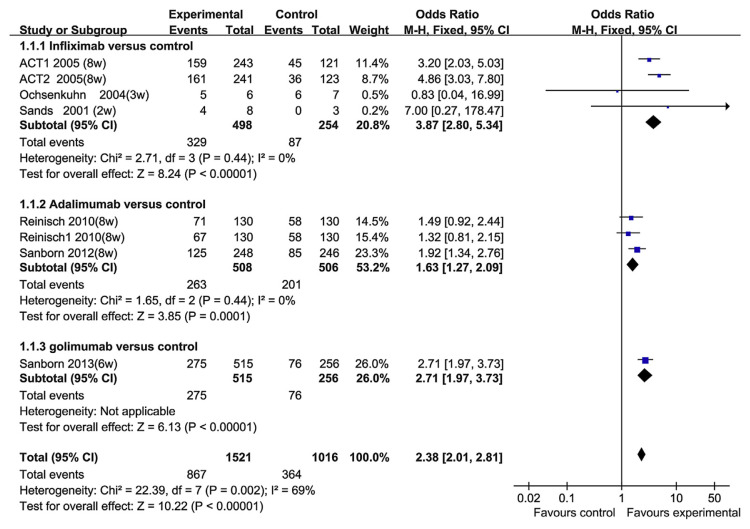
Six RCT papers reported the short-time response (2–8 weeks) and remission. The heterogeneity test indicated that χ2 = 22.39 and p = 0.002, demonstrating heterogeneity. Therefore, the random-effects model was adopted, and the OR value was 2.34 (95% CI, 1.66–3.29; p < 0.00001; Fig. 2). To investigate the factors that may result in the heterogeneity, we performed subgroup analyses based on the TNF-α blocker groups. In the infliximab group, there were three RCT papers that investigated the short-term response. The heterogeneity test indicated that χ2 = 2.71 and p = 0.44, and the OR value was 3.87 (95% CI, 2.80–5.34; p < 0.0001). There was no statistical heterogeneity. In the adalimumab group, there were only two studies, comprising 1014 patients. The heterogeneity test indicated that χ2 = 1.65 and p = 0.44, and the OR value was 1.63 (95% CI, 1.27–2.09). There was no statistical heterogeneity. In the golimumab group, golimumab showed better performance than placebo for short-term response (Fig. 3). There was a substantial heterogeneity among different TNF-α blocker groups, therefore, we think that was the primary reason for the heterogeneity. We did not conduct a meta-regression analysis to explore the factors causing heterogeneity. But in the overall analysis, the results of the short-term response (2–8 weeks) of these RCTs were consistent. Therefore, the TNF-α blockers were effective for the rapid induction of response.
Fig. 2.
The forest plot of short-term response to tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
Fig. 3.
The short-term response to tumor necrosis factor-alpha blockers in subgroup patients.
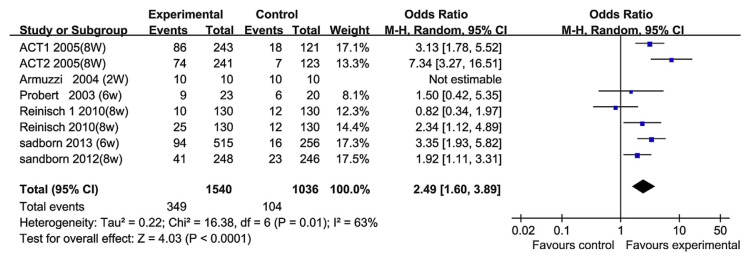
Short-term remission (2–8 weeks) was reported in six RCT papers. The heterogeneity test indicated that χ2 = 16.38 and p = 0.01, demonstrating heterogeneity. Therefore, a random-effects model was adopted, and the OR value was 2.49 (95% CI, 1.60–3.89; Fig. 4). In clinical practice, one of the therapeutic goals for a new biological agent is the rapid induction of response or remission. But in those six RCT papers, the heterogeneity showed significant differences between the TNF-α blocker groups. The factor that caused the heterogeneity may be that almost every RCT uses a unique clinical or endoscopic index. Moreover, the definitions of remission or improvement differ across the studies. This limits the conclusion of studies that compare RCTs in UC patients. For example, in the work of Rutgeerts et al [17] (ACT1), clinical remission was defined as a total Mayo score of ≤ 2 points, with no individual subscore exceeding 1 point. But in the paper of Probert et al [8], clinical remission was defined as ulcerative colitis symptom score (UCSS) < 2, and sigmoidoscopic remission was defined as a Baron score of 0. In the overall analysis, however, the short-term remission rate was significantly higher than that in the control group. We did not conduct metaregression analysis to explore the factors causing the heterogeneity.
Fig. 4.
The forest plot of short-term remission to tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
3.4. Long-term remission
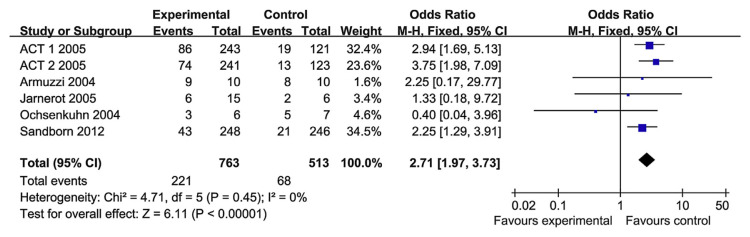
Long-term remission (12 weeks–10.9 months) was reported in five RCT papers. The heterogeneity test indicated that χ2 = 4.71 and p = 0.45, demonstrating homogeneity. Therefore, a fixed-effects model was adopted, and the OR value was 2.71 (95% CI, 1.97–3.73) (Fig. 5). So the TNF-α blockers performed better than the control groups in terms of long-term remission.
Fig. 5.
The forest plot of long-term remission to tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
3.5. Mucosal healing
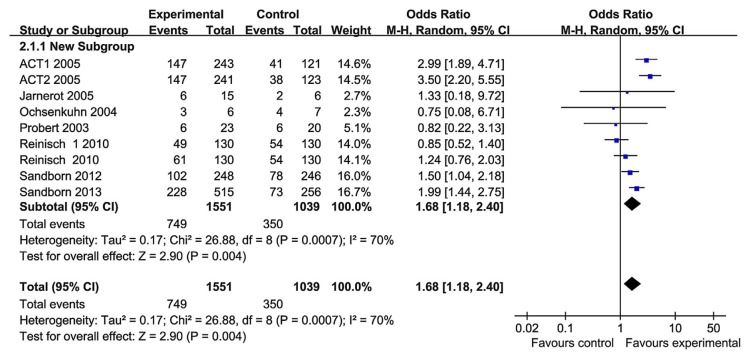
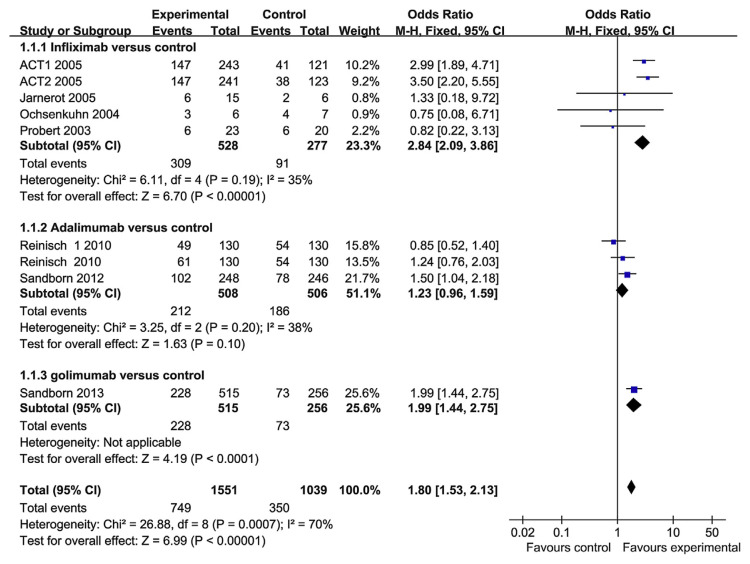
The therapeutic goals in UC had evolved to include mucosal healing as a measure of treatment efficacy. Mucosal healing was reported in seven RCT papers. The heterogeneity test indicated that χ2 = 26.88 and p = 0.0007, demonstrating heterogeneity. Therefore, a random-effects model was adopted, and the OR value was 1.68 (95% CI, 1.18–2.40; Fig. 6). So we performed subgroup analysis based on the TNF-α blocker groups (Fig. 7). In the infliximab group, there are four RCT papers that investigated the mucosal healing. The heterogeneity test indicated that χ2 = 6.11 and p = 0.19, and the OR value was 2.84 (95% CI, 2.09–3.86; p < 0.0001). There was no statistical heterogeneity. Infliximab performed better than the control group in terms of mucosal healing. In the adalimumab group, there were only two studies. The heterogeneity test indicated that χ2 = 3.25 and p = 0.20, and the OR value was 1.23 (95% CI, 0.96–1.59). There was no statistical heterogeneity. However, adalimumab did not perform better than the control group in terms of mucosal healing, a result that is different from that of adalimumab for CD treatment [21]. There was only one paper about golimumab, and golimumab performed significantly better than the control group for mucosal healing. So the hterogeneity may be a result of the different TNF-α blockers have been used. In the overall analysis, however, TNF-α blockers were superior to placebo in mucosal healing.
Fig. 6.
The forest plot of mucosal healing rate following the use of tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
Fig. 7.
The mucosal healing rate following the use of tumor necrosis factor-alpha blockers in subgroup patients.
3.6. Serious adverse reactions
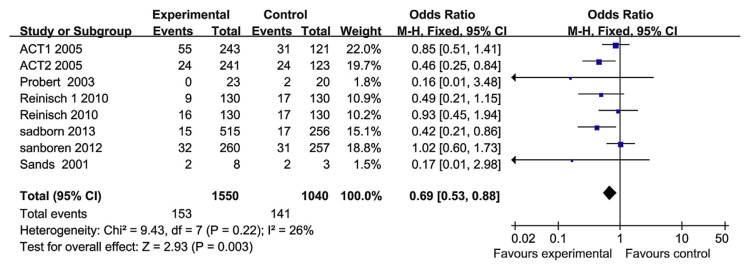
Six papers have reported the safety of the TNF-α blockers. Serious adverse events, which qualified as life-threatening or severe, were recorded. The serious adverse events included abdominal pain, nausea, arthralgia and upper respiratory tract infection, and malignant tumors. The heterogeneity test indicated that χ2 = 9.43 and p = 0.22, demonstrating homogeneity. Therefore, a fixed-effects model was adopted, and the OR value was 0.69 (95% CI, 0.53–0.88; Fig. 8). There was no apparent relationship between the treatment using TNF-α blockers and the incidences of all treatment-emergent adverse events. Therefore, the TNF-α blockers did not increase the risk of serious adverse events.
Fig. 8.
The forest plot of serious adverse reactions with regard to the use of tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
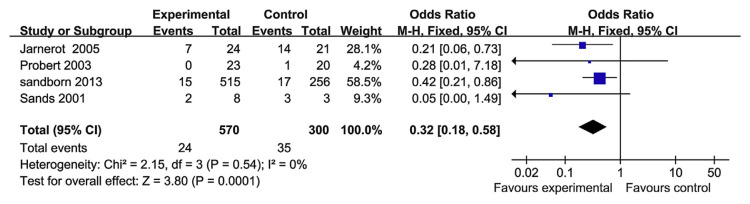
3.7. Colectomy
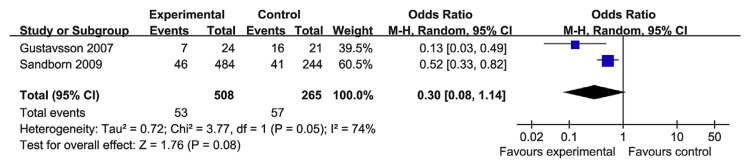
Colectomy was reported in four RCT papers. The heterogeneity test indicated that χ2 = 2.15 and p = 0.54, demonstrating homogeneity. Therefore a fixed-effects model was adopted, and the OR value was 0.32 (95% CI, 0.18–0.58; p = 0.0001; Fig. 9). Therefore, TNF-α blockers can reduce colectomy rates when compared with the control group, but the risk of colectomy, in the long term, is not modified. Moreover, there were only two studies that evaluated the long-term colectomy rate [22,23]; here, the heterogeneity test indicated χ2 = 3.77 and p = 0.05, demonstrating heterogeneity (Fig. 10).
Fig. 9.
The forest plot of colectomy rate following the use of tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
Fig. 10.
The forest plot of long-term colectomy rate following the use of tumor necrosis factor-alpha blockers for the treatment of ulcerative colitis.
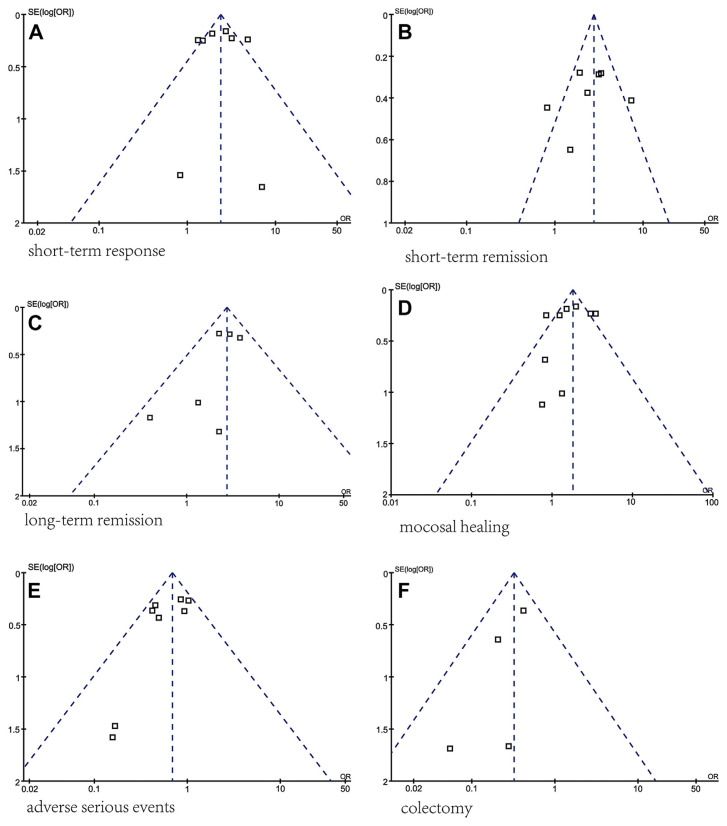
3.8. Publication bias
A funnel plot was used to assess the presence of publication and other reporting biases by plotting the standard error against the log OR (Fig. 11). The shape of the funnel plot was symmetrical, indicating no significant publication bias. All analyses were performed using the software Review Manager 5.0 (Cochrane Collaboration, Oxford, UK).
Fig. 11.
Assessment of publication bias using a funnel plot. Funnel plot analysis of the (A) short-term response, (B) short-term remission, (C) long-term remission, (D) mucosal healing, (E) serious adverse events, and (F) colectomy between TNF-α blocker groups and control groups.
In the TNF-α blocker and control groups, all funnel plots of short-term response, short-term remission, long-term remission, mucosal healing, severe adverse reactions, and colectomy rate were roughly symmetrical, suggesting the absence of publication bias. But in the analysis of each parameter, only a small number of studies were included, so publication bias was of concern.
4. Discussion
UC is a chronic relapsing inflammatory disorder of the large bowel, and the cause of the disorder is not known. The condition is thought to arise from dysregulation of both the innate and adaptive immune systems, leading to an abnormal inflammatory response to commensal bacteria in a genetically susceptible individual [24]. Cytokines, including proinflammatory cytokines and anti-inflammatory cytokines, play an important role in regulating intestinal immunity [25]. The cytokine profiles of CD and UC are usually different. CD is associated with an overexpression of Th1-related proinflammatory cytokines, whereas the latter is associated with an increased production of Th2-related inflammatory molecules [26]. However, increased serum and colonic mucosa concentrations of TNF-α have also been reported in patients with UC, suggesting a possible role in the pathogenesis of the disease [27,28]. Until recently, the management of UC consisted of the stepwise use of mesalazine, corticosteroids, and immunomodulators, or consideration of surgery [29,30]. In the past decade, anti-TNF-α agents made an important contribution to the management of CD, especially in patients who were refractory to conventional therapies. The role of TNF-α blocking agents in UC, however, is unclear, and recent studies have yielded conflicting results.
There are several differences in the therapeutic mechanism of various TNF-α blockers. Infliximab, an immunoglobulin (Ig) G1 chimeric monoclonal antibody, binds with high affinity to free and membrane-bound TNF-α, neutralizing its biological activity [29]. In 2010, infliximab was the only biological agent approved for the treatment of UC in the United States and Europe [31]. Adalimumab is a fully human IgG1 monoclonal antibody directed against TNF-α that inhibits the activity of this cytokine by blocking the interaction of TNF-α with its p55 and p75 cell surface receptors. Adalimumab is approved in the United States, Europe, and Japan for CD [32]. Recently, two RCTs have demonstrated the ability of adalimumab to induce clinical remission in patients with moderate-to-severe UC, and found that adalimumab might be an effective therapy for UC. Golimumab represents another relatively new human monoclonal anti-TNF IgG1 antibody; it is a fully human monoclonal antibody to TNF-α and is subcutaneously administered [33]. This drug has only been tested in active UC. Certolizumab pegol is a more recently developed TNF-α antagonist with a unique physical structure that may provide an alternative tolerability profile to other TNF-α antagonists, and currently approved in the US as a treatment for CD [34]. To date, no RCT has compared the efficacy/safety of certolizumab with active treatments for UC. There may be clinically important differences among anti-TNF-α agents. Immunogenicity is directly related to the structure of protein therapeutics and may result in increased adverse effects and diminished efficacy. Thus, adalimumab, golimumab, and certolizumab may be safer and more effective than infliximab because they have weaker immunogenicity than infliximab.
The authors conducted a systematic review and meta-analysis of the available published RCTs to examine the efficacy and safety of the TNF-α blockers in the treatment of UC. A total of nine RCTs met the criteria and were included in the meta-analysis, and the quality of many of these studies was very high (Jadad score ≥ 3). Six of the included studies compared infliximab with controls (4 compared infliximab with placebo and 2 compared infliximab with corticosteroids), two studies compared adalimumab with placebo, and one study compared golimumab with placebo. For the short-term response and mucosal healing, we found that TNF-α blockers were superior to controls, but heterogeneity across studies was found. Exploring the sources of heterogeneity using subgroup analysis, we found that the various TNF-α blockers influenced the heterogeneity. Therefore, we analyzed the effects in the subgroups. Heterogeneity was not found in infliximab, adalimumab, and glimumab. The results suggested that infliximab, adalimumab, and golimumab were superior to placebo in achieving short-term response. For the mucosal healing, however, adalimumab was not more effective than placebo. For short-term remission (2–8 weeks), the heterogeneity was significantly different between the TNF-α blocker groups. The reason for this heterogeneity was that different short-term remission measurements were used. But in the overall analysis, TNF-α blockers performed significantly better than the control group in terms of short-term remission rate.
In this meta-analysis, long-term remission, serious adverse reactions, and colectomy rate were also considered. Overall, the TNF-α blockers did not increase the risk of death, serious infections, or malignancy. And for long-term remission and colectomy rate, the TNF-α blockers were superior to controls, and heterogeneity across studies had not been found. However, whether TNF-α blockers can prevent colectomy in the long term remains to be elucidated and will require further long-term prospective studies.
In contrast to previous meta-analyses examining the efficacy and safety TNF-α blockers for UC, the current analysis included the findings from a recently published RCT reported by Sandborn and colleagues [11], who first introduced golimumab for the treatment of UC [12].
All papers selected for this study were high-quality RCTs (Jadad score ≥ 3 points) and fulfilled the inclusion criteria. However, our meta-analysis has several limitations. First, there was only one RCT that compared the golimumab with placebo. A second limitation is that all studies included in this meta-analysis were written in English. Hence, it is possible that we may have missed potentially relevant trials from non-English language literature. Third, these data need to be interpreted with caution because the patients in these clinical trials might not be representative of patients seen in clinical practice, and follow-up might not be sufficiently long for some serious events such as malignancy (if they occurred). Fourth, we did not perform metaregression to assess the factors that resulted in heterogeneity; we specifically performed subgroup analyses on patients assigned to various TNF-α blocker groups.
5. Conclusion
In the past decade, infliximab has made an important contribution to the management of UC, and it is the only biological agent approved to treat patients with UC, but as time goes by, more and more studies have found that numerous patients have shown diminished or loss of response to infliximab, owing to the development of antibodies directed against the drug. Thus, new biological agents should be developed and used for the treatment of UC. In our study, adalimumab and golimumab have also been found to be effective in UC, because they are capable of inducing response, remission, and even mucosal healing.
In conclusion, our study suggested that TNF-α blockers were superior to controls in achieving short-term clinical response/remission, long-term remission, and mucosal healing, and decreased the colectomy rate and serious adverse reactions. Although TNF-α blockers were largely safe, it is important to understand that serious adverse events were probably associated with anti-TNF-α therapies. Because anti-TNF-α therapies are nonselective, a new technology can be exploited for selective detection as well as noninvasive therapy of UC. Nanomedicine is one of the most rapidly developing fields in the 21st century [35]. It has the ability for highly selective accumulation in the diseased tissue and is capable of delivering an effective therapeutic action selectively. Thus, we believe nanomedicine combined with anti-TNF-α therapies will lead breakthroughs for the treatment of UC.
Owing to the limitations of this meta-analysis, more prospective randomized trials are needed to confirm the results.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–8. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanauer SB. Medical therapy for ulcerative colitis 2004. Gastroenterology. 2004;126:1582–92. doi: 10.1053/j.gastro.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 3. Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–9. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 5. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 6. Hommes DW, van de Heisteeg BH, van der Spek M, Bartelsman JF, van Deventer SJ. Infliximab treatment for Crohn's disease: one-year experience in a Dutch academic hospital. Inflamm Bowel Dis. 2002;8:81–6. doi: 10.1097/00054725-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 7. Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, Targan SR, Podolsky DK. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83–8. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 8. Probert CS, Hearing SD, Schreiber S, Kuhbacher T, Ghosh S, Arnott ID, Forbes A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52:998–1002. doi: 10.1136/gut.52.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Databa se Syst Rev. 2006;19:1–30. doi: 10.1002/14651858.CD005112.pub2. [DOI] [PubMed] [Google Scholar]
- 10. Huang X, Lv B, Jin HF, Zhang S. A meta-analysis of the therapeutic effects of tumor necrosis factor-alpha blockers on ulcerative colitis. Eur J Clin Pharmacol. 2011;67:759–66. doi: 10.1007/s00228-011-1079-3. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W, Gibson PR, Collins J, Jarnerot G, Hibi T, Rutgeerts P. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2013;146:85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B, Kampman W, Lazar A, Thakkar R. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–7. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 17. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 18. Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, Vilien M, Strom M, Danielsson A, Verbaan H, Hellstrom PM, Magnuson A, Curman B. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–11. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19. Ochsenkuhn T, Sackmann M, Goke B. Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot study. Eur J Gastroenterol Hepatol. 2004;16:1167–71. doi: 10.1097/00042737-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 20. Armuzzi A, De Pascalis B, Lupascu A, Fedeli P, Leo D, Mentella MC, Vincenti F, Melina D, Gasbarrini G, Pola P, Gasbarrini A. Infliximab in the treatment of steroid-dependent ulcerative colitis. Eur Rev Med Pharmacol Sci. 2004;8:231–3. [PubMed] [Google Scholar]
- 21. Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A, Lomax KG, Pollack PF, D'Haens G. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–11. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, Lu J, Horgan K, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–60. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 23. Gustavsson A, Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, Vilien M, Strom M, Verbaan H, Hellstrom PM, Magnuson A, Halfvarson J, Tysk C. Clinical trial: colectomy after rescue therapy in ulcerative colitis — 3-year follow-up of the Swedish–Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32:984–9. doi: 10.1111/j.1365-2036.2010.04435.x. [DOI] [PubMed] [Google Scholar]
- 24. Ardizzone S, Bianchi Porro G. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475–96. doi: 10.1046/j.1365-2796.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 25. Cominelli F. Cytokine-based therapies for Crohn's disease—new paradigms. N Engl J Med. 2004;351:2045–8. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 26. D'Haens G. Infliximab for ulcerative colitis: finally some answers. Gastroenterology. 2005;128:2161–4. doi: 10.1053/j.gastro.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 27. Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913–7. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–53. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 30. Travis SP, Stange EF, Lemann M, et al. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohns Colitis. 2008;2:24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 31. Peters BJ, Capelle MA, Arvinte T, et al. Validation of an automated method for compounding monoclonal antibody patient doses: case studies of Avastin (bevacizumab), Remicade (infliximab) and Herceptin (trastuzumab) mAbs. 2013;5:162–70. doi: 10.4161/mabs.22873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burmester GR, Mease P, Dijkmans BA, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68:1863–9. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shealy DJ, Cai A, Staquet K, Baker A, Lacy ER, Johns L, Vafa O, Gunn G, Tam S, Sague S, Wang D, Brigham-Burke M, Dalmonte P, Emmell E, Pikounis B, Bugelski PJ, Zhou H, Scallon BJ, Giles-Komar JM. Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor alpha. mAbs. 2010;2:4. doi: 10.4161/mabs.2.4.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrante M, Vermeire S, Rutgeerts P. Certolizumab pegol in the treatment of Crohn's disease. Expert Opin Biol Ther. 2013;13:595–605. doi: 10.1517/14712598.2013.777039. [DOI] [PubMed] [Google Scholar]
- 35. Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3–17. doi: 10.1016/j.jfda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]