Abstract
Polygala tenuifolia root is used as a functional food due to its attractive health benefits. In this study, ultrahigh-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) and gas chromatography–mass spectrometry (GC-MS) were utilized to characterize the bioactive compounds in P. tenuifolia root. The UPLC-MS/MS information revealed 36 bioactive compounds, including oligosaccharide esters, polygalasaponins, and polygalaxanthones. GC-MS identified 34 volatile compounds with fatty acids as the main chemicals. The leading compound judged by UPLC-MS/MS was tenuifoliside A, and oleic acid was the leading volatile from GC-MS profiles. All samples tested showed similar bioactive compound compositions, but the level of each compound varied. Principal component analysis revealed the principal bioactive compounds with significant level variations between samples. These principal chemicals could be used for quality judgment of P. tenuifolia root, instead of measuring the levels of all compositional compounds.
Keywords: bioactive compound, gas chromatography-mass spectrometry, Polygala tenuifolia, ultrahigh-performance liquid, chromatography-tandem mass spectrometry
1. Introduction
The genus Polygala includes herbaceous plants, shrubs, and small trees, which are distributed over the world. Some Polygala species are used in functional foods and folk medicines against inflammation and disorders of the central nervous system [1]. As a widely used medicinal plant, Polygala tenuifolia has attracted much attention due to its good pharmaceutical properties against insomnia, neurosis, and dementia [2]. Moreover, P. tenuifolia root can attenuate cognitive dysfunction and relieve depression [3,4]. As cognitive dysfunction and depression are common disorders, the preventative effect of P. tenuifolia root has attracted much interest by scientists in the functional food field. Many functional food products formulated with P. tenuifolia root have been developed.
Natural bioactive compounds, such as phenolics and carbohydrates, are responsible for the pharmaceutical activities of medicinal herbs [5,6]. It has been reported that oligosaccharides, saponins, and xanthones in P. tenuifolia root are critical chemicals involved in disease treatment and health benefits [7]. The antidepressant effect is mainly attributed to the occurrence of oligosaccharide esters, such as 3,6′-disinapolysucrose. Polygalasaponins possess good capability against cognitive impairments [8]. Xanthones have diverse biological profiles, including anticancer, antihypertension, and antioxidation [9]. It is obvious that the bioactive compound composition determines the quality of P. tenuifolia root. More than 200 chemicals have been identified from the Polygala genus. However, the chemical composition of P. tenuifolia root is still not clear. Determination of phytochemical profile by ultrahigh-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) and gas chromatography–mass spectrometry (GC-MS) is a good choice to understand the chemistry nature of medicinal plant.
Metabolomics is being increasingly utilized to gain insight into the chemical composition of biological materials [10]. Two types of analytical techniques, based on MS and nuclear magnetic resonance spectroscopy (NMR), are widely applied in metabolomics. The MS technique shows better precision and resolution than NMR. Therefore, in this work, the bioactive compounds profile of P. tenuifolia root was analyzed using both UPLC-MS/MS and GC-MS. It is useful to judge the quality of commercial P. tenuifolia root.
2. Materials and methods
2.1. Plant material
Four P. tenuifolia roots were collected from Sanmenxia in Henan province (Roots 1 and 2) and Yuncheng in Shanxi province (Roots 3 and 4). Each sample was pulverized into powder and screened through a 60-mesh sieve.
2.2. UPLC-MS/MS analysis
Two grams of P. tenuifolia rootpowderwereextractedwith20mL of methanol for 1 week in the dark at room temperature. The extract was centrifuged at 8000 × g for 10 minutes. Each sample was extracted in triplicate. The supernatant was collected and subjected to UPLC-MS/MS analysis. Acquity UPLC equipped with a BEH C18 column (1.7 μm, 2.1 mm × 150 mm; Waters, Milford, MA, USA) was used for chemicals isolation. A gradient elution program was conducted as follows: 0–45 minutes, from 5% to 50% solvent B; 45–48 minutes, from 50% to 100% solvent B; 48–51 minutes, and from 100% to 5% solvent B; 51–53 minutes, 5% solvent B. The flow rate was 0.25 mL/min. Solvent A was 0.1% formic acid in water, and solvent B was acetonitrile. Detection was performed on a triple quadrupole mass spectrometer using an electrospray ionization (ESI) interface. Ionization of the analytes was achieved using ESI in negative mode. The interface conditions were as follows: capillary voltage, 1.0 kV; cone voltage, 10 eV; collision voltage, 10 eV; source temperature, 150°C; desolvation temperature, 500°C; cone gas, nitrogen at a flow rate of 50 L/h; desolvation gas, nitrogen at a flow rate of 800 L/h; collision gas, argon at a flow rate of 0.2 mL/min. Mass scan was set in the range of m/z 50–2000. The daughter ion mode was monitored at a collision voltage of 10–40 eV.
2.3. GC-MS analysis
Two grams of P. tenuifolia root powder were extracted with 20 mL of hexane/acetone (7:3, v/v) for 1 week in the dark at room temperature. The extracts were centrifuged at 8000 × g for 15 minutes. The supernatants were collected and subjected to trimethylsilyl derivatization due to the occurrence of fatty acids following the method of Yang et al [11]. A gas chromatography/mass spectrometer (GCMS-QP 2010; Shimadzu, Kyoto, Japan) was used to analyze the chemical composition. The derivatives were loaded into a RTX-5 capillary column. The temperature program was set as follows: the initial temperature of column was 50°C, holding for 1 minute, increasing to 250°C at 3°C/min, holding for 17 minutes, and increasing to 280°C at 10°C/min; injection temperature: 250°C. The ion source of the mass spectrometer was set at 250°C. The scanning m/z range was 20–550 atomic mass units. A 1-μl aliquot of sample was injected and the split ratio was 10:1. The carrier gas was helium with a flow rate of 1.0 mL/min. The peaks were identified using the NIST database and retention index.
2.4. Data analysis
For each sample, the peak heights of metabolites in mass spectra were recorded and averaged over three replicates. An unsupervised multivariate statistical method, principal component analysis (PCA), was used on UV-scaling data by software SIMCA-P version 11.5 (Umetrics, Umeå, Sweden). PCA was performed to visualize the clustering of different samples without any knowledge of their group membership.
3. Results
3.1. Semipolar metabolites composition identified by UPLC-MS/MS
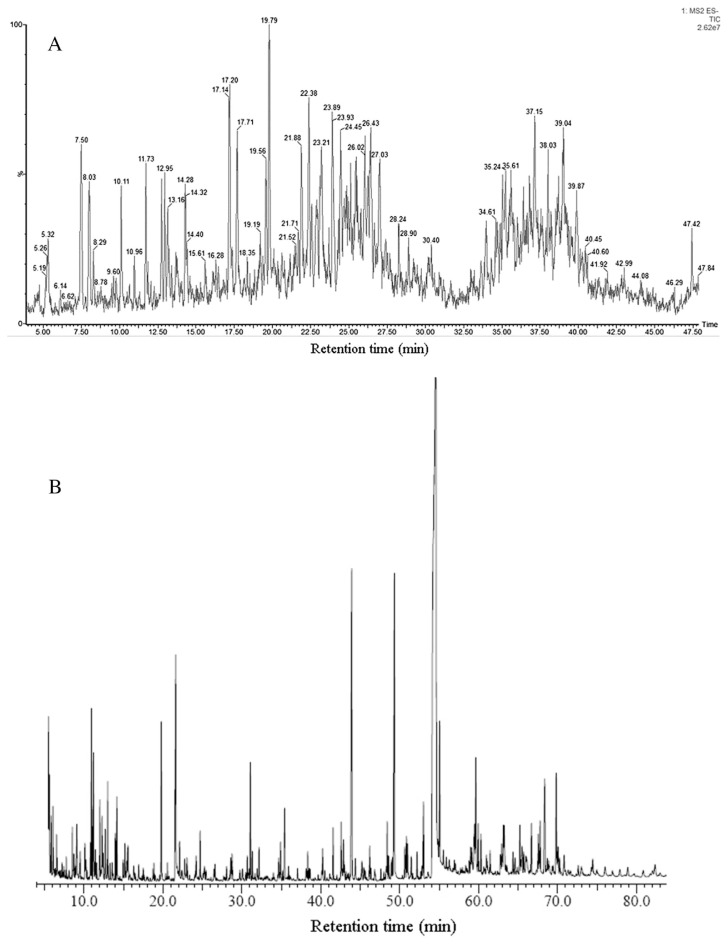
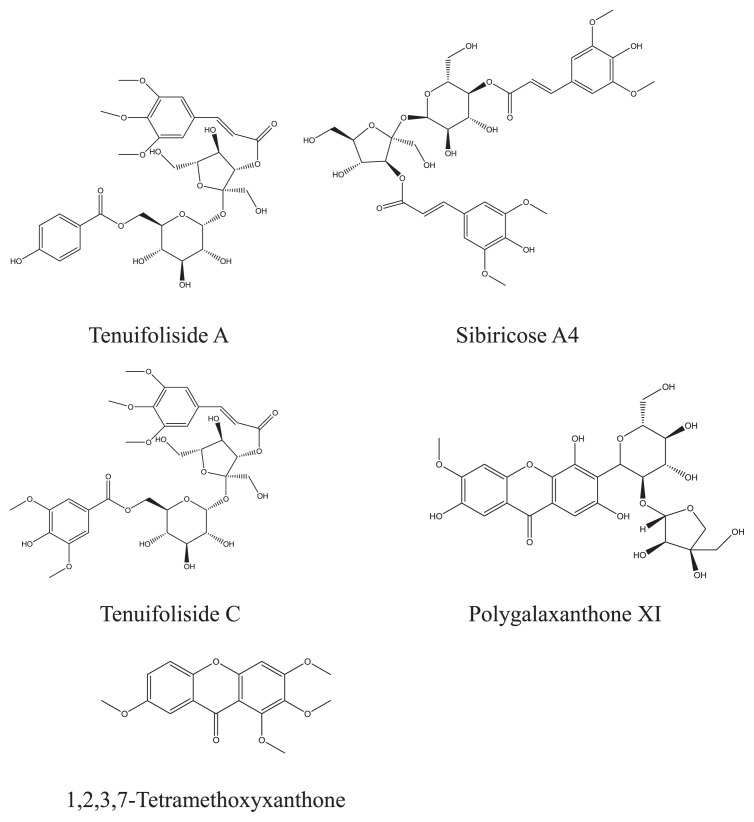
The methanolic extract of P. tenuifolia root was analyzed using UPLC-MS/MS to identify the semipolar metabolites. Fig. 1 shows the full scan ESI-MS spectra in negative mode. The identified chemicals of main peaks are listed in Table 1. Those metabolites with relatively high peaks were interpreted here. The leading peak was tenuifoliside A (retention time, 19.79 minutes; Fig. 2). The MS spectra had an [M−H] at m/z 680.7 and [2M−H] at m/z 1362.7. The fragment ions were interpreted as follows: m/z 442.6 (loss of trimethoxycinnamoyl and hydroxyl group), 281.0 (loss of trimethoxycinnamoyl and fructose), 238.9 (breaking of glucose), and 136.7 (p-hydroxy benzoic acid). Peak at 17.16 minutes gave [M−H] and [2M−H] at m/z 752.8 and m/z 1506.9, respectively, which was identified as sibiricose A4. Its fragment ion at m/z 223.1 indicated synapic acid, and m/z 546.7 was due to the loss of a synapoyl group. Peak at 22.36 minutes gave [M−H] and [2M−H] at m/z 766.8 and m/z 1535.8, respectively. The fragment ions at m/z 528.8 (loss of sinapoyl and hydroxyl), m/z 237.3 (trimethoxy cinnamic acid), and m/z 222.6 (synapic acid) further confirmed this peak as tenuifoliside C. A peak at 12.96 minutes was polygalaxanthone XI (MW 568), a xanthone C-glycoside, which gave an [M−H] and [2M−H] at m/z 567.0 and m/z 1134.5, respectively. The fragment ions at m/z 435.1 (loss of apiose), m/z 416.9 (loss of apiose and H2O), and m/z 344.8 (breaking of glucose at 0,3 bonds) further confirmed this structure. 1,2,3,7-Tetramethoxyxanthone (MW 316) was detected at 47.40 minutes with [M−H] at m/z 314.9, and fragment ions at m/z 214.4 (loss of C5H9O2) and m/z 116.8 (loss of C10H14O4). The chemical structures of the above compounds are shown in Fig. 2. Some other chemicals, including polygalasaponins, xanthones, and sucrose esters, were also identified, as has been described previously [12–15].
Fig. 1.
Ultrahigh-performance liquid chromatography–tandem mass spectrometry and gas chromatography–mass spectrometry profiles of Polygala tenuifolia root. (A) Total ion chromatogram analyzed by ultrahigh-performance liquid chromatography–tandem mass spectrometry; (B) total ion chromatogram analyzed by gas chromatography–mass spectrometry.
Table 1.
Semipolar metabolites found in the methanolic extract of Polygala tenuifolia root measured by ultrahigh-performance liquid chromatography–tandem mass spectrometry.
| No. | Retention time (min) | MW | [M−H]− (m/z) | [2M−H]− (m/z) | Fragment ions (m/z) | Chemicals |
|---|---|---|---|---|---|---|
| 1 | 5.29 | 462 | 460.9 | 922.9 | 299.1, 136.9 | Sibiricose A3 |
| 2 | 7.5 | 518 | 516.7 | 1034.9 | 340.6, 192.8 | Arillalose B |
| 3 | 7.96 | 548 | 546.8 | 1094.8 | 222.6, 204.9 | Sibiricose A6/A1 |
| 4 | 10.11 | 548 | 546.9 | 1094.3 | 222.8, 204.8 | Sibiricose A6/A1 |
| 5 | 10.96 | 568 | 566.8 | 435.2, 314.5 | Polygalaxanthone III or VIII | |
| 6 | 11.73 | 538 | 536.7 | 386.7, 266.7 | Sibiricaxanthone B | |
| 7 | 12.75 | 568 | 566.5 | 434.8, 314.9 | Polygalaxanthone III or VIII | |
| 8 | 12.96 | 568 | 567.0 | 1134.5 | 435.1, 416.9, 344.8 | Polygalaxanthone XI |
| 9 | 13.16 | 562 | 560.7 | 1123.2 | 399.0, 341.0, 236.8 | Sibiricose A2 |
| 10 | 13.69 | 256 | 255.0 | 511.0 | 212.9, 168.5 | 1-Hydroxy-2,3-methylenedioxyxanthone |
| 11 | 13.77 | 550 | 548.8 | 467.3, 254.9 | — | |
| 12 | 14.3 | 668 | 666.7 | 1334.9 | 461.1, 280.8, 239.1 | Tenuifoliside B |
| 13 | 14.4 | 754 | 752.7 | 1506.7 | 705.2, 528.5, 205.0 | — |
| 14 | 16.3 | 582 | 580.9 | 549.0, 323.0, 272.7 | Polygalaxanthone V | |
| 15 | 16.45 | 754 | 752.9 | 1507.1 | 705.0, 692.5, 630.8 | Telephiose E |
| 16 | 17.16 | 754 | 752.8 | 1506.9 | 546.7, 223.1 | Sibiricose A4 |
| 17 | 17.71 | 724 | 722.7 | 1447.3 | 696.5, 546.8, 516.6 | Arillanin A |
| 18 | 19.21 | 652 | 650.7 | 528.8, 222.5, 204.5 | Acylsucroses 3′-O-(4-hydroxy-3,5-dimethoxy-E-cinnamoyl), 6-O-benzoyl | |
| 19 | 19.56 | 1484 | 1482.8 | Tenuifoliose Ga | ||
| 20 | 19.79 | 682 | 680.7 | 1362.7 | 442.6, 281.0, 238.9, 136.7 | Tenuifoliside A |
| 21 | 21.85 | 1526 | 1525.4 | 688.7 | Tenuifoliose Fa | |
| 22 | 21.9 | 1496 | 1494.9 | Tenuifoliose La | ||
| 23 | 22.36 | 768 | 766.8 | 1535.8 | 528.8, 237.3, 222.6 | Tenuifoliside C |
| 24 | 22.87 | 1660 | 1659.1 | Polygalasaponin XLVa | ||
| 25 | 23.12 | 1296 | 1294.8 | Tenuifoliose Ca | ||
| 26 | 23.19 | 738 | 736.9 | 1477.5 | 704.8 | Reiniose Aa |
| 27 | 23.94 | 1308 | 1306.8 | 1160.8, 163.3, 145.0 | Tenuifoliose I | |
| 28 | 24.45 | 1338 | 1336.9 | 894.5, 320.6 | Senegose L | |
| 29 | 24.69 | 1296 | 1294.9 | 1118.5, 881.4 | Senegose K | |
| 30 | 25.48 | 1308 | 1306.8 | 964.7 | Reinioside Ba | |
| 31 | 26.07 | 1338 | 1336.8 | Tenuifoliose Ba | ||
| 32 | 26.38 | 1350 | 1348.9 | 1308.4, 1250.1 | Polygalasaponin XXXV | |
| 33 | 26.47 | 1526 | 1525.2 | Tenuifoliose Fa | ||
| 34 | 26.99 | 1380 | 1379.0 | 1336.6, 390.8 | Tenuifoliose A | |
| 35 | 39.91 | 1732 | 1731.2 | 1587.8, 425.4 | Onjisaponin W | |
| 36 | 47.40 | 316 | 314.9 | 214.4, 116.8 | 1,2,3,7-Tetramethoxyxanthone |
— = unknown;
= possible structure; MW = molecular weight.
Fig. 2.
Structures of characteristic metabolites in Polygala tenuifolia root.
3.2. Volatile metabolites composition identified by GC-MS
The volatile metabolites were extracted by hexane/acetone, trimethylsilylated, and determined by GC-MS, which gave 34 volatile compounds, including alcohol, carboxylic acids, esters, and terpenoids (Table 2). The leading volatile chemical was oleic acid in all samples tested. Other volatile compounds with percentage higher than 1% were 2,5-dihydroxy-3,6-bis(hydroxymethyl)-1,4-dioxane, hexadecanoic acid, 9-octadecenoic acid methyl ester, 9,12-octadecadienoic acid, stearic acid, 11-eicosenoic acid, eicosanoic acid, and eicosanoic acid 2-glycerol ester.
Table 2.
Volatile metabolites occurred in hexane/ acetone extract of Polygala tenuifolia root determined by gas chromatography–mass spectrometry.
| No. | Retention time (min) | Chemicals |
|---|---|---|
| 1 | 5.86 | isobutonol |
| 2 | 6.55 | 2-ethoxyethyl acetate |
| 3 | 9.10 | 2,2′,5,5′-tetrahydro-2,2′-bifuran |
| 4 | 10.95 | octyl-3-ol |
| 5 | 11.44 | phenol |
| 6 | 12.02 | propanedioic acid |
| 7 | 12.29 | 4-oxo-pentanoic acid |
| 8 | 12.71 | o-cymene |
| 9 | 13.00 | benzenamine |
| 10 | 13.98 | 5-ethyl-m-xylene |
| 11 | 14.16 | 1,2,3,4-tetramethyl-benzene |
| 12 | 15.19 | 2,3-dihydroxyl-propanoic acid |
| 13 | 22.11 | 2-benzyl-1,3-dioxolane |
| 14 | 24.72 | decanoic acid |
| 15 | 31.08 | 2-hydroxyl benzoic acid |
| 16 | 34.89 | 2,3-dimethyl-3-hydroxyglutaric acid |
| 17 | 38.35 | 9H-carbazole |
| 18 | 41.56 | undecanedioic acid |
| 19 | 42.58 | cinoxate |
| 20 | 43.89 | 2,5-dihydroxy-3,6-bis(hydroxymethyl)-1,4-dioxane |
| 21 | 48.41 | palmitelaidic acid |
| 22 | 49.29 | hexadecanoic acid |
| 23 | 50.87 | 9-octadecenoic acid, methyl ester |
| 24 | 54.34 | 9,12-octadecadienoic acid |
| 25 | 54.52 | oleic acid |
| 26 | 55.06 | stearic acid |
| 27 | 59.62 | 11-eicosenoic acid |
| 28 | 60.29 | eicosanoic acid |
| 29 | 63.12 | isooctyl phthalate |
| 30 | 65.22 | tetracosanoic acid |
| 31 | 66.68 | thymol-glucoside |
| 32 | 68.38 | eicosanoic acid,2-glycerol ester |
| 33 | 69.82 | squalene |
| 34 | 89.42 | 22,23-dibromostigmasterol acetate |
3.3. PCA of P. tenuifolia root based on their metabolites profiles
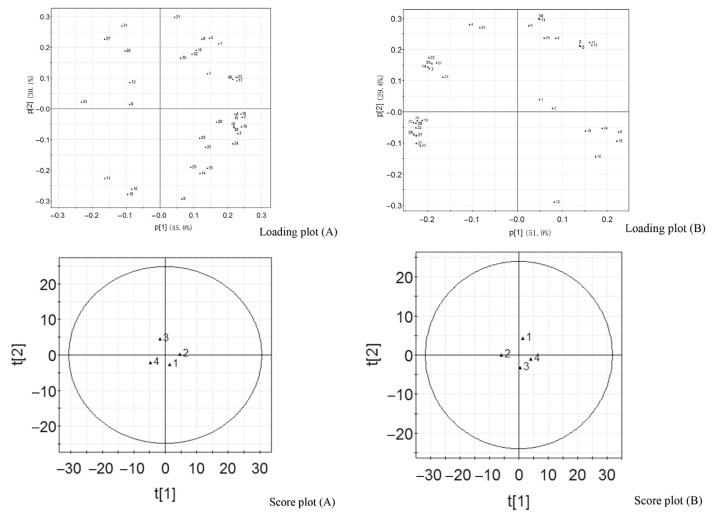
PCA was conducted on methanolic extract of P. tenuifolia root samples. The first two principal components accounted for 75.9% of the total variance, in which the first principal component accounted for 45.8% of the total variance and the second principal component 30.1%. The loading plot (Fig. 3A) revealed the main compounds responsible for the sample difference. The first component was represented by arillalose B, sibiricoses A1, A6, tenuifolisides B, C, arillanin A, 6-O-benzoyl 3′-O-(4-hydroxy-3,5-dimethoxy-E-cinnamoyl) acylsucroses, tenuifolioses A, E, L, polygalasaponin XLV, and reinioside B, which are characterized as saponins and oligosaccharide esters. The second component was mainly defined by 1-hydroxy-2,3-methylenedioxyxanthone, sibiricose A2, tenuifolioses B, F, and G, which were characterized as xanthone and oligosaccharide esters. Score plot showed the statistical similarity between samples. P. tenuifolia Root 1 was characterized by large amounts of arillalose B, sibiricose A2, and telephiose E, while Root 3 showed high levels of tenuifoliose B and I. They were located in two opposite quadrants in the score plot, which indicated significant differences. Root 2 was located in the proximity of Root 1 in the score plot.
Fig. 3.
Plots of PCA on semipolar and volatile metabolites of Polygala tenuifolia root. (A) Loading plot and score plot are the results of semipolar metabolites; (B) loading plot and score plot are the results of volatile metabolites.
Fig. 3B shows the results of PCA on volatile metabolites levels. The first two principal components accounted for 81.5% of the total variance, 51.9% by the first component and 29.6% by the second component. Major metabolites defining the first component were propanedioic acid, 2-hydroxyl benzoic acid, hexadecanoic acid, palmitelaidic acid, 9-octadecenoic acid methyl ester, stearic acid, 11-eicosenoic acid, eicosanoic acid, tetracosanoic acid, and eicosanoic acid 2-glycerol ester. The second component was mainly characterized by 5-ethyl-m-xylene, 1,2,3,4-tetramethyl-benzene, and 2,3-dihydroxyl-propanoic acid. P. tenuifolia Root 1 was characterized by high levels of 5-ethyl-m-xylene and 1,2,3,4-tetramethyl-benzene. Root 3 was characterized by high level of 2,3-dihydroxyl-propanoic acid.
4. Discussion
4.1. Phytochemical profile of P. tenuifolia root
Phytochemical analysis is a good way to evaluate the quality of commercial biological resources, such as medicinal herbs and fruits. At present, LC-MS and GC-MS are two efficient and precise techniques to resolve the phytochemical profile of plant resources [16,17]. The former technique can identify semipolar metabolites with advantages of high precision and short time consumed. It is widely applied to the characterization of plant secondary metabolites. GC-MS can reveal the chemistry nature of volatile metabolites [18], which define the flavor of the plant [19]. GC-MS also provides complementary information to LC-MS analysis to obtain a complete phytochemical profile. From the bioactive compounds profile obtained in this work, it can be concluded that four samples had similar metabolites profiles, but the level of each metabolite varied.
More than 70 metabolites were identified from P. tenuifolia root in this study. Three sucrose esters, including sibiricose A4 and tenuifoliside A and C, were detected as the leading metabolites in the UPLC-MS/MS profile. They have been reported as the bioactive compounds responsible for the pharmaceutical effect of P. tenuifolia root against depression [20]. Several saponins and xanthones were also detected as important constituents, including 1,2,3,7-tetramethoxyxanthone, 1-hydroxyl-2,3-methylenedioxyxanthone, and polygalaxanthones III, V, and VIII, as well as polygalasaponins XLV and XXXV. Polygalasaponins play a critical role in the characteristic bioactivities of P. tenuifolia root, such as neuroprotection and memory improvement. Cognitive dysfunction is a symptom of central nervous system disorder, which endangers the life quality of people. Xue et al [8] pointed out that polygalasaponins can significantly prevent scopolamine-induced cognitive impairments in mice. Even though the numbers and levels of saponins and xanthones in the UPLC-MS profile were less than oligosaccharide esters (Table 1 and Fig. 1), they work as prerequisites to the health-beneficial effects of P. tenuifolia root. GC-MS analysis showed that the major volatile compounds were fatty acids, consistent with the determinations of Wang et al [21]. Among these, oleic acid was the leading volatile metabolite. It is also a pharmacologically active chemical. Although the contribution of volatile compounds to the pharmaceutical activities of P. tenuifolia root is much less than nonvolatile compounds, they influence the flavor characteristics to some extent.
Based on the level and importance to the pharmacological functions, the oligosaccharide esters, polygalasaponins and xanthones identified in the UPLC-MS profile could be selected to evaluate the quality of P. tenuifolia root. A P. tenuifolia root with relatively high levels of bioactive compounds is considered as having good quality. Through analysis of variance of representative metabolites levels (data not shown), the results indicate that four P. tenuifolia roots had no significant differences in the levels of tenuifoliside A, tenuifoliose A, and onjisaponin W. For sibiricose A4, Root 2 sample showed a significantly (p < 0.05) higher level than Root 1, but no significant (p > 0.05) differences between Roots 2, 3, and 4 were observed. The results showed that Root 1 had a worse quality compared with the other three samples.
4.2. Multivariate statistical analysis of P. tenuifolia root metabolites
PCA is an unsupervised clustering technique for identifying patterns in data, and expressing the data in a way to emphasize their similarities and differences. Through reducing the number of dimensions, it can define a limited number of principal components that describe independent variation in the results [10,22]. In this study, PCA was carried out on semipolar and volatile metabolites of P. tenuifolia root to find principal variables defining the pharmacological quality. In the loading plot, it displays the influence of individual spectral peaks in each principal component and describes the separation shown in the score plot [23]. The points in the loading plot far away from zero point represent characteristic markers with most confidence to each group. Fig. 3A shows the characteristic compounds for each sample. The first two principal components accounted for most of the total variance. The first principal components were labeled by tenuifolioses (A, E, and L) and polygalasaponin XLV. The second principal components were labeled by tenuifolioses (B, F, and G) and 1-hydroxy-2,3-methylene-dioxyxanthone. It was unexpected that tenuifoliside A had the leading peak in the UPLC-MS profile. However, it was not the representive chemical in the two first principal components due to the insignificant variance. Only those metabolites having significant variance will be calculated in the PCA model. Tenuifolioses are the characteristic oligosaccharide esters in P. tenuifolia root [24], which are constructed by pentasaccharide, phenolic acid, and acetyl moieties. It is very rare to find these oligosaccharides in other plant resources. They show good neuroprotective and antioxidant activities, which make P. tenuifolia root a good choice for traditional folk medicine. Seven xanthones were detected in the UPLC-MS profile, and only 1-hydroxy-2,3-methylenedioxyxanthone was included among the principal components. Xanthones show good potential for treatment against inflammation, asthma, and allergy [1]. They also exhibit bioactivity against neroblastoma [25]. Polygalaxanthones are a type of xanthone characterized by a C-glycoside moiety, which contribute partially to the bioactivity of P. tenuifolia root. Through PCA calculation, some oligosaccharide esters, xanthones, and saponins were selected as principal components. These metabolites can be chosen to determine the quality of commercial P. tenuifolia roots, instead of measuring the levels of all metabolites. This result also proved that UPLC-MS/MS and GC-MS were efficient for quality evaluation of medicinal plants [26].
5. Conclusion
Analysis of bioactive compounds profile was an efficient way to define the quality of P. tenuifolia root. By using UPLC-MS/MS and GC-MS, the semipolar and volatile metabolites were identified and their levels were statistically compared. Tenuifoliside A was the leading metabolite in P. tenuifolia root. Xanthones and saponins are two other important types of metabolite found in P. tenuifolia root. PCA plot indicated the representative metabolites for each sample, and these metabolites could be utilized to judge the commercial quality of P. tenuifolia root.
Acknowledgments
We are grateful for financial support from Guangdong Natural Science Foundation for Distinguished Young Scholars (No. S2013050014131), Youth Innovation Promotion Association of Chinese Academy of Sciences, Pearl River Science and Technology New Star Fund of Guangzhou (No. 2014J2200081), Guangdong Natural Science Foundation (No. S2011020001156), and International Foundation for Science (No. F/4451-2).
Funding Statement
We are grateful for financial support from Guangdong Natural Science Foundation for Distinguished Young Scholars (No. S2013050014131), Youth Innovation Promotion Association of Chinese Academy of Sciences, Pearl River Science and Technology New Star Fund of Guangzhou (No. 2014J2200081), Guangdong Natural Science Foundation (No. S2011020001156), and International Foundation for Science (No. F/4451-2).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. El Sayah M, Cechinel V, Pinheiro TR, Yunes RA, Calixto JB. In vitro effect of the extract and the 1,7-dihydroxy-2,3-dimethoxy xanthone from Polygala cyparissias on the contractions induced by inflammatory mediators and ovalbumin in normal and actively sensitised trachea from guinea pig. Inflamm Res. 1999;48:218–23. doi: 10.1007/s000110050449. [DOI] [PubMed] [Google Scholar]
- 2. Park HJ, Lee K, He H, Lee M, Kim JW, Whang WW, Kwon YK, Kwon H. Effects of Polygala tenuifolia root extract on proliferation of neural stem cells in the hippocampal CA1 region. Phytother Res. 2008;22:1324–9. doi: 10.1002/ptr.2488. [DOI] [PubMed] [Google Scholar]
- 3. Hu Y, Liao HB, Liu P, Guo DH, Rahman K. A bioactive compound from Polygala tenuifolia regulates efficiency of chronic stress on hypothalamic-pituitary-adrenal axis. Pharmazie. 2009;64:605–8. [PubMed] [Google Scholar]
- 4. Ikeya Y, Takeda S, Tunakawa M, Karakida H, Toda K, Yamaguchi T, Aburada M. Cognitive improving and cerebral protective effects of acylated oligosaccharides in Polygala tenuifolia. Biol Pharm Bull. 2004;27:1081–5. doi: 10.1248/bpb.27.1081. [DOI] [PubMed] [Google Scholar]
- 5. Yang B, Chen F, Hua YL, Huang SS, Lin S, Wen L, Jianga Y. Prooxidant activities of quercetin, p-courmaric acid and their derivatives analysed by quantitative structure-activity relationship. Food Chem. 2012;131:508–12. [Google Scholar]
- 6. Zhu QQ, Jiang YM, Lin S, Wen L, Wu D, Zhao M, Chen F, Jia Y, Yang B. Structural identification of (1→6)-α-D-glucan, a key responsible for the health benefits of longan, and evaluation of anticancer activity. Biomacromolecules. 2013;14:1999–2003. doi: 10.1021/bm400349y. [DOI] [PubMed] [Google Scholar]
- 7. Klein LC, de Andrade SF, Cechinel V. A pharmacognostic approach to the Polygala genus: phytochemical and pharmacological aspects. Chem Biodivers. 2012;9:181–209. doi: 10.1002/cbdv.201000319. [DOI] [PubMed] [Google Scholar]
- 8. Xue W, Hu JF, Yuan YH, Sun JD, Li BY, Zhang DM, Li CJ, Chen NH. Polygalasaponin XXXII from Polygala tenuifolia root improves hippocampal-dependent learning and memory. Acta Pharm Sinic. 2009;30:1211–9. doi: 10.1038/aps.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Na Y. Recent cancer drug development with xanthone structures. J Pharm Pharmacol. 2009;61:707–12. doi: 10.1211/jpp/61.06.0002. [DOI] [PubMed] [Google Scholar]
- 10. Bernillon S, Biais B, Deborde C, Maucourt M, Cabasson C, Gibon Y, Hansen TH, Husted S, de Vos RCH, Mumm R, Jonker H, Ward JL, Miller SJ, Baker JM, Burger J, Tadmor Y, Beale MH, Schjoerring JK, Schaffer AA, Rolin D, Hall RD, Moing A. Metabolomic and elemental profiling of melon fruit quality as affected by genotype and environment. Metabolomics. 2013;9:57–77. [Google Scholar]
- 11. Yang B, Jiang YM, Zhao MM, Chen F, Wang R, Chen Y, Zhang D. Structural characterisation of polysaccharides purified from longan (Dimocarpus longan Lour.) fruit pericarp. Food Chem. 2009;115:609–14. [Google Scholar]
- 12. Dao TT, Dang TT, Nguyen PH, Kim E, Thuong PT, Oh WK. Xanthones from Polygala karensium inhibit neuraminidases from influenza A viruses. Bioorg Med Chem Lett. 2012;22:3688–92. doi: 10.1016/j.bmcl.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 13. Fu J, Zuo L, Yang JZ, Chen R, Zhang D. Oligosaccharide polyester and triterpenoid saponins from the roots of Polygala japonica. Phytochemistry. 2008;69:1617–24. doi: 10.1016/j.phytochem.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 14. Zhang DM, Miyase T, Kuroyanagi M, Umehara K, Noguchi H. Polygalasaponins XLII–XLVI from roots of Polygala glomerata. Phytochemistry. 1998;47:459–66. doi: 10.1016/s0031-9422(97)00439-1. [DOI] [PubMed] [Google Scholar]
- 15. Ling Y, Li ZX, Chen MC, Sun Z, Fan M, Huang C. Analysis and detection of the chemical constituents of Radix polygalae and their metabolites in rats after oral administration by ultrahigh-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Pharmaceut Biomed. 2013;85:1–13. doi: 10.1016/j.jpba.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 16. Mari A, Lyon D, Fragner L, Montoro P, Piacente S, Wienkoop S, Egelhofer V, Weckwerth W. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics. 2013;9:599–607. doi: 10.1007/s11306-012-0473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen RC, Wei KJ, Wang TM, Yu YM, Li JY, Lee SH, Wang WH, Ren TJ, Tsai CW. Simultaneous quantification of antibiotic dyes in aquatic products and feeds by liquid chromatography-tandem mass spectrometry. J Food Drug Anal. 2013;21:339–46. [Google Scholar]
- 18. Chen HC, Peng LW, Sheu MJ, Lin LY, Chiang HM, Wu CT, Wu CS, Chen YC. Effects of hot water treatment effects on the essential oils of calamondin. J Food Drug Anal. 2013;21:363–8. [Google Scholar]
- 19. Besada C, Sanchez G, Salvador A, Granell A. Volatile compounds associated to the loss of astringency in persimmon fruit revealed by untargeted GC-MS analysis. Metabolomics. 2013;9:157–72. [Google Scholar]
- 20. Hu Y, Liao HB, Dai-Hong G, Liu P, Wang YY, Rahman K. Antidepressant-like effects of 3,6-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem Int. 2010;56:461–5. doi: 10.1016/j.neuint.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Chang L, Zhao X, Meng X, Liu Y. Gas chromatography–mass spectrometry analysis on compounds in volatile oils extracted from Yuan Zhi (Radix polygalae) and Shi Chang Pu (Acorus tatarinowii) by supercritical CO2. J Trad Chinese Med. 2012;32:459–64. doi: 10.1016/s0254-6272(13)60055-2. [DOI] [PubMed] [Google Scholar]
- 22. Park MK, Cho IH, Lee S, Choi HK, Kwon DY, Kim YS. Metabolite profiling of Cheonggukjang, a fermented soybean paste, during fermentation by gas chromatography-mass spectrometry and principal component analysis. Food Chem. 2010;122:1313–9. [Google Scholar]
- 23. Yuk J, McIntyre KL, Fischer C, Hicks J, Colson KL, Lui E, Brown D, Arnason JT. Distinguishing Ontario ginseng landraces and ginseng species using NMR-based metabolomics. Anal Bioanal Chem. 2013;405:4499–509. doi: 10.1007/s00216-012-6582-6. [DOI] [PubMed] [Google Scholar]
- 24. Miyase T, Iwata Y, Ueno A. Tenuifolioses A–F, oligosaccharide multi-esters from the roots of Polygalatenuifolia Willd. Chem Pharm Bull. 1991;39:3082–4. [Google Scholar]
- 25. Mak NK, Li WK, Zhang M, Wong RN, Tai LS, Yung KK, Leung HW. Effects of euxanthone on neuronal differentiation. Life Sci. 2000;66:347–54. doi: 10.1016/s0024-3205(99)00596-2. [DOI] [PubMed] [Google Scholar]
- 26. Yang B, Yang HS, Chen F, Hua YL, Jiang YM. Phytochemical analyses of Ziziphus jujuba Mill. var. spinosa seed by ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry. Analyst. 2013;138:6881–8. doi: 10.1039/c3an01478a. [DOI] [PubMed] [Google Scholar]