Abstract
Chun Mee tea is a kind of green tea produced in China mainly for export purposes. Foam quantity is usually used as an index for evaluating the quality of Chun Mee tea. In the current study, we compared the concentrations of total saponin and flavonoids between foamy and low-foam Chun Mee tea. Our research confirmed that the total saponin and O-glycosylated flavonoid concentrations were related to the foam quantity of Chun Mee teas. We also studied the short-term safety effects of extract supplementation with foamy and low-foam Chun Mee tea in rats by routine blood tests and analysis of liver and kidney function, and blood lipids. Our results showed that both types of tea extract supplementations did not cause any observable adverse effects or impair either liver or kidney function. Additionally, this study confirmed the beneficial effects of Chun Mee tea extract supplementation on the decrease of total plasma cholesterol.
Keywords: chemical analysis, Chun Mee tea, foam, short-term safety effect
1. Introduction
Tea [Camellia sinensis (L.) O. Kuntze] is an important commercial produce consumed worldwide, primarily as a beverage, which is made from processed leaves. Chun Mee tea has uniquely shaped leaves, with each leaf shaped like an eyebrow (the term “Chun Mee” means eyebrow in Chinese). Today, Chun Mee tea is one of the widely exported Chinese green teas, mainly transported to Africa and the Middle East. Chun Mee tea is known for its sweetness and smoothness. The foam quality (the quantity and size of foam) is used as a parameter for evaluating the quality of this tea. In West African countries, including Senegal, Gambia, and Mauritania, Chun Mee tea is boiled with mint leaves and sugar. The boiled tea water is then poured back and forth from a glass to a teapot several times, because of which the tea becomes foamy. A thicker foam indicates better quality tea. For example, the market price of 41022 (high-foam Chun Mee tea, US dollars 4.17/kg in China) is 47.35% higher than that of 9371 (low-foam Chun Mee tea, US dollars 2.83/kg).
Tea flavonoids, especially epigallocatechin gallate, are the main functional components and constitute over 10% of the dry weight of green tea [1]. Laboratory studies demonstrated inhibition of tumorigenesis in different animal models by tea and tea polyphenols [2,3]. Some epidemiological studies have suggested that consumption of black or green tea has a potentially protective effect against cancers of the lung [4,5], breast [6], gastric tract [7], and prostate [8] in humans. In addition, saponins constitute an important group of glycosides mainly functioning as surface-active and foaming agents in plants [9].Many pharmacological activities have been attributed to saponins, including hypotensive [10] and antibiotic [11] effects. However, ingestion of high concentrations of saponins can cause cytotoxicity and hemolysis [12]. It is necessary to determine the potential effects of drinking foamy Chun Mee tea.
Previously, we used the liquid chromatography–mass spectrometry method to determine the concentrations of catechins, flavonols, and phenolic acids in tea leaves [13]. The present study focuses on analyzing the quantitative composition of flavonoids and the total saponin concentration in Chun Mee teas, and particularly the differences in these aspects between foamy and low-foam tea. The precise short-term effects of exposure to different Chun Mee teas, as measured by routine blood tests and analysis of liver and kidney function, have, to our knowledge, not been previously examined. The results of this study will be useful for further elucidating the chemical composition of the foam of Chun Mee tea and for quantitatively estimating the risk of drinking foamy Chun Mee tea.
2. Materials and methods
2.1. Materials and standard chemicals
Foamy Chun Mee tea (41022) and low-foam Chun Mee tea (9371) were obtained from Shitai Rixing Tea Co. (Shitai, Anhui, China). Compounds such as (+)-catechin, (+)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin 3-O-gallate, (−)-epigallocatechin 3-O-gallate, gallic acid, and β-glucogallin were obtained from Shanghai RongHe Pharmaceutical Co. (Shanghai, China). Myricetin 3-O-glucoside, quercetin 3-O-glucoside, and kaempferol were purchased from Sigma Chemicals Co. (St Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade methanol, acetonitrile, and formic acid were purchased from Shanghai GuoMei Pharmaceutical Co. (Shanghai, China). HPLC-grade water was prepared from distilled water using a Milli-Q system (Millipore Laboratory, Bedford, MA, USA).
2.2. Analysis of catechins, flavonols, phenolic acids, and saponins in Chun Mee tea
Chun Mee tea samples were ground to a fine powder in liquid nitrogen. The powdered samples (1 g) were extracted with 5mL methanol by sonication at room temperature for 10 minutes, followed by centrifugation at 4000g for 15 minutes. The residues were extracted twice by this method. The supernatants were then filtered through a 0.22 μm membrane. Catechins, flavonols, and phenolic acids were analyzed by HPLC.
All samples were separated on a Phenomenex Synergi 4u Fusion-RP80 column (5 μm, 250 × 4.6 mm2) with detection at 280 nm and 345 nm using an HPLC-UV detector (Waters 2478; Waters Instruments, Minneapolis, USA). The mobile phase consisted of 1% acetic acid in water (A) and 100% acetonitrile (B). The gradient increased linearly from 0% B to 10% B (v/v) at 10 minutes, 13% B at 30 minutes, 16% B at 65 minutes, 33% B at 81 minutes, and 90% B at 85 minutes, and stayed at 90% B at 90 minutes. Peaks were identified and measured according to the method of Wang et al [13]. All samples were run in triplicate for both quantitation and multivariate statistical analysis. The concentration of total saponins was determined according to the spectrophotometry method of vanillin–sulfuric acid [14]. Saponin amounts were determined by measuring absorbance at 430 nm, based on the color reactions with anisaldehyde, sulfuric acid, and ethyl acetate.
2.3. Animals and treatment
The tea samples (1 kg) were extracted with 5 L of water by sonication at 80°C for 1 hour. The extracts were then filtered with a quantitative filter paper. Spray-dried powders were prepared using a BUCHI spray dryer (Buchi Co., Oldham, UK). Solutions of dry powders (1 g/L) were prepared for intragastric administration to rats.
Sprague–Dawley male (190–240 g) and female (170–220 g) rats were obtained from the Center of Laboratory Animals of Anhui province, China (Grade II, SCXK: 2011-002). Rats were given normal chow diet and water ad libitum, and were housed under standard conditions of 12 h/light:12 h/dark. Four rats were housed per cage for the chronic toxicity study. All protocols were approved by the committee for care and use of laboratory animals at Anhui Medical University, Anhui, China.
After an acclimatization period, the rats were randomly assigned to the control group, low-foam tea group, and foamy tea group (8 animals, 4 female and 4 male, in each group). The rats in the tea groups were orally administered spray powders of low-foam tea and foamy tea at a dose of 50 mg/kg body weight, once per day, 6 days per week, for 13 weeks. The rats in the control group were administered water in the same quantities. The rats were weighed prior to the initiation of treatment, and then weekly for the next 13 weeks.
For the final administration, food was removed overnight from the rats. The animals were anesthetized with 10% chloral hydrate (3.0 mL/kg) and killed by bleeding, and the blood was collected for subsequent routine tests. An aliquot of the blood was also used for hematological examination. The remainder of the blood was allowed to clot, and the serum was collected. The following parameters were analyzed: white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, differential blood count, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, glucose, chloride, calcium, sodium, potassium, blood pH, blood urea nitrogen, creatinine, total proteins, albumin, globulin, triglycerides, and cholesterol. At necropsy, weights of the testes, ovaries, kidneys, liver, heart, lung, adrenals, brain, spleen, and thymus were determined. The relative organ weight (weight per 100 g body weight) was calculated.
2.4. Statistical analysis
Statistical differences were analyzed using one-way analysis of variance. Student t test was used to determine significant differences between the groups. Results are expressed as the mean ± standard error of the mean; differences were considered statistically significant at a value of p < 0.05.
3. Results and discussion
3.1. Analyses of flavonoid and saponin concentrations in Chun Mee teas
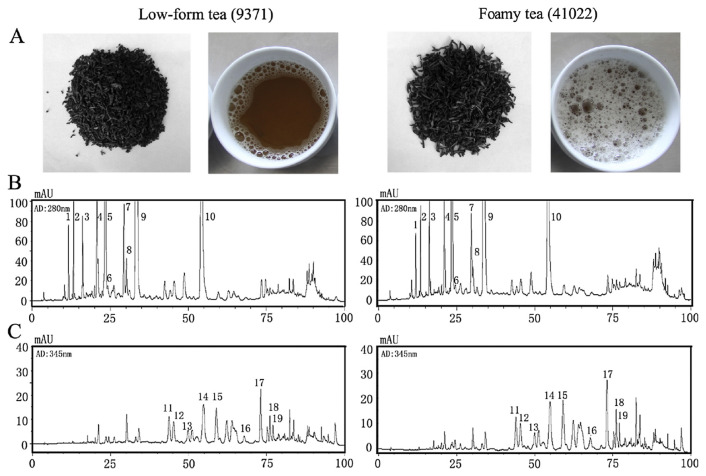
The shapes and total amounts of foam were notably different between the two tea samples. As shown in Fig. 1A, 41022 had a thicker foam than 9371. Results showed that the concentration of total saponins was notably higher in 41022 than in 9371, with the former having 10.26% more saponins than the latter (Table 1). HPLC methods were applied to determine the flavonoid compounds in the tea leaves (Fig. 1B). Peak identification was based on comparison with standards and published data [13]. As shown in Table 1, Chun Mee tea had the highest amount of flavan-3-ols (catechins), accounting for 19% of the dry weight. However, there was no significant difference in catechins and phenolic acids between 41022 and 9371 (p = 0.514 and p = 0.202, respectively). As with saponin, the concentration of O-glycosylated flavonoid was higher in 41022 (by 15.93%) than in 9371. Chemical evaluation studies of licorice roots involving quantitative analyses of saponin and O-glycosylated flavonoid constituents also showed that these constituents were affected simultaneously [15]. Glycosylation of flavonoids reduced the 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) free radical scavenging potential [16]. However, glycosylation can decrease the inhibitory potential against inflammation, renal failure, aging, and especially antidiabetes [17]. Wang et al [18] illustrated the glycosylation of flavonoids can significantly reduce the binding constants for bovine serum albumin (BSA).
Fig. 1.
Comparison of the shape, foam amount, and HPLC chromatogram of different Chun Mee teas. (A) Shape and foam amount of Chun Mee tea. (B and C) Representative HPLC chromatograms registered at 280 nm and 345 nm, respectively. HPLC = high-performance liquid chromatography.
Table 1.
Concentrations of flavonoids and saponins in Chun Mee tea.a
| Compoundb | 9371 (low-foam tea) | 41022 (foamy tea) |
|---|---|---|
| Saponins* (p < 0.005) | 15.279 ± 0.205 | 16.847 ± 0.082 |
| Flavan-3-ols (p = 0.514) | ||
| Gallocatechin (4) | 2.337 ± 0.417 | 2.536 ± 2.149 |
| Epigallocatechin (5) | 63.244 ± 8.004 | 62.680 ± 4.645 |
| Catechin (6) | 0.220 ± 0.112 | 0.163 ± 0.057 |
| Epicatechin (7) | 5.152 ± 0.825 | 4.893 ± 0.386 |
| Epigallocatechin gallate (9) | 44.769 ± 8.086 | 46.210 ± 3.812 |
| Epicatechin gallate (10) | 11.863 ± 2.141 | 11.765 ± 1.421 |
| Total | 187.034 ± 19.064 | 188.193 ± 12.328 |
| Phenolic acids (p = 0.202) | ||
| β-Glucogallin (1) | 0.639 ± 0.089 | 0.606 ± 0.770 |
| Galloyl acid (2) | 0.941 ± 0.258 | 0.838 ± 0.131 |
| Galloylquinic acid* (3) | 0.993 ± 0.100 | 1.339 ± 0.153 |
| p-Coumaroylquinic acid (8) | 0.752 ± 0.164 | 0.622 ± 0.732 |
| Total | 4.866 ± 0.515 | 4.986 ± 0.138 |
| O-glycosylated flavonols* (p < 0.005) | ||
| Myricetin 3-O-galactoside or glucoside (11) | 2.079 ± 0.294 | 2.195 ± 0.155 |
| Myricetin 3-O-galactoside or glucoside (12) | 1.641 ± 0.276 | 1.812 ± 0.139 |
| Quercetin 3-O-glucosylrutinoside or galactosylrutinoside* (13) | 1.198 ± 0.153 | 1.347 ± 0.088 |
| Quercetin 3-O-glucosylrutinoside or galactosylrutinoside (14) | 4.376 ± 0.590 | 4.738 ± 0.457 |
| Quercetin 3-O-dirhamnosylglucoside or kaempferol 3-O-galactosylrutinoside* (15) | 2.933 ± 0.438 | 3.509 ± 0.254 |
| Quercetin 3-O-dirhamnosylglucoside or kaempferol 3-O-galactosylrutinoside* (16) | 0.557 ± 0.101 | 0.966 ± 0.141 |
| Kaempferol 3-O-galactoside or glucoside (17) | 3.535 ± 0.452 | 3.811 ± 0.790 |
| 6,8-C-Diglucosylapigenin or kaempferol 3-O-rhamnosylgalactoside* (18) | 1.007 ± 0.009 | 1.464 ± 0.055 |
| Quercetin 3-O-rhamnoside or kaempferol* (19) | 0.643 ± 0.112 | 0.893 ± 0.059 |
| Total | 17.969 ± 1.470 | 20.735 ± 1.702 |
Significant correlation (p < 0.05).
DW = dry weight; SEM = standard error of the mean.
mg/g DW, means ± SEM of duplicate assays.
The number of every compound corresponds to the peak number in Fig. 1.
3.2. Effect of Chun Mee tea on body weight and relative organ weights
In the present study, the administered dose of tea was approximately similar to the estimated mean daily intake of catechins in the UK [19]. There was no treatment-related mortality in this study, and body weight was measured weekly. The results did not show any biologically or toxicologically significant differences in the mean body weights between the control and the treated animals of the same sex at any time point (data not shown). Further, there were no significant differences in the relative organ weights of testes, ovaries, kidneys, liver, heart, lung, adrenals, brain, spleen, and thymus between the treatment and the control groups (Table 2).
Table 2.
Effect of Chun Mee tea extract on the relative organ weight in rats.
| Organ (g) | Control | 9371 (little foam tea) | 41022 (foam tea) |
|---|---|---|---|
| Heart | 0.293 ± 0.032 | 0.283 ± 0.035 | 0.305 ± 0.034 |
| Liver | 2.653 ± 0.369 | 2.564 ± 0.282 | 2.547 ± 0.273 |
| Spleen | 0.274 ± 0.051 | 0.227 ± 0.041 | 0.220 ± 0.051 |
| Lung | 0.500 ± 0.089 | 0.451 ± 0.080 | 0.849 ± 0.885 |
| Kidney | 0.248 ± 0.044 | 0.259 ± 0.021 | 0.267 ± 0.027 |
| Adrenal | 0.012 ± 0.006 | 0.009 ± 0.004 | 0.011 ± 0.002 |
| Thymus | 0.091 ± 0.019 | 0.089 ± 0.031 | 0.080 ± 0.034 |
| Brain | 0.360 ± 0.075 | 0.384 ± 0.072 | 0.389 ± 0.068 |
| Ovary (female) | 0.034 ± 0.003 | 0.032 ± 0.007 | 0.039 ± 0.004 |
| Testis (male) | 0.387 ± 0.011 | 0.284 ± 0.103 | 0.384 ± 0.029 |
Data are presented as mean ± SEM; for ovary and testis, n = 4; for the other organs, n = 8.
SEM = standard error of the mean.
3.3. Effect of Chun Mee tea extract on routine blood and electrolytes indexes in rats
The rats were treated orally with low-foam tea and foamy tea at doses of 50 mg/kg once per day for a period of 13 weeks. Routine blood examinations were performed after the final treatment day. Results from the routine blood tests showed that the hemoglobin concentration was significantly increased in both the foamy tea and low-foam tea groups (p < 0.01). It has been reported that tea drinking reduces iron absorption from the gut in healthy adults [20,21] and in patients with thalassemia [22]. By contrast, our results showed that consumption of both types of tea extracts led to a significant increase in hemoglobin concentration. Disler et al [21] inferred that drinking tea with meals may contribute to the pathogenesis of iron deficiency if the diet consists largely of vegetable products. In the present study, both the Chun Mee teas were administered (intragastric administration) at 9:00–10:00 AM, without affecting iron absorption. The mechanisms of how the Chun Mee teas contribute to the increase in hemoglobin concentration remain unknown.
Tables 3 and 4 show that no significant abnormality was observed in other routine blood parameters (including white blood cell count, red blood cell count, and platelets) and electrolyte parameters (including potassium, sodium, chloride, and calcium) in treated rats compared with the control group.
Table 3.
Effect of Chun Mee tea extract on routine blood indexes in rats.
| Parameters | Control | 9371 (little foam tea) | 41022 (foam tea) |
|---|---|---|---|
| WBC (109/L) | 8.36 ± 2.54 | 6.98 ± 1.98 | 7.88 ± 1.21 |
| Lymphocytes (109/L) | 4.24 ± 1.18 | 3.90 ± 1.45 | 3.83 ± 0.89 |
| Monocytes (109/L) | 0.41 ± 0.16 | 0.34 ± 0.19 | 0.34 ± 0.11 |
| Lymphocytes (%) | 52.38 ± 9.96 | 55.73 ± 8.10 | 48.29 ± 8.46 |
| Monocytes (%) | 4.99 ± 0.86 | 4.71 ± 1.73 | 4.90 ± 0.65 |
| RBC (1012/L) | 12.60 ± 1.42 | 12.44 ± 1.76 | 14.09 ± 1.51 |
| MCV (fL) | 54.93 ± 2.94 | 57.48 ± 2.14 | 53.90 ± 1.86 |
| Hematocrit (L/L) | 69.19 ± 9.02 | 71.48 ± 10.72 | 75.90 ± 7.83 |
| Hemoglobin (g/L) | 139.88 ± 9.06 | 160.50 ± 15.63* | 162.75 ± 13.20* |
| Platelets (109/L) | 279.00 ± 175.49 | 143.25 ± 59.72 | 251.63 ± 135.90 |
| Mean platelets volume (fL) | 8.21 ± 0.99 | 8.39 ± 0.94 | 9.36 ± 1.56 |
Data are presented as mean ± SEM; n = 8.
p < 0.01, compared with the control group.
MCV = mean corpuscular volume; RBC = red blood cell count; SEM = standard error of the mean; WBC = white blood cell count.
Table 4.
Effect of Chun Mee tea extract on electrolytes in rats.
| Electrolytes | Control | 9371 (little foam tea) | 41022 (foam tea) |
|---|---|---|---|
| Potassium (mmol/L) | 1.50 ± 0.43 | 1.43 ± 0.46 | 1.54 ± 0.59 |
| Sodium (mmol/L) | 137.15 ± 1.30 | 138.94 ± 3.21 | 138.10 ± 3.64 |
| Chloride (mmol/L) | 104.69 ± 2.11 | 107.05 ± 3.16 | 106.98 ± 2.33 |
| Calcium (mmol/L) | 0.95 ± 0.09 | 0.98 ± 0.10 | 0.87 ± 0.24 |
Data are presented as mean ± standard error; n = 8.
3.4. Effects of Chun Mee tea extract on liver and kidney function and blood lipid in rats
Liver function, as assessed by the alanine aminotransferase and aspartate aminotransferase levels, total protein, albumin, and total bilirubin, of rats treated with foamy and low-foam tea extracts did not differ from the levels observed for control rats (Table 5). Kidney function was assessed by measuring the urea nitrogen and creatinine levels. The results showed that there was no significant difference in creatinine levels between the treated and control groups. However, both types of tea resulted in a significant increase of urea nitrogen, although the increase was within normal levels [23].
Table 5.
Effect of Chun Mee tea extract on liver and kidney function and blood lipid in rats.
| Parameters | Control | 9371 (little foam tea) | 41022 (foam tea) |
|---|---|---|---|
| ALT (U/L) | 33.25 ± 8.38 | 30.63 ± 5.95 | 28.38 ± 3.54 |
| AST (U/L) | 99.25 ± 22.83 | 87.63 ± 23.00 | 85.88 ± 16.53 |
| Total proteins (g/L) | 70.09 ± 3.32 | 72.43 ± 4.02 | 69.80 ± 5.94 |
| Albumin (g/L) | 33.30 ± 2.56 | 34.96 ± 3.70 | 34.34 ± 4.77 |
| Total bilirubin (%) | 24.46 ± 0.18 | 24.26 ± 0.24 | 24.76 ± 0.36 |
| Alkaline phosphatase (U/L) | 6.63 ± 3.07 | 7.50 ± 5.15 | 7.38 ± 6.67 |
| Glucose (mmol/L) | 8.06 ± 0.75 | 9.59 ± 1.53* | 9.04 ± 1.37 |
| Urea nitrogen (mmol/L) | 5.26 ± 0.54 | 6.71 ± 0.83** | 6.26 ± 1.13* |
| Creatinine (μmol/L) | 2174.50 ± 17.02 | 2171.25 ± 22.80 | 2185.13 ± 18.18 |
| Total cholesterol (mg/dL) | 1.34 ± 0.22 | 1.06 ± 0.11** | 1.03 ± 0.20* |
| Triglyceride (mmol/L) | 1.04 ± 0.21 | 1.01 ± 0.23 | 1.22 ± 0.41 |
Data are presented as mean ± standard error of the mean; n = 8.
p < 0.05.
p < 0.01, compared with the control group.
ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Our results, similar to other studies [24], showed that both low-foam tea and foamy tea induced a significant decrease of plasma total cholesterol in rats. An epidemiological study revealed that compared to no tea consumption, habitual green tea consumption was associated with a lower risk of death from cardiovascular disease [25]. The beneficial effects of green tea on cardiovascular health may be due to the high concentration of green tea catechins, which have been proved to modulate the plasma lipid profile favorably. In animal experiments, catechins with gallate esters were shown to influence the biliary micelle system in the intestine by forming insoluble coprecipitates of cholesterol and increasing the fecal excretion of cholesterol [26]. In addition, Singh et al [27] further demonstrated that green and black tea extracts are potent and selective inhibitors of HMG-CoA reductase, which is likely a rate-limiting enzyme in cholesterol biosynthesis.
Studies on healthy humans have shown that green tea supplementation does not have significant effects on plasma glucose and urea nitrogen [28]; however, our results showed that low-foam Chun Mee tea significantly increased plasma glucose levels such that they fell within the normal range. Further, both low-foam and foamy tea extracts significantly increased the plasma urea nitrogen concentration within the normal range. We confirmed that daily consumption of green tea does not affect plasma glucose and urea nitrogen in healthy people. However, further study is required to examine the effects of daily consumption of Chun Mee tea on plasma glucose and urea nitrogen in diabetics and nephrotics.
4. Conclusion
We have analyzed the quantitative composition of flavonoids and the total saponin concentration in Chun Mee teas. Our research confirmed that the saponins and O-glycosylated flavonoids were related to the foam quantity of Chun Mee teas. Concentrations of total saponins and O-glycosylated flavonols in 41022 were higher than those in 9371, by 10.26% and 15.93%, respectively. We further studied the subchronic effects of low-foam tea and foamy Chun Mee tea extracts on hematologic parameters in rats. The results showed that both types of tea extract supplementations did not cause adverse effects or impair liver and kidney function in rats. The study also showed the beneficial effects of Chun Mee tea extract supplementation such as decreasing plasma total cholesterol, which contributed to a reduction in cardiovascular risk factors.
Acknowledgments
This work was supported by the Nature Science Foundation of China (Projects 31270730 and 31000314); Science and Technology Projects of Anhui Province, China (Project 13Z03012); and the Agricultural Technology Transfer Foundation of Anhui Province, China (Projects 1404032014 and 1404032029).
Funding Statement
This work was supported by the Nature Science Foundation of China (Projects 31270730 and 31000314); Science and Technology Projects of Anhui Province, China (Project 13Z03012); and the Agricultural Technology Transfer Foundation of Anhui Province, China (Projects 1404032014 and 1404032029).
REFERENCES
- 1. Friedman M, Mackey BE, Kim HJ, Lee IS, Lee KR, Lee SU, Kozukue E, Kozukue N. Structure–activity relationships of tea compounds against human cancer cells. J Agric Food Chem. 2007;55:243–53. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 2. Katiyar SK, Agarwal R, Wang ZY, Bhatia AK, Mukhtar H. (−)-Epigallocatechin-3-gallate in Camellia sinensis leaves from Himalayan region of Sikkim: inhibitory effects against biochemical events and tumor initiation in Sencar mouse skin. Nutr Cancer. 1992;18:73–83. doi: 10.1080/01635589209514207. [DOI] [PubMed] [Google Scholar]
- 3. Xu GP, Song PJ, Reed PI. Effects of fruit juices, processed vegetable juice, orange peel and green tea on endogenous formation of N-nitrosoproline in subjects from a high-risk area for gastric cancer in Moping County, China. Eur J Cancer Prev. 1993;2:327–35. doi: 10.1097/00008469-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 4. Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio JC, Ronco A. Consumption of tea and coffee and the risk of lung cancer in cigarette-smoking men: a case-control study in Uruguay. Lung Cancer. 1998;19:101–7. doi: 10.1016/s0169-5002(97)00075-5. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Yu X, Wu Y, Zhang D. Coffee and tea consumption and risk of lung cancer: a dose-response analysis of observational studies. Lung Cancer. 2012;78:169–70. doi: 10.1016/j.lungcan.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6. Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–61. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sasazuki S, Tamakoshi A, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Inoue M, Tsugane S. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Green tea consumption and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:335–46. doi: 10.1093/jjco/hys009. [DOI] [PubMed] [Google Scholar]
- 8. Jain MG, Hislop GT, Howe GR, Burch JD, Ghadirian P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int J Cancer. 1998;78:707–11. doi: 10.1002/(sici)1097-0215(19981209)78:6<707::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9. Stanimirova R, Marinova K, Tcholakova S, Denkov ND, Stoyanov S, Pelan E. Surface rheology of saponin adsorption layers. Langmuir. 2011;27:12486–98. doi: 10.1021/la202860u. [DOI] [PubMed] [Google Scholar]
- 10. Sagesaka-Mitane Y, Sugiura T, Miwa Y, Yamaguchi K, Kyuki K. Effect of tea-leaf saponin on blood pressure of spontaneously hypertensive rats. Yakugaku Zasshi. 1996;116:388–95. doi: 10.1248/yakushi1947.116.5_388. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 11. Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M. Antimicrobial activity of saponins from Medicago sp.: structure–activity relationship. Phytother Res. 2006;20:454–7. doi: 10.1002/ptr.1876. [DOI] [PubMed] [Google Scholar]
- 12. Liu Z, Gao W, Jing S, Zhang Y, Man S, Wang Y, Zhang J, Liu C. Correlation among cytotoxicity, hemolytic activity and the composition of steroidal saponins from Paris L. J Ethnopharmacol. 2013;149:422–30. doi: 10.1016/j.jep.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Gao L, Shan Y, Liu Y, Tian Y, Xia T. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze) Sci Hortic. 2012;141:7–16. [Google Scholar]
- 14. Uematsu Y, Hirata K, Saito K, Kudo I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J AOAC Int. 2000;83:1451–4. [PubMed] [Google Scholar]
- 15. Kuwajima H, Taneda Y, Chen W, Kawanishi T, Hori K, Taniyama T, Kobayashi M, Ren J, Kitagawa I. Variation of chemical constituents in processed licorice roots: quantitative determination of saponin and flavonoid constituents in bark removed and roasted licorice roots. Yakugaku Zasshi. 1999;119:945–55. doi: 10.1248/yakushi1947.119.12_945. [DOI] [PubMed] [Google Scholar]
- 16. Om A, Kim JH. A quantitative structure-activity relationship model for radical scavenging activity of flavonoids. J Ned Food. 2008;11:29–37. doi: 10.1089/jmf.2007.048. [DOI] [PubMed] [Google Scholar]
- 17. Xiao J, Chen T, Cao H. Flavonoid glycosylation and biological benefits. Biotechnol Adv. 2014 doi: 10.1016/j.biotechadv.2014.05.004. [in press] [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Zhao Y, Yang F, Yuan Y, Wang H, Xiao J. Influences of glucose on the dietary hydroxyflavonoid–plasma protein interaction. J Agric Food Chem. 2012;60:12116–21. doi: 10.1021/jf303094e. [DOI] [PubMed] [Google Scholar]
- 19. Khokhar S, Magnusdottir SG. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 2002;50:565–70. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- 20. Derman D, Sayers M, Lynch SR, Charlton RW, Bothwell TH, Mayet F. Iron absorption from a cereal-based meal containing cane sugar fortified with ascorbic acid. Br J Nutr. 1977;38:261–9. doi: 10.1079/bjn19770087. [DOI] [PubMed] [Google Scholar]
- 21. Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, Walker RB, Walker RB, Mayet F. The effect of tea on iron absorption. Gut. 1975;16:193–200. doi: 10.1136/gut.16.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Alarcon PA, Donovan ME, Forbes GB, Landaw SA, Stockman JA., 3rd Iron absorption in the thalassemia syndromes and its inhibition by tea. N Engl J Med. 1979;300:5–8. doi: 10.1056/NEJM197901043000102. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo M, Morikawa Y, Hashimoto Y, Baratz RS. Changes in blood urea nitrogen (BUN) concentration during pregnancy in the rat with or without obstructive uremia. Exp Pathol. 1986;30:203–8. doi: 10.1016/s0232-1513(86)80078-0. [DOI] [PubMed] [Google Scholar]
- 24. Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–10. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]
- 25. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–65. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 26. Chan PT, Fong WP, Cheung YL, Huang Y, Ho WK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129:1094–101. doi: 10.1093/jn/129.6.1094. [DOI] [PubMed] [Google Scholar]
- 27. Singh DK, Banerjee S, Porter TD. Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells. J Nutr Biochem. 2009;20:816–22. doi: 10.1016/j.jnutbio.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, Rimbach G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139:58–62. doi: 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]