Abstract
The present paper describes the development of a high performance liquid chromatography-ultraviolet (HPLC-UV) detection method for quantitative determination of peimine and peiminine in Fritillariae Thunbergii Bulbus (FTB). Separation was achieved using a conventional XBridge™Shield RP 18 column (250 mm × 4.6 mm, internal diameter 3.5 μm) with photodiode array detection at 190–400 nm for UV spectra and 220 nm for quantification. The mobile phase consisted of (A) 0.03% diethylamine aqueous solution and (B) acetonitrile eluted by an isocratic procedure at 45:55 (A:B) over 25 minutes. The method was validated for linearity, limits of detection (LOD) and quantification (LOQ), inter- and intra-day precisions, repeatability, stability, and recovery. All the validation results were satisfactory. The developed method was then applied to assay the contents of the two chemical markers in all the FTB samples collected. Based on the contents of the two analytes, hierarchical clustering analysis (HCA) was performed to reveal the similarities and differences of the samples.
Keywords: Fritillariae Thunbergii Bulbus, hierarchical clustering analysis, HPLC-UV, quality assessment
1. Introduction
Fritillariae Thunbergii Bulbus (FTB), the dried bulb of Fritillaria thunbergii Miq., is one of the traditional Chinese medicines commonly used for detoxification, to eliminate phlegm, and to relieve cough [1,2]. Pharmacological studies indicated that FTB showed good effects in the treatment of hyperthyroidism [3] and prostatitis [4] and could enhance the multidrug resistance (MDR) reversal effect of cisplatin (DDP) on A549/DDP cells in vitro and in vivo as well as downregulate MDR1 mRNA and P-glycoprotein (P-gp) expression in A549/DDP cells [5]. Peimine and peiminine are regarded as the main bioactive compounds in FTB. Intraperitoneal administration of peimine and peiminine resulted in a significant antitussive effect in mice [6]. Peimine was found to reverse MDR in the A549/DDP cell line [7]. According to Chinese Pharmacopeia (2010 edition) and Taiwan Herbal Pharmacopeia (2nd edition), the total content of peimine and peiminine in qualified FTB should not be less than 0.08% [1,2]. However, the quantification of peimine and peiminine is difficult and challenging because the chemical structures of the two compounds are similar. There is also a lack of UV-conjugated systems (Fig. 1). In the Chinese Pharmacopeia analysis, an evaporative light scattering detector (ELSD) was used for quantification of the two analytes in FTB samples [1,2]; however, the expensiveness, poor repeatability, and poor stability of the detector make it be unacceptable in routine analyses.
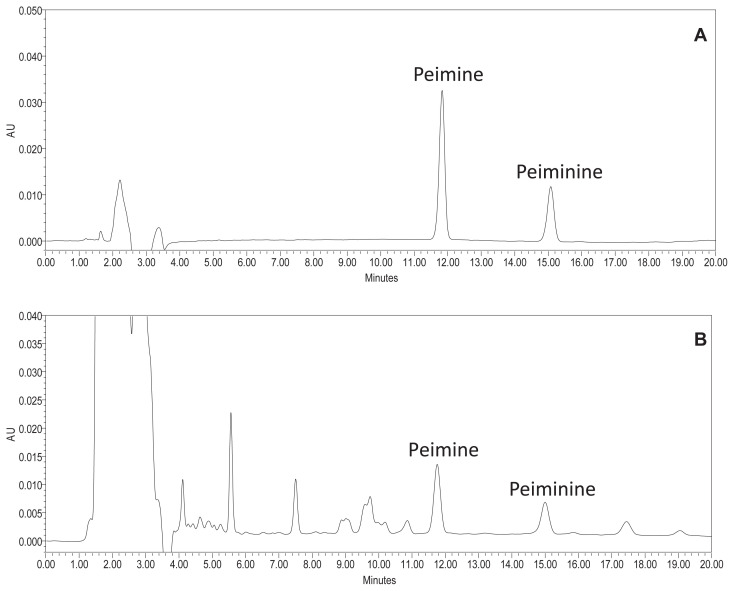
Fig. 1.
Representative chromatograms of (A) mixed standards of peimine and peiminine and (B) FTB-01 detected at 220 nm.
Therefore, in the present study, we established and validated a high performance liquid chromatography coupled with ultraviolet (HPLC-UV) detector for quantitative determination of peimine and peiminine. FTB samples from different pharmacies and markets in Taiwan were analyzed. The data enabled us to ascertain the stability and homogeneity of the herb in Taiwan markets.
2. Methods
2.1. Chemicals, solvents, and herbal materials
Peimine and peiminine were purchased from the National Institute for Control of Pharmaceutical and Biological Products (Beijing, People’s Republic of China). LC-grade methanol and acetonitrile were purchased from Merck (Taipei, Taiwan). Purified water was prepared with the Milli-Q system (Millipore, Milford, MA, USA). All other reagents used in the present study were of analytical grade. Herbal materials of FTB were purchased from local markets in Taiwan, which were marked as FTB-01 to FTB-10, respectively. All the specimens were deposited at the Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, School of Pharmacy, China Medical University.
2.2. Sample and reference preparation
FTB samples were dried in a shaded place and were ground into fine powder (20 mesh) using a grinder with a knife blade. For each sample, 2 g of FTB powder was carefully weighed into a 125-mL conical flask. Ammonium hydroxide (5 mL) was then added and each sample was soaked for 1 hour, after which 50 mL of dichloromethane-methanol (4:1, v/v) was added to the flask. Each sample was extracted using heat reflux at 65°C for 2 hours. The extract was filtered and transferred to a 100 mL-evaporation pan, and evaporated to dryness. The residue was dissolved and made up to 10 mL with methanol. The final volume was filtered through a 0.45-μm polyvinylidene fluoride (PVDF) syringe filter (VWR Scientific, Seattle, WA, USA) before analysis.
The reference compounds of peimine and peiminine were accurately weighed and were dissolved in methanol at 1165 mg/L and 1044 mg/L (stock solutions), respectively. The stock solutions were then diluted to appropriate concentrations for establishment of calibration curves. An aliquot of 10 μL of each solution was used for HPLC analysis.
2.3. HPLC analysis
HPLC analyses were performed on a Waters 2695 HPLC system (Milford, USA) equipped with a Waters 2998 photodiode array detector (PDA), a Waters e2695 separation module, and a column heater module. An XBridge Shield RP 18 column (250 mm × 4.6 mm, internal diameter 3.5 μm) (Milford, USA) was used. The mobile phase consisted of water containing (A) 0.03% diethylamine and (B) acetonitrile. The isocratic program applied 55% of B over 0–20 minutes. The flow rate was set at 1 mL/min and the injection volume was 10 μL. UV spectra were acquired from 190 nm to 400 nm. The autosampler and column compartments were maintained at 25°C and 35 °C, respectively.
2.4. Method development
The limits of detection (LOD) and quantification (LOQ) were defined as the lowest concentrations of analytes in the sample that can be detected and quantified, which were determined on the basis of signal-to-noise ratios (S/N) at 3:1 and 10:1, respectively. Intra- and inter-day variations were chosen to evaluate the precision of the developed method. The intra-day variation was determined by analyzing one of the mixed stock solutions five times within 1 day. For the inter-day variability test, the same solution was examined in triplicate for 3 consecutive days. Repeatability was confirmed with five different working solutions prepared from one sample. Stability was tested with the same sample solution at 0, 2, 4, 8, 12, and 24 hours. The recovery was performed by adding known amounts of the two analytes into the samples, which were then extracted, processed, and quantified in accordance with the methods mentioned above.
2.5. Hierarchical clustering analysis
Hierarchical clustering analysis (HCA) was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) based on the contents of peimine and peiminine in different FTB samples.
3. Results and discussion
3.1. Optimization of the extraction method
The extraction method was optimized taking the extraction efficiency of peimine and peiminine as indicators. Ultrasonic and reflux extraction were investigated at room temperature using dichloromethane-methanol (4:1, v/v) as the extraction solvent. As a result, the total contents of peimine and peiminine obtained by using heat reflux extraction were both higher than that obtained by using ultrasonic extraction (p < 0.05). Furthermore, the two compounds were almost extracted completely (>99%) for 2 hours. Finally, the optimal extraction procedure was finalized as described in the “Sample and reference preparation” section.
3.2. Optimization of chromatographic conditions
To develop a reliable chromatographic fingerprinting method, an optimized strategy for HPLC conditions was performed. To obtain sharp and symmetrical peaks, different mobile phase systems, methanol–water, acetonitrile–water, acetonitrile–-water (0.5% formic acid), and acetonitrile–water (0.03% diethylamine) elution systems were tested. Good resolution and baseline, sharp, and symmetrical peaks were obtained by using the acetonitrile–water (0.03% diethylamine) system. A few columns (Waters XTerra RP18, Thermo Ascentis C18, and Grace Alltima C18) were tested before the Waters XBridge C18 column (250 mm × 4.6 mm, internal diameter 3.5 μm) was finally selected as the column of choice. To obtain a sufficiently large number of detectable peaks on the chromatographic finger-prints, a photodiode array detector (PAD) full scan (190–400 nm) was used for investigating all the main peaks and finally 220 nm was selected as the detection wavelength. Column temperatures of 20°C, 25°C, 30°C, and 35°C were investigated. Although chromatograms detected at different temperatures did not show obvious differences, 35°C was selected as the preferable one in order to minimize the influences from room temperature on the chromatograms. In the process of gradient optimization, the gradient time, gradient procedure, and initial composition of the mobile phase were taken into consideration. Finally, the gradient procedure was finalized as described in the “HPLC analysis” section. A representative chromatographic fingerprint obtained from FTB-01 is shown in Fig. 1.
3.3. Validation of the quantitative analytical method
The HPLC method was validated by defining the LOD and LOQ, linearity, inter-day and intra-day precisions, repeatability, stability, and recovery.
The calibration curves were plotted on the basis of linear regression analysis of the integrated peak areas (y) versus concentrations (x, mg/L) of the two analytes at five different levels. LOD and LOQ values for each analyte under the present chromatographic conditions were determined in terms of baseline noise, according to the International Union of Pure and Applied Chemistry (IUPAC) definition. LOD was determined as the analyte concentration yielding a signal with an S/N ratio of 3:1, whereas the LOQ was defined as the analyte concentration yielding a signal with an S/N ratio of 10:1. The results of regression equations, correlation coefficients, linear ranges, LODs, and LOQs for peimine and peiminine are shown in Table 1. The correlation coefficient (R2) of the regression equation for each analyte indicates good linearity, being better than 0.999.
Table 1.
The results of LODs, LOQs, regression equations, correlation coefficients, and linearity ranges of peimine and peiminine.
| Analyte | Regression equation | Correlation coefficient | Linearity range (mg/L) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Peimine | y = 889972x + 2447.4 | 0.9999 | 24.3–388.3 | 1.67 | 6.07 |
| Peiminine | y = 895452x − 1376.1 | 0.9999 | 10.9–174.0 | 5.44 | 14.26 |
LOD = limit of detection; LOQ = limit of quantification.
Intra- and inter-day variations were chosen to determine the precision of the developed method. For the intra-day variability test, one of the mixed standard solutions (peimine, 97.1 mg/L; peiminine, 43.5 mg/L) was analyzed five times within 1 day, while for the inter-day variability test, the mixed standard solution was examined in triplicate each day on 3 consecutive days. The relative standard deviations (RSDs) for the peak areas were calculated as measurements of precision. The RSDs of intra-day variation for peimine and peiminine were less than 3.50%, and the RSDs of inter-day variation for peimine and peiminine were less than 4.00%, as shown in Table 2. Repeatability was evaluated by analyzing five different working solutions prepared from the same sample (FTB-01). RSD values were 1.86% and 2.17% for peimine and peiminine, respectively (Table 2). Stability was determined using repeated analyses of the same sample solution at different times during storage at room temperature (approx. 25°C) for 24 hours. The RSD values of peak areas of peimine and peiminine were 3.99% and 1.68%, respectively (Table 2), indicating that the stability of the sample solution within 1 day was good. The recovery test was determined using spiked FTB samples. A portion of 1 g of FTB sample was individually spiked with 0.5010 mg of peimine and 0.3132 mg of peiminine, respectively. Five replicate samples were extracted and analyzed according to the procedures described above. As shown in Table 3, the mean recovery values [n = 5, mean ± standard deviation (SD)] were found to be 94.18% ± 3.75 and 91.56 ± 2.11, respectively.
Table 2.
The results of the precision, repeatability, and stability tests.
| Analyte | Precision (RSD, %) | Repeatability (RSD, %) | Stability (RSD, %) | |
|---|---|---|---|---|
|
| ||||
| Intra-day (n = 5) | Inter-day (n = 5) | |||
| Peimine | 1.99 | 2.06 | 1.86 | 3.99 |
| Peiminine | 3.39 | 3.51 | 2.17 | 1.68 |
RSD = relative standard deviation.
Table 3.
The accuracy of the HPLC-UV method for the determination of peimine and peiminine.
| Analyte | Sample weight (g) | Original (mg) | Spiked (mg) | Found (mg) | Recovery (%) | Mean recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Peimine | 0.9755 | 0.4841 | 0.5010 | 0.9827 | 99.53 | 94.18 | 3.75 |
| 0.9702 | 0.4815 | 0.5010 | 0.9332 | 90.17 | |||
| 0.9712 | 0.4820 | 0.5010 | 0.9454 | 92.51 | |||
| 0.9901 | 0.4914 | 0.5010 | 0.9537 | 92.30 | |||
| 0.9961 | 0.4944 | 0.5010 | 0.9620 | 93.34 | |||
| Peiminine | 0.9755 | 0.2915 | 0.3132 | 0.5741 | 90.25 | 91.56 | 2.11 |
| 0.9702 | 0.2899 | 0.3132 | 0.5820 | 93.25 | |||
| 0.9712 | 0.2902 | 0.3132 | 0.5728 | 90.22 | |||
| 0.9901 | 0.2958 | 0.3132 | 0.5808 | 90.99 | |||
| 0.9961 | 0.2976 | 0.3132 | 0.5936 | 94.49 |
HPLC-UV = high performance liquid chromatography-ultraviolet detection; RSD = relative standard deviation.
These RSD values indicate that the proposed methodology is reproducible and suitable for the quantitative determination of peimine and peiminine in FTB samples.
3.4. Quantitative determination of peimine and peiminine in FTB samples
The developed HPLC-UV analytical method was applied for the quantitative determination of peimine and peiminine in 10 FTB samples. The calibration curves were used to calculate the contents of the two compounds in the samples (data are shown in Table 4). Firstly, the contents of peimine and peiminine in different samples varied from 0.0135% to 0.1331% and from 0.0321% to 0.0605%, respectively. FTB-09 was found to have the highest content of peimine at 0.1331%; meanwhile, FTB-06 had the highest content of peiminine at 0.0605%. The lowest content of peimine and peiminine were both found in FTB-12 at 0.0135% and 0.0321, respectively. Secondly, the highest total content of peimine plus peiminine was found in FTB-09 at 0.1934%, however, FTB-12 gave the lowest one at 0.0456%. Thirdly, according to the specification in Chinese Pharmacopeia (2010 edition) that the total content of peimine and peiminine should be not less than 0.080%, FTB-12 was an unqualified herb that could not be used clinically.
Table 4.
The contents of peimine and peiminine in the FTB samples.
| Sample no. | Content (%)a | ||
|---|---|---|---|
|
| |||
| Peimine | Peiminine | Total | |
| FTB-01 | 0.0550 | 0.0331 | 0.0881 |
| FTB-02 | 0.0781 | 0.0506 | 0.1287 |
| FTB-03 | 0.0685 | 0.0492 | 0.1177 |
| FTB-04 | 0.0576 | 0.0401 | 0.0977 |
| FTB-05 | 0.0589 | 0.0451 | 0.1040 |
| FTB-06 | 0.0833 | 0.0605 | 0.1438 |
| FTB-07 | 0.0669 | 0.0509 | 0.1178 |
| FTB-08 | 0.0663 | 0.0418 | 0.1081 |
| FTB-09 | 0.1331 | 0.0603 | 0.1934 |
| FTB-10 | 0.0674 | 0.0466 | 0.1140 |
| FTB-11 | 0.0688 | 0.0492 | 0.1179 |
| FTB-12 | 0.0135 | 0.0321 | 0.0456 |
Calculated with dried Fritillariae Thunbergii Bulbus (FTB) samples.
3.5. HCA
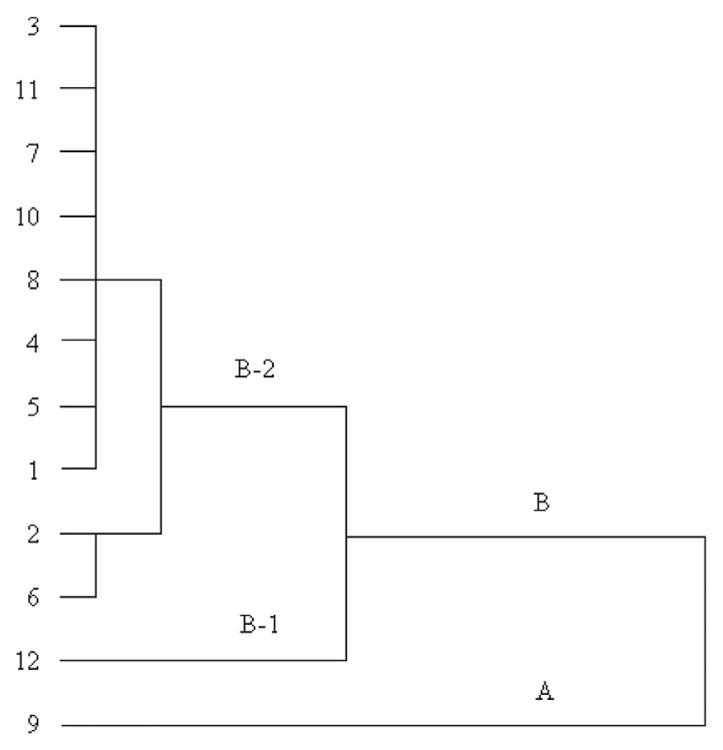
HCA is a statistical method for finding relatively homogeneous clusters of cases based on measured characteristics [8,9]. The contents of peimine and peiminine were defined as the variables in the analysis so as to analyze, differentiate, and classify the 12 batches of FTB samples. Ward’s method, which is a very efficient method for the analysis of variance between clusters, was applied, and Square Euclidean distance was selected as a measurement. A dendrogram was generated (Fig. 2), which revealed the relationships between the samples. The 12 tested samples of FTB were divided into two main clusters, cluster A and cluster B. Sample no. 9 was in cluster A and other samples were in cluster B, which was then divided into two subgroups. Sample no. 12 was in group B-1, and other samples were in group B-2. The results indicated the similarities and differences between the samples. Sample no. 9 had the highest content of peimine at 0.1331% and the second highest content of peiminine at 0.0603%—this is why it locates far away from other samples. Sample no. 12 had the lowest contents of the two analytes at 0.0135% and 0.0321%, respectively, explaining why it was separated from others in group B.
Fig. 2.
Dendrogram of HCA for the 12 batches of FTB samples. The 12 tested samples of FTB were divided into two main clusters, cluster A and cluster B. HCA was carried out using SPSS 13.0 software. Ward’s method was applied, and Squared Euclidean distance was selected as a measurement. FTB = Fritillariae Thunbergii Bulbus; HCA = hierarchical clustering analysis.
All in all, from the dendrogram, we could easily and intuitively see the similarities and differences of the tested FTB samples.
4. Conclusion
An HPLC-UV method was developed and validated after detailed investigation on extraction of chemical compounds from FTB using different solvents and methods. The method was validated to be accurate and reliable with good repeatability. It was then applied to analyze the two chemical markers in different FTB samples. The contents of peimine and peiminine were then used as variables to perform HCA. From the dendrogram obtained, we could easily see the similarities and differences of the samples. Because the ELSD detector has many disadvantages, including expensiveness, poor repeatability, and poor stability of the data, the method established in the present study using a UV detector is very valuable for routine analyses.
Acknowledgments
We like to thank Department of Chinese Medicine and Pharmacy, Ministry of Welfare and Health, Taiwan for the grants support (CCMP100-RD-106; CCMP101-CP-11; CCMP102-RD-001)
Funding Statement
We like to thank Department of Chinese Medicine and Pharmacy, Ministry of Welfare and Health, Taiwan for the grants support (CCMP100-RD-106; CCMP101-CP-11; CCMP102-RD-001)
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1.State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. 2010 ed. Beijing: Chemical Industry Press; 2010. [Google Scholar]
- 2.Committee of Chinese Medicine and Pharmacy, Department of Health. Taiwan herbal pharmacopeia. 2nd ed. Taipei: Department of Health, Executive Yuan of Republic of China Press; 2013. [Google Scholar]
- 3. Lin MB, Wan LL, Zhou ZY. Protection effect of Fritillaria thunbergii against hyperthyroidism in rats and mice. Zhongguo Yao Fang. 2010;21:1362–3. [Google Scholar]
- 4. Xia JX, Han L, Zhou XH, Wei B. The effect of Zhejiang Fritillaria thunbergii against immunological CP/CPPS. Zhonghua Zhong Yi Yao Xue Kan. 2011;29:1023–5. [Google Scholar]
- 5. Li ZH, An C, Hu KW, Zhou KH, Duan HH, Tang MK. Multidrug resistance reversal activity of total alkaloids from Fritillaria thunbergii on cisplatin-resistant human lung adenocarcinoma A549/DDP cells. Zhongguo Yao Li Xue Yu Du Li Xue Za Zhi. 2013;27:315–20. [Google Scholar]
- 6.Ou M. Chinese-English manual of common-used in traditional chinese medicine. Guangdong: Guangdong Science and Technology; 1992. [Google Scholar]
- 7. Tang XY, Tang YX. Efficiency and mechanisms of peimine reversing multi-drug resistance of A549/DDP cell line. Shandong Yi Yao. 2012;52:4–6. [Google Scholar]
- 8. Dong WW, Au D, Cao XW, Li XB, Yang DJ. Discriminating Astragali Radix from its adulterants using HPLC coupled with chemometric clustering techniques. J Food Drug Anal. 2011;19:495–501. [Google Scholar]
- 9. Dong WW, Zeng ZD, Au D, Chan CO, Yang DJ, Li XB. Quality control of Colla corii asini using near-infrared spectroscopy and chemometrics clustering techniques. J Food Drug Anal. 2012;20:152–8. [Google Scholar]