Abstract
Safflower (Carthamus tinctorius L.) is a traditional medicinal and edible herb with a long history of use in China. In this study, a model of hepatotoxicity induced by carbon tetrachloride (CCl4) in mice was used to investigate the hepatoprotective effects of kaempferol 3-O-rutinoside (K-3-R) and kaempferol 3-O-glucoside (K-3-G), two kaempferol glycosides isolated from C. tinctorius L. K-3-R and K-3-G, at doses of 200 mg/kg and 400 mg/kg, were given orally to male mice once/d for 7 days before they received CCl4 intraperitoneally. Our results showed that K-3-R and K-3-G treatment increased the level of total protein (TP) and prevented the CCl4-induced increases in serum aspartate aminotransferase (AST), serum alkaline phosphatase (ALP), and hepatic malondialdehyde (MDA) levels. Additionally, mice treated with K-3-R and K-3-G had significantly restored glutathione (GSH) levels and showed normal catalase (CAT) and superoxide dismutase (SOD) activities, compared to CCl4-treated mice. K-3-R and K-3-G also mitigated the CCl4-induced liver histological alteration, as indicated by histopathological evaluation. These findings demonstrate that K-3-R and K-3-G have protective effects against acute CCl4-induced oxidative liver damage.
Keywords: Carthamus tinctorius L., carbon tetrachloride, hepatoprotective effects, kaempferol glycosides
1. Introduction
The liver is the first organ to metabolize all foreign compounds and hence it is susceptible to injury that can result in different diseases such as hepatitis, cirrhosis, or hepatocellular carcinoma. A major cause of these disorders is exposure to different environmental pollutants and chemicals, e.g., paracetamol, carbon tetrachloride (CCl4), thioacetamide, alcohol, etc. [1]. CCl4 is a xenobiotic that produces hepatotoxicity in humans, as well as in various experimental animals [2]. It has been well established that CCl4 accumulates in hepatic parenchyma cells and is metabolically activated by cytochrome P450-dependent monooxygenases to form highly reactive radicals, mainly the trichloromethyl radical (CCl3*). These radicals bind covalently to cell components, inhibit the lipoprotein secretion, and react with free oxygen to form CCl3O2* radicals, thereby resulting in protein and DNA damage and lipid peroxidation [3]. Oxidative stresses play a central role in the pathology and progression of liver toxicity. Conventional drugs used in pharmacotherapy, such as steroids, vaccines, and antiviral drugs, have shown limited therapeutic benefits and are associated with serious risks of toxicity, especially if administered chronically or subchronically [4]. As an alternative, herbal medicines and their active compounds have attracted great attention as potential functional ingredients to protect liver injuries, due to their mild actions and fewer adverse effects.
Safflower (Carthamus tinctorius L.), a flowering annual plant of the family Asteraceae widely grown in the world, has been traditionally used, especially in oriental countries, to prepare herbal medicines, food colorings, cosmetics, and even for dyeing fabrics and in paintings [5]. As a traditional Chinese medicine, safflower has been used in combination with Salvia and Chuanxiong to soothe the liver and relieve jaundice for the treatment of old and new liver disorders [6]. Further studies revealed that the plant possessed powerful antioxidant and hepatoprotective effects against CCl4-induced liver injury [7–9], but research has tended to focus on its mixed extracts and Hydroxysafflor yellow A.
Kaempferol, the most common flavonol present in different glycosidic forms in several plants including C. tinctorius L., has been chosen together with Hydroxysafflor yellow A as the phytochemical marker and legal standard component for controlling the quality of safflower in Chinese Pharmacopoeia 2010 [10] in China. In our study, two kaempferol glycosides were isolated from safflower flowers: kaempferol 3-O-rutinoside (K-3-R) and kaempferol 3-O-glucoside (K-3-G). Previous studies have shown that K-3-R isolated from safflower had protective effects against cerebral ischemic damage and multi-infarct dementia [11,12]. In addition, it was reported that K-3-G possessed hepatoprotective activity on tacrine-induced cytotoxicity in human liver-derived HepG2 cells [13]. However, to our best knowledge, no in vivo studies regarding the hepatoprotective activity of these two single compounds (K-3-R and K-3-G) have been conducted. Considering the fact that flavonoids have significant antioxidant activity [14], and that one of the main constituents of the species are kaempferol glycosides, the present study was focused on investigating the functional roles of K-3-R and K-3-G from safflower against CCl4-induced oxidative hepatic injury and providing evidence for the possibility of using K-3-R and K-3-G as a source of natural drugs for the treatment of liver disorders.
2. Materials and methods
2.1. Plant material
Flowers of safflower (C. tinctorius L.) were harvested at Jianyang County (104.64°E, 30.43°N), Sichuan Province of China in June 2011 and supplied by Sichuan Academy of Agricultural Science, Jianyang, China. The reference specimens were deposited at the Department of Pharmacognosy, West China School of Pharmacy, Sichuan University, Chengdu, China.
2.2. Extraction and isolation
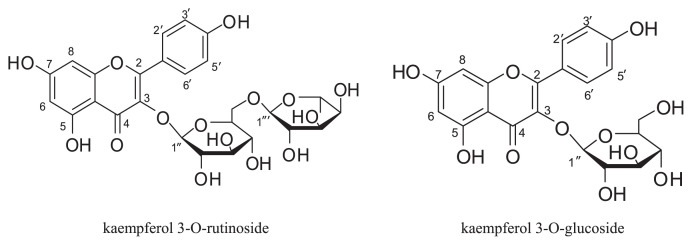
Air dried and grounded flowers of safflower (2000 g) were extracted with 75% Ethanol (EtOH) (v/v) at room temperature. After evaporating EtOH under reduced pressure (< 40°C), the residue was dissolved in H2O and then subjected to a D101 macroporous resin column previously equilibrated with water. After the column had been rinsed with H2O (discarded), fractions were collected by increasing the EtOH content of the eluent (20~80%, v/v, successively). The dried residue of 50% EtOH-eluted fraction was dissolved in H2O and applied to a polyamide column eluting with 10~80% EtOH. Collected fractions were combined based on their Thin Layer Chromatography (TLC) profiles and were concentrated at reduced pressure. After EtOH evaporation of the 10% EtOH-eluted fraction, a precipitate developed consisting mainly of K-3-R. Impurities were removed by recrystallization of the precipitate with EtOH, and 3.5 g of the pure K-3-R was obtained. The 50% EtOH-eluted fraction was further purified on a Sephadex LH-20 (Pharmacia Biotech, Uppsala, Sweden) column using 50% Methanol (MeOH) as the mobile phase to yield 2.4 g of K-3-G. The structures of the isolated compounds were elucidated by spectroscopic [mass spectrometry (MS) and 1H-nuclear magnetic resonance (1H-NMR)] data by comparison with published data [15,16] (Fig. 1).
Fig. 1.
Chemical structure of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside.
K-3-R: ESI-MS m/z: 595.42 [M + H]. 1H-NMR (CD3OD, 600 MHz): δ: 8.06 (2H, d, J = 9.6 Hz, H-2′, 6′), 6.89 (2H, d, J = 9.6 Hz, H-3′, 5′), 6.41 (1H, d, J = 1.8 Hz, H-6), 6.21 (1H, d, J = 1.8 Hz, H-8), 5.13 (1H, d, J = 7.2 Hz, glucose H-1′), 4.51(1H, d, J = 1.2 Hz, rhamnose H-1″), and 1.11 (3H, d, J = 6.0 Hz, rham-nose 5 CH3). 13C-NMR (CD3OD, 150 MHz): δ: 16.5 (C-5‴), 76.8(C-1″), 75.8(C-2″), 74.3(C-3″), 72.5(C-4″), 70.9(C-6″), 70.7(C-1‴), 70.0(C-2‴), 68.3(C-3‴), and 67.1(C-4‴).
K-3-G: ESI-MS m/z: 449.22 [M + H]. 1H-NMR (DMSO-d6, 600 MHz): δ: 12.62 (br. s, 1H, C5-OH), 10.30 (br. s, 1H, C7-OH), 8.06 (2H, d, J = 9.6 Hz, H-2′, 6′), 6.89 (2H, d, J = 9.6 Hz, H-3′, 5′), 6.43 (1H, d, J = 1.2 Hz, H-6), 6.20 (1H, d, J = 1.2 Hz, H-8), and 5.46 (1H, d, J = 7.2, glucose H-1′).
2.3. Animals and treatments
Male Kunming mice (20–25 g) were obtained from the Experimental Animal Center of Sichuan University. The animals were housed at 25 ± 2°C under a 12 hour light/12 hour dark cycle with access to food and water ad libitum. All the animal experimental procedures are approved by the Animal Care and Use Committee of Sichuan University for Nationalities.
The CCl4-induced oxidative toxicity test was performed according to the previous study with some modifications [17]. After acclimation for 1 week, the animals were randomly divided into seven groups (n = 8). Group I (normal control) and Group II (CCl4 model) were given distilled water (10 mL/kg b.w.). Groups III and IV were administrated K-3-R (200 mg/kg and 400 mg/kg, b.w.). Groups V and VI were administrated K-3-G (200 mg/kg and 400 mg/kg, b.w.). Group VII was administrated biphenyldicarboxylate pills (BP, 100 mg/kg, b.w.). After the oral administration for 7 days, all mice except those in the control group were simultaneously given a CCl4-peanut oil mixture (1:100, intraperitoneally, 10 mL/kg b.w.) 2 hours after the last administration, while the control group received peanut oil alone. Then, all of the animals were fasted for 18 hours and sacrificed by cervical dislocation. Blood was collected, allowed to clot, and serum was separated for assessment of enzyme activity. Livers were dissected out immediately, and a portion was transferred into 10% formalin for histopathological investigation; the rest was properly stored at −80°C for pending tests.
2.4. Measurement of serum total protein, aspartate aminotransferase, and alkaline phosphatase levels
Collected blood samples were placed at 4°C for 2 hours and centrifuged at 2000 rpm for 10 minutes at 4°C to obtain the serum. The level of serum total protein (TP), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities were detected using commercial reagent kits obtained from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China) according to the instruction manuals.
2.5. Measurement of hepatic malondialdehyde, glutathione, superoxide dismutase, and catalase levels
Liver homogenates (10.0%, w/v) were prepared with 50mM cold potassium phosphate buffer (pH 7.4). The resulting suspension was centrifuged at 2000 rpm for 10 minutes, and the supernatants were collected for further analysis. All treatments were done at 4°C. Protein concentration and malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) levels were assayed using commercial reagent kits obtained from the Institute of Biological Engineering of Nanjing Jiancheng according to the instruction manuals.
2.6. Histopathological examination
Livers slices were fixed with 10% formalin in phosphate buffered saline for 24 hours and embedded in paraffin. Sections of 5 μm in thickness were cut, deparaffinized, dehydrated, and stained with hematoxylineosin (H&E) and studied under a microscope to observe histopathological changes in the liver. Photographs of each of the slides were taken at 100× magnification.
2.7. Statistical analysis
The data obtained were analyzed using SPSS version 19 (SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard deviation. The data were statistically analyzed by the one way analysis of variance test and p <0.05 was considered significant.
3. Results
3.1. Effects of K-3-R and K-3-G on serum biochemical marker levels
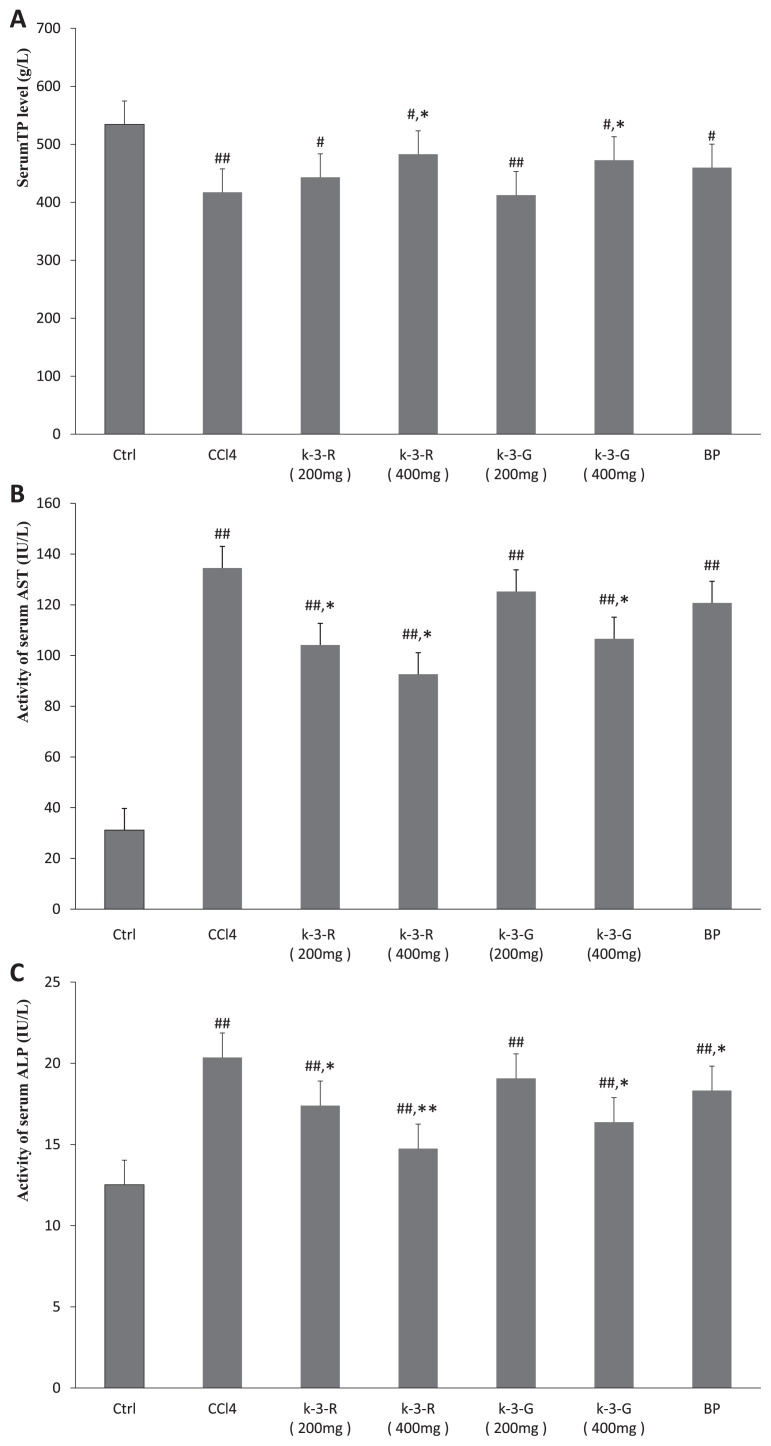
As shown in Fig. 2, enzymatic activities of serum AST and ALP in the normal mice were 31.18 U/L and 12.52 IU/L, respectively. After administration of CCl4, the levels of these biochemical markers in mice were markedly increased compared to the normal control group (p < 0.01). Treatment with K-3-R and K-3-G showed dose-dependent inhibition in this elevation of AST and ALP in comparison with the CCl4 model group (p < 0.01, p < 0.05), while BP treatment only significantly decreased the enzymatic activity of serum ALP (p < 0.05). The CCl4 model group had considerably lower TP levels than the normal group (p < 0.01); K-3-R and K-3-G treatments at 400 mg/kg resulted in a significant improvement in TP level (p < 0.05). However, low dose K-3-R, K-3-G, and BP treatments did not observably affect the TP level, although there was a slight increase. Hence, treatments with K-3-R and K-3-G (400 mg/kg) had significant protective effects against the acute hepatotoxicity induced by CCl4 in mice that were superior to the standard BP (100 mg/kg).
Fig. 2.
Effects of kaempferol 3-O-rutinoside (K-3-R) and kaempferol 3-O-glucoside (K-3-G) on (A) serum total protein (TP) level, (B) alkaline phosphatase (ALP) activity, and (C) aspartate aminotransferase (AST) activity. Data are expressed as mean ± SD (n = 6). ##p < 0.01, #p < 0.05, statistically significant relative to the normal control group. **p < 0.01, *p < 0.05, statistically significant relative to the carbon tetrachloride (CCl4) model group.
3.2. Effects of K-3-R and K-3-G on CCl4-induced oxidative stress
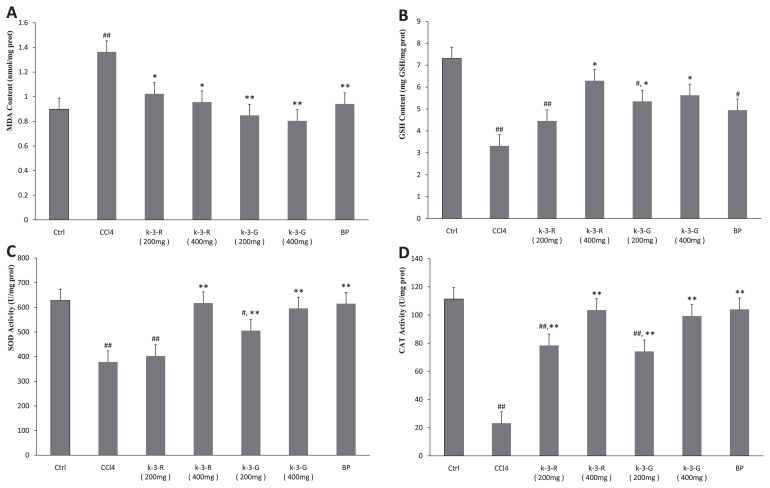
Fig. 3 depicts the effects of K-3-R and K-3-G on CCl4-induced oxidative stress. The level of MDA in the CCl4 model group was significantly elevated from 0.90 ± 0.14 nmol/mg protein of normal mice to 1.36 ± 0.33 nmol/mg protein (p < 0.01). Treatment with K-3-Rand K-3-G considerably lowered the level of the CCl4-elevated MDA (p < 0.05, 0.01). Intraperitoneal injection of CCl4 dramatically reduced the level of GSH in the liver of mice when compared with the normal group (p < 0.01). This decrease was significantly reversed through K-3-R and K-3-G treatments (p < 0.05, 0.01), especially when the dosage was increased to 400 mg/kg; there were no significant differences compared to the normal group in GSH level (p <0.05). In contrast with normal mice, the activities of the two hepatic antioxidant enzymes, SOD and CAT, were decreased significantly in mice treated with CCl4 alone (p < 0.01). However, administration of both K-3-R and K-3-G remarkably avoided these decreases in a dose-dependent manner (p < 0.05, 0.01), except for SOD in mice given 200 mg/kg K-3-R (p > 0.05). Treatment with BP at 100 mg/kg increased the levels of SOD and CAT activities and decreased MDA content significantly (p < 0.01).
Fig. 3.
Effects of kaempferol 3-O-rutinoside (K-3-R) and kaempferol 3-O-glucoside (K-3-G) on (A) hepatic malondialdehyde (MDA) content, (B) glutathione (GSH) content, (C) superoxide dismutase (SOD) activity, and (D) catalase (CAT) activity. Data are presented as mean ± SD (n = 7). ##p < 0.01, #p < 0.05, statistically significant relative to the normal control group. **p < 0.01, *p < 0.05 statistically significant relative to the carbon tetrachloride (CCl4) model group.
3.3. Histological analyses
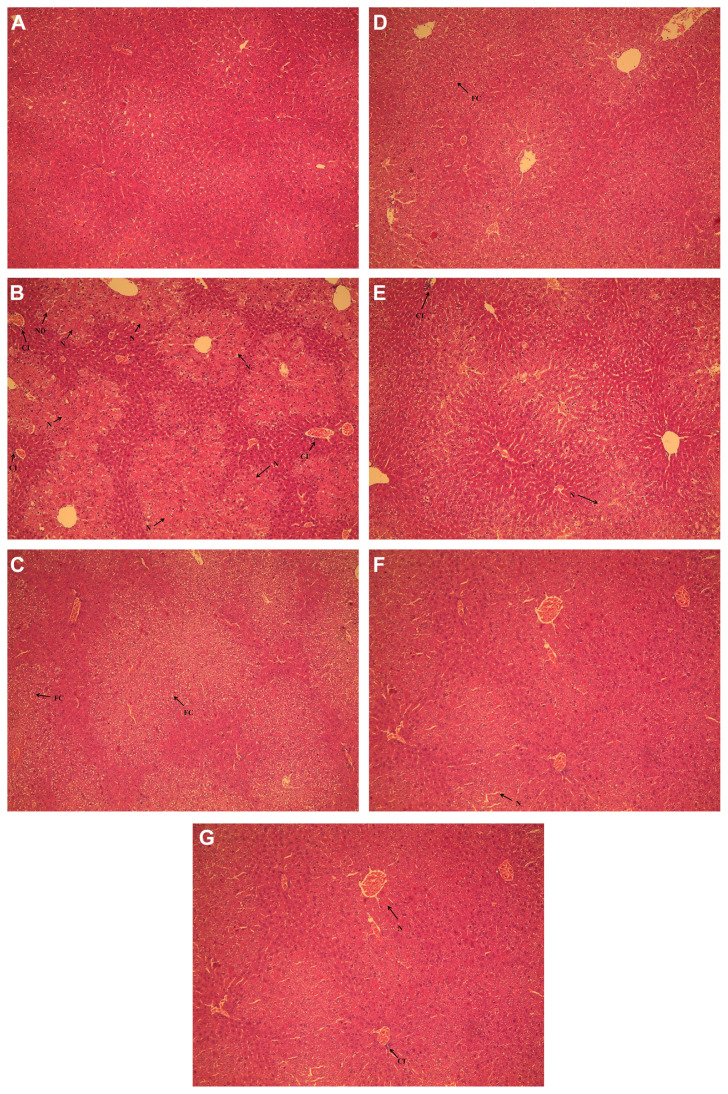
To assess histological changes, H&E staining of liver tissue sections from each group was examined. When compared with the normal group, liver tissue in the CCl4 model group revealed the most severe damage of all the groups; the liver sections showed hydropic degeneration, fatty changes, a mild degree of cellular infiltration, and widespread hepatocellular necrosis (Fig. 4B). The groups treated with K-3-R (Fig. 4C and D) and K-3-G (Fig. 4E and F) exhibited a more or less improvement in the liver histopathology against CCl4-induced histological alteration, supporting the results obtained from serum and hepatic biochemical markers.
Fig. 4.
Effects of kaempferol 3-O-rutinoside (K-3-R) and kaempferol 3-O-glucoside (K-3-G) on liver histopathology of mice using hematoxylin-eosin (H&E) staining (original magnification of 100×). (A) Normal control group; (B) carbon tetrachloride (CCl4) model group; (C) K-3-R (400 mg/kg) + CCl4 group; (D) K-3-R (200 mg/kg) + CCl4 group; (E) K-3-G (400 mg/kg) + CCl4 group; (F) K-3-G (200 mg/kg) + CCl4 group; and (G) biphenyldicarboxylate (BP) (100 mg/kg) + CCl4 group. CI = cellular infiltration; FC = fatty changes; HD = hydropic degeneration; N = hepatocellular necrosis.
4. Discussion
CCl4 intoxication is a commonly used and best characterized animal model of liver injury, since the pathological lesions developed in CCl4-treated animals closely resemble the symptoms of cirrhosis in human [18]. Once the liver is exposed to CCl4, AST which is normally localized to the cytoplasm is released into the circulation, and causes the level of these serum marker enzymes to increase significantly. Membrane disintegration of hepatocytes with subsequent release of marker enzymes of hepatotoxicity was the result of CCl4-induced lipid peroxidation. Loss of cell viability is most often measured as loss of membrane integrity [19]. In addition, ALP is excreted normally in bile. Hepatic injury induced by CCl4 could result in defective excretion of bile by hepatocytes and so ALP levels could reflect a pathological alteration in biliary flow [20]. Treatments with both K-3-R and K-3-G significantly prevented CCl4-induced elevation of AST and ALP, implying that these two kaempferol glycosides can increase stabilization of the plasma membrane and ameliorate biliary dysfunction effectively, thereby repairing hepatic tissue damage caused by CCl4. In the present study, TP level in the serum was also measured. Albumin and globulin are the main components of TP in the plasma and serum albumin is mainly synthesized by the liver. In drug-induced hepatotoxicity, albumin synthesis will be depleted due to cirrhosis and this will lead to a reduction in TP [21]. CCl4 expectedly reduced serum TP, while treatment with K-3-R and K-3-G restored the protein content well, especially at a high dose of 400 mg/kg, which may reflect liver disease, nutritional state, proteins synthesis state, and others.
Lipid peroxide is a primary parameter which can be considered as a marker of oxidative injury, and hepatic MDA formation is commonly used as an indicator of liver tissue damage involving a series of chain reactions [22]. An increase in MDA levels in the liver suggested the enhancement of oxidative stress leading to tissue damage and failure of the antioxidant defense mechanisms to prevent the formation of excessive free radicals [23]. In the present study, the elevated hepatic MDA levels were significantly decreased by administration of K-3-R and K-3-G. Therefore, these results provided evidence which implied that K-3-R and K-3-G could provide protective effects against CCl4-induced liver damage in terms of preventing lipid peroxide formation and blocking oxidative chain reactions.
In order to resist the oxidative stress, the defense systems in the body possess a host of antioxidant systems, including the nonenzymatic system (such as GSH) and a series of anti-oxidant enzymes (such as SOD, CAT) which work in concert to control the cascades of uncontrolled oxidation and protect cells from oxidative damage by scavenging of overproduced reactive oxygen species (ROS) [24]. As a nonenzymatic anti-oxidant, GSH is an important regulator of intracellular redox homeostasis, which can reduce H2O2, hydroperoxides (ROOH), and xenobiotic toxicity. As enzymatic antioxidant systems, SOD and CAT constitute a mutually supportive defense against ROS to maintain cellular redox balance [25,26]. SOD can catalyze the dismutation of superoxide anions into hydrogen peroxide (H2O2) [27]. CAT can further catalyze the decomposition of H2O2 to H2O and O2 [28]. In our study, we observed that the levels of GSH, SOD, and CAT were significantly lower in CCl4-induced liver injury mice as compared with those of normal mice, representing severe oxidative stress status to hepatic cells. Treatment with K-3-R and K-3-G significantly restored GSH levels as well as SOD and CAT activities, suggesting that K-3-R and K-3-G have considerable antioxidant activities and suppressed CCl4-induced oxidative liver injury.
Finally, the hepatoprotective effects of K-3-R and K-3-G were confirmed by histological observations. When compared to the CCl4 model group, hepatocytes were found to be normalized, with minimum cellular necrosis, fatty changes, and cellular infiltration with 400 mg/kg of K-3-R and K-3-G treatments, thus suggesting that K-3-R and K-3-G were able to alleviate liver toxicity produced by CCl4 in mice.
In conclusion, the present study demonstrated the strong hepatoprotective effects of K-3-R and K-3-G, two kaempferol glycosides isolated from safflower flowers, in a CCl4-induced liver damage model, probably mediated via the reduction of oxidative stress and apoptotic cell death. These findings support the uses of this plant in ethnomedicine and suggest that K-3-R and K-3-G may be useful as natural sources to protect liver from oxidative damage.
Acknowledgments
This research was supported by the Research Foundation for the Sichuan Science and Technology Support Programme of China (2011NZ0098-12-01).
Funding Statement
This research was supported by the Research Foundation for the Sichuan Science and Technology Support Programme of China (2011NZ0098-12-01).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2. Rudnicki M, Silveira MM, Pereira TV, et al. Protective effects of Passiflora alata extract pretreatment on carbon tetrachloride induced oxidative damage in rats. Food Chem Toxicol. 2007;45:656–61. doi: 10.1016/j.fct.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 3. Sun F, Hamagawa E, Tsutsui C, Ono Y, Ogiri Y, Kojo S. Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim Biophys Acta. 2001;1535:186–91. doi: 10.1016/s0925-4439(00)00098-3. [DOI] [PubMed] [Google Scholar]
- 4. Jaishree V, Badami S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J Ethnopharmacol. 2010;130:103–6. doi: 10.1016/j.jep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 5. Clementi C, Basconi G, Pellegrino R, Romani A. Carthamus tinctorius L.: a photophysical study of the main coloured species for artwork diagnostic purposes. Dyes Pigments. 2014;103:127–37. [Google Scholar]
- 6. Zhou XD, Tang LY, Xu YL, Zhou G, Wang Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2014;151:27–43. doi: 10.1016/j.jep.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 7. Paramesha M, Ramesh CK, Krishna V, Ravi Kumar YS, Parvathi KM. Hepatoprotective and in vitro antioxidant effect of Carthamus tinctorious L., var Annigeri-2-, an oil-yielding crop, against CCl(4)-induced liver injury in rats. Pharmacogn Mag. 2011;7:289–97. doi: 10.4103/0973-1296.90406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu SC, Yue Y, Tian H, Li Z, Li X, He W, Ding H. Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl4-induced liver damage in rats via the Nrf2 pathway. J Ethnopharmacol. 2013;148:570–8. doi: 10.1016/j.jep.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 9. Zhang YB, Guo J, Dong HY, Zhao X, Zhou L, Li X, Liu J, Niu Y. Hydroxysafflor yellow A protects against chronic carbon tetrachloride-induced liver fibrosis. Eur J Pharmacol. 2011;660:438–44. doi: 10.1016/j.ejphar.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 10.The Pharmacopoeia Committee of China. Honghua Chinese Pharmacopoeia 2010. 1st ed. Beijing, China: China Medical Science and Technology Press; 2010. pp. 141–2. [Google Scholar]
- 11. Li R, Guo M, Zhang G, Xu X, Li Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J Ethnopharmacol. 2006;107:143–50. doi: 10.1016/j.jep.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 12. Huang JL, Fu ST, Jiang YY, Cao YB, Guo ML, Wang Y, Xu Z. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol Biochem Behav. 2007;86:741–8. doi: 10.1016/j.pbb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13. Kim SM, Kang K, Jho EH, Jung YJ, Nho CW, Um BH, Pan CH. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tertbutyl hyperoxide. Phytother Res. 2011;25:1011–7. doi: 10.1002/ptr.3387. [DOI] [PubMed] [Google Scholar]
- 14. Hu LH, Li LR, Xu DM, Xia X, Pi R, Xu D, Wang W, Du H, Song E, Song Y. Protective effects of neohesperidin dihydrochalcone against carbon tetrachloride-induced oxidative damage in vivo and in vitro. Chem Biol Interact. 2014;213:51–9. doi: 10.1016/j.cbi.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15. Li F, He ZS, Ye Y. Flavonoids from Carthamus tinctorius. Chinese J Chem. 2000;20:699–702. [Google Scholar]
- 16. Kazunma K, Tkahashi T, Sato K, Takeuchi H, Matsumoto T, Okuno T. Quinochalcones and flavonoids from fresh florets in different cultivars of Carthamus tinetorius L. Biosci Biotech Bioch. 2002;64:1588–99. doi: 10.1271/bbb.64.1588. [DOI] [PubMed] [Google Scholar]
- 17. Dong DS, Zhang S, Yin LH, Tang X, Xu Y, Han X, Qi Y, Peng J. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol. 2013;62:120–30. doi: 10.1016/j.fct.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 18. Lin YC, Cheng KM, Huang HY. Hepatoprotective activity of Chhit-Chan-Than extract powder against carbon tetrachloride induced liver injury in rats. J Food Drug Anal. 2014;22:220–9. [Google Scholar]
- 19. Naik SR, Panda VS. Hepatoprotective effect of Ginkgo select Phytosome in rifampicin induced liver injury in rats: evidence of antioxidant activity. Fitoterapia. 2008;79:439. doi: 10.1016/j.fitote.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 20. Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother. 2012;3:149–55. doi: 10.4103/0976-500X.95515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravichandrab VD, Ramesha C, Sridhar KA. Hepatoprotective potentials of aqueous extract of Convolvulus pluricaulis against thioacetamide induced liver damage in rats. Biomed Aging Pathol. 2013;3:131–5. [Google Scholar]
- 22. Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66:2583–91. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 23. Dreissgacker U, Suchy MT, Maassen N, Tsikas D. Human plasma concentrations of malondialdehyde (MDA) and the F2-isoprostane 15(S)-8-iso-PGF2a may be markedly compromised by hemolysis: evidence by GC-MS/MS and potential analytical and biological ramifications. Clin Biochem. 2010;43:159–67. doi: 10.1016/j.clinbiochem.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26. Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27. Chen KN, Peng WH, Hou CW, Chen CY, Chen HH, Kuo CH, Korivi M. Codonopsis javanica root extracts attenuate hyperinsulinemia and lipid peroxidation in fructose-fed insulin resistant rats. J Food Drug Anal. 2013;21:347–55. [Google Scholar]
- 28. Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]