Abstract
Flavor is the most important aspect of food. Based on the complex matrix of the food system and the flavor structure themselves, one important factor that plays a key role in the quality attribute of food is flavor stability. Not surprisingly, there is a large volume of published research investigating the stability of different food flavor compounds, since understanding flavor stability is crucial to creating greater awareness of dietary flavor application. This review presents a variety of factors that are thought to be involved in the stability of several selected important flavor compounds and the approach to improve the stability of different flavors. Some mechanisms of chemical degradation of flavor compounds were also provided.
Keywords: chemical stability, flavor-matrix interaction, food flavor, mechanism
1. Introduction
The most important parameter that maximizes food quality and global competitiveness is flavor, and increasing attention is being given to the stability of flavor. The list of known flavoring agents, used as food additives, includes thousands of molecular compounds, both synthetic and natural, and flavorists can mix these together to produce many of the common flavors. The quality attribute of food aroma is influenced by three factors: (1) chemical reactivity of food flavor; (2) environment of food such as availability of light and atmospheric oxygen; and (3) food matrix system and its constituents such as protein, fat, carbohydrate, transition metal, radical, and other polymers in food such as brown melanoidins formed during thermal processing of food. Among the many factors related to flavor quality, flavor stability is the most important one. The chemical structure of individual flavor compounds is associated with the chemical reaction that is responsible for its stability. The presence of active functional groups, such as carbonyl, hydroxyl, and thiol functional groups, affects the chemical reactivity of these compounds. Both high- and low-volatility flavor compounds, regardless of whether they are neutral, acidic, or nitrogen-and sulfur-containing compounds, can be susceptible to chemical changes occurring in various kinds of interactions, including oxidation, hydrolysis, thermal degradation, photo-oxidation, polymerization of unsaturated compounds, and interaction with protein in food systems. For instance, aldehyde can be readily oxidized to acid, amine can form a complex with metal ions, and terpenes are capable of undergoing rearrangement and isomerization under acidic condition. These vulnerable consequences have impacts on the overall flavor quality of food [1].
Because the perception of flavor involves integration of several flavor compounds and not a single compound and a variety of factors affect the stability of flavor compounds, it is not easy to study the chemical stability of individual flavor compounds. In the literature, flavor stability studies have generally focused on the whole food, and not on a single molecular flavor compound. As demonstrated by several studies available in the literature, several mechanisms have been recognized to contribute to flavor reactivity, and later to stability [1]. An understanding of the structure and reactivity of a particular flavor compound is important for understanding its stability, and this will assist in creating greater awareness of dietary flavor application.
To-date, there is no article summarizing the stability of flavor compounds, which would be important information and an important quality criterion for flavor application in food. Hence, our interest in this review was to focus on the chemical reactivity of 10 common food flavor compounds including citral, allyl isothiocyanate (AITC), methional, furaneol, homofuraneol, norfuraneol, 2-methyl-3-furanthiol (MFT), furfurylthiol, 2-acetyl-1-pyrroline (2-AP), and vanillin (see Fig. 1 for structures). Some mechanisms proposed in various studies have also been discussed.
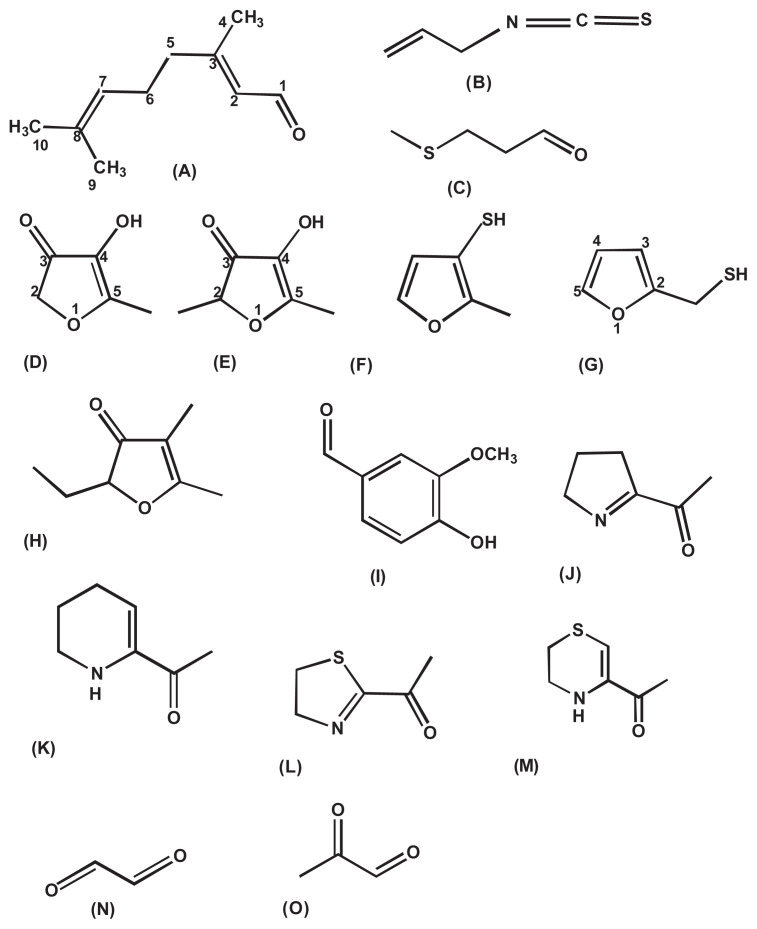
Fig. 1.
Structure of selected reactive flavor compounds: (A) citral, (B) allyl isothiocyanate, (C) methional, (D) norfuraneol, (E) furaneol, (F) 2-methyl-3-furanthiol, (G) 2-furfurylthiol, (H) homofuraneol/ethyl furanol, (I) vanillin, (J) 2-acetyl-1-pyrroline, (K) 6-acetyl-1,2,3,4-tetrahydropyridine, (L) 2-acetyl-2-thiazoline, (M) 5-acetyl-2, 3-dihydro-4H-1, 4-thiazine, (N) glyoxal, and (O) methylglyoxal.
2. Stability of selected flavor compounds
2.1. Citral
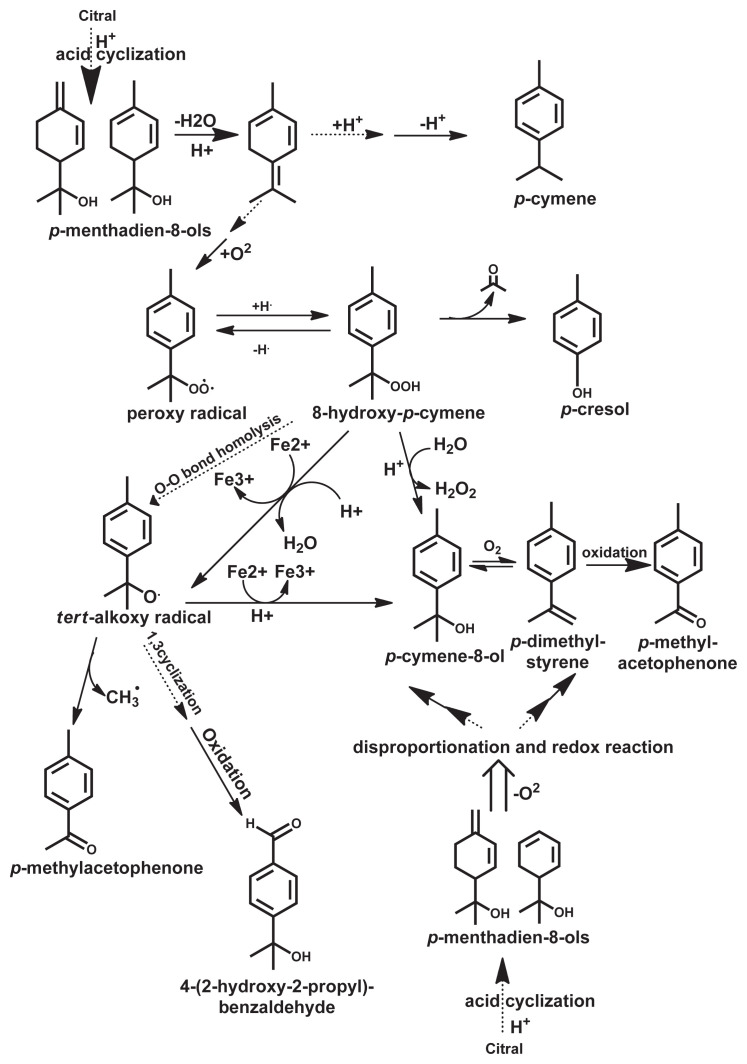
Citral, 3,7-dimethyl-2,6-octadienal, is the most important flavor compounds in citrus oils, and the structure is shown in Fig. 1. This monoterpene, naturally found in oils such as lemongrass (Cymbopogon citratus) and Litsea cubeba Pers, consists of two geometrical isomers, neral (E-isomer) and geranial (Z-isomer), in a ratio of about 1:2 or 3:2, in which the E-isomer is more stable than the Z-isomer [2–7]. Because citral is an α,β-unsaturated aldehyde, it is highly susceptible to acid-catalyzed cyclization and oxidative degradation, particularly in the presence of light and heat, leading to off-flavor formation, particularly in lime and citrus juice products [2,3,5,8]. The degradation process of citral under acidic conditions is accelerated by high temperature, light, and availability of oxygen [2,9]. Based on the degradative chemical reaction, Hideki [5] divided the degradation products of citral into three groups: acid-catalyzed cyclization products, photochemical cyclization products, and oxidation products. After citral cyclization, diol dehydration reaction takes place and the major intermediate compounds are monoterpene alcohols, particularly p-mentha-1,5-dien-8-ol and p-mentha-1,2-dien-8-ol [4,6,10]. Both unstable monoterpene alcohols can be deteriorated with disproportionation and redox reactions under acidic condition, and more stable aromatic compounds (p-cymene, p-cymene-8-ols, and α-p-dimethylstyrene) can be obtained later in the presence or absence of oxygen [2,4,6]. However, p-cymene-8-ols can undergo a dehydration reaction and transform to aroma compounds, α,p-dimethylstyrene, p-cymene, p-methylacetophenone, and p-cresol, in which the last two compounds are the most potent off-flavor compounds [2,4,6,8,11]. In the presence of oxygen, α,p-dimethylstyrene can be further oxidized to p-methylacetophenone [11].
Ueno et al [11] found that 4-(2-hydroxy-2-propyl)benzaldehyde is one of the oxidation products of citral, leading to off-flavor, in addition to α,p-dimethylstyrene, p-cymene, p-methylacetophenone, and p-cresol. They also pointed out that p-methylacetophenone and 4-(2-hydroxy-2-propyl)benzaldehyde can be formed from not only the O–O homolysis of 8-hydroperoxy-p-cymene by tert-alkoxy radical [p-CH3C6H4C(CH3)2O•] but also the Fe2+-induced decomposition of 8-hydroperoxy-p-cymene through the same radical intermediate. Using aroma extraction dilution assay, Schieberle et al [12] found that p-methylacetophenone as well as p-cresol were more potent off-flavor compounds in citral degradation than α-p-dimethylstyrene [13]. The proposed mechanisms of citral degradation through acid-catalyzed cyclization and oxidation described in previous studies are summarized in Fig. 2 [4,6,9,10,12].
Fig. 2.
Possible pathway of citral decomposition.
A considerable number of studies have shown that various antioxidants inhibit and slow the unstable chemical reactivity of citral. Kimura et al [4] showed that the antioxidants BHT, BHA, n-propyl gallate, α-tocopherol, nordihydroguaiaretic acid, and n-tritriacontane, had no effect on the formation of undesirable flavor compounds from citral degradation, which were p-cymene-8-ols, α-p-dimethylstyrene, p-cymene, p-methylacetophenone, and p-cresol. By contrast, isoascorbic acid in a carbonated system was found to inhibit the formation of citral oxidation products p-cymene-8-ols and α,p-dimethylstyrene [9]. Using plant extracts including grape seed, pomegranate seed, green tea, and black tea, Liang et al [2] revealed their inhibitory effects on citral off-odor formation. The study found that all four types of plant extracts dramatically decreased p-methylacetophenone, p-cresol, and 8-hydroxy-p-cymene formation, leading to the conclusion that the oxygen-scavenging effect of phenolic compounds in plant extracts, regardless of their water-soluble attributes, blocked the pathway from p-cymene-8-ol to p-methylacetophenone, the key undesirable off-flavor compounds in citral degradation [2]. Ueno et al [11] investigated the effect of the antioxidant activity of pure compounds including catechin, quercitrin (quercitrin 3-O-rhamnoside), and ascorbic acid on the formation pathways of citral oxidation products. The results indicated that catechin exhibited a stronger inhibitory effect on the formation of p-methylacetophenone and 4-(2-hydroxy-2-propyl)benzaldehyde than quercitrin and ascorbic acid. This finding came to the conclusion that the competition reaction between H donation at C-ring and phenoxy–peroxy coupling reactions at B-rings of catechin to peroxy radical promoted the formation of 8-hydroxy-p-cymene and p-cymene-8-ol, respectively, and led to inhibit the formation of 4-(2-hydroxy-2-propyl)benzaldehyde and p-methylacetophenone. Recently, Yang et al [14] systematically investigated the effects of six different natural antioxidants on the stability of citral in oil-in-water nanoemulsions, and found that β-carotene, tanshinone, and black tea extract could greatly enhance citral’s chemical stability during storage as well as inhibit some of the potent off-flavor compounds.
However, application of the abovementioned antioxidants in the food industry is limited due to their own unique taste profiles, intense colors, and cost ineffectiveness since they are not commercially available. Therefore, the research on available or commercial antioxidants that can effectively inhibit citral degradation and off-flavor formation is one of the topics that researchers pay attention to. Ubiquinone (2,3-dimethoxy-5-methyl-6-multiprenyl-1,4-benzoquinone or Q10), widely known as coenzyme Q, is getting attention recently, as it is an important antioxidant especially in biological system and is now a commercialized nutraceutical used in many dietary supplements available in the market. Zhao et al [15] elucidated the effect of ubiquinol (Q10H2), the fully reduced form, as an antioxidant in the oil-in-water nanoemulsion system to protect citral from chemical degradation and off-flavor generation, and found that the optimum concentration of Q10H2 in the formulation was around 0.10 wt% in the system (Q10H2/citral ratio 1:1), which can effectively protect citral from chemical degradation and oxidation. Lower and higher Q10H2 concentrations gave contrast results. They also concluded that ubiquinone-10, the fully oxidized form, had a negligible effect on citral’s chemical stability and off-flavor generation [15].
Since citral is widely used as an additive in food, beverage, perfumery, and pharmaceutical industries in the form of an oil-in-water emulsion, several studies have focused on the development of strategies to prevent or retard chemical degradation of citral. Major methods were investigated, including spray-dry encapsulation [16], oil-in-water emulsion/nanoemulsion systems [17], engineering of the interface of emulsion droplets with different emulsifiers [8], multilayer coatings [13,18], surface charges [19], use of solid lipids as the oil phase [20], and use of micelles and reverse micelles to entrap citral in the oil phase [19]. Encapsulation of citral in the form of an emulsion or micelles is a technique used to increase its stability, since it can isolate citral from reactive molecules in an aqueous phase, such as protons, metals, and free radicals. Djordjevic et al [8] compared the effect of two emulsifiers, whey protein isolate and gum arabic to stabilize oil-in-water emulsion, on the oxidation of citral and concluded that whey protein isolate was more effective than gum arabic. In the oil-in-water emulsion system, they also found that slowing of the formation of citral degradation product, p-cymene, was much more effective in sodium dodecyl sulfate–chitosan-stabilized emulsion than in gum arabic-stabilized emulsion. This inhibition of citral oxidative degradation can be attributed to the formation of a cationic and thick emulsion droplet interface [13]. Citral molecules in oil droplets show greater stability against chemical degradation than those in the aqueous phase, since they are protected by the emulsifier surrounding them [21].
In an acidic aqueous solution where citral degradation is favored, increasing citral partition in the oil phase decreases the rate of citral degradation. In a nonionic surfactant emulsion, triacetin (glycerol triacetate) or medium-chain triglyceride in the aqueous phase (the flavor solvent used in food industries) increases the stability of citral. Medium-chain triglyceride presents as an oil droplet incorporating citral within the hydrophobic internal region (medium-chain triglyceride oil droplet) and is therefore protected from the aqueous acidic environment, while triacetin presents as a microemulsion that causes citral to be incorporated within the nonpolar regions of the triacetin/nonionic surfactant structure, leading to stabilization of citral against acid-catalyzed degradation [17]. The result here suggests that either triacetin or nonionic surfactant retards citral degradation by interfering directly with the chemical reaction, and interfacial properties, especially surface charge of surfactant, play an important role in citral stability in an emulsion system. Based on the pH condition, the rate of citral degradation was faster in anionic surfactant-stabilized emulsions than in cationic or nonionic surfactant-stabilized emulsions at pH 3.5 [19].
The above results indicate that a cationic emulsion system is favored to protect citral from degradation. The explanation is that the positive charge of the interfacial layer can repel reactive species such as protons, metal ions, and free radicals away from emulsion droplets, inducing citral degradation. Recently, multilayer emulsion systems have been proposed to improve citral stability [18]. Multilayer emulsions are prepared by the layer-by-layer deposition technique based on the electrostatic interaction between negatively charged emulsion droplets, the primary emulsions, and positively charged biopolymers such as proteins and polysaccharides, secondary emulsions. The stability of citral could be improved based on the secondary emulsifier used. Yang et al [18] reported that chitosan, as a secondary biopolymer coating in cationic lecithin-CS multilayer emulsion, was more effective in improving citral stability than ɛ-polylysine in a lecithin–ɛ-polylysine multilayer emulsion, where phase separation of the emulsion took place following creaming and coalescence.
2.2. Allyl isothiocyanate
AITC, 3-isothiocyanato-1-propene, is the major pungent flavor compound naturally found in plants of the Brassicaceae family such as horseradish, mustard, wasabi, and cruciferous vegetables including cabbage and cauliflower. It can be generated from the chemical conversion of a glucosinolate, sinigrin, by enzymatic hydrolysis of myrosinase released when the plant tissue is disrupted [22–25]. AITC is an unstable compound and has been reported to gradually decompose to compounds with a garlic-like odor in water at 37°C and even at room temperature [26], and the chemical reactivity can be generated through various chemical reactions such as hydrolysis, oxidation, thermal degradation, and reaction with proteins.
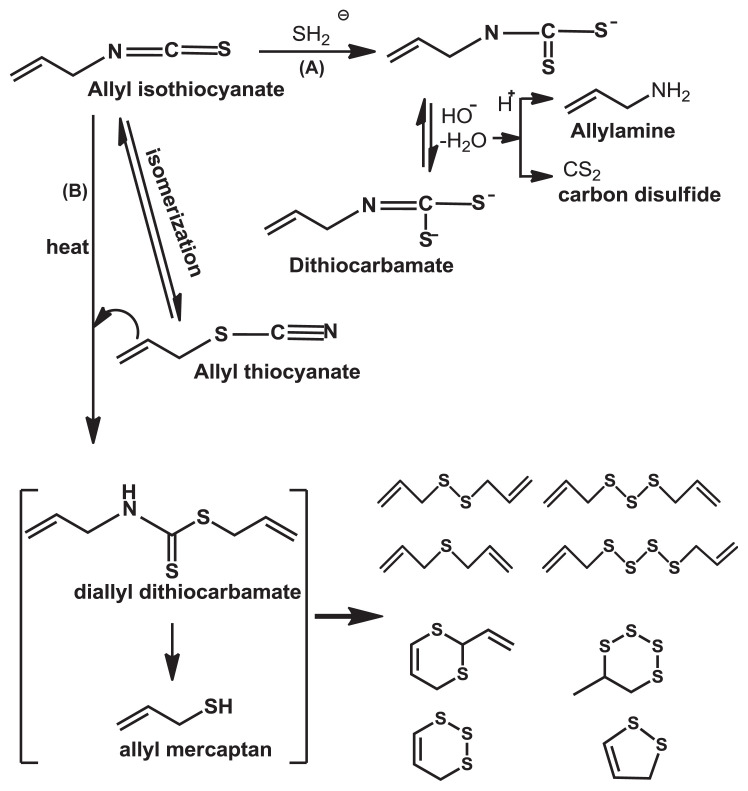
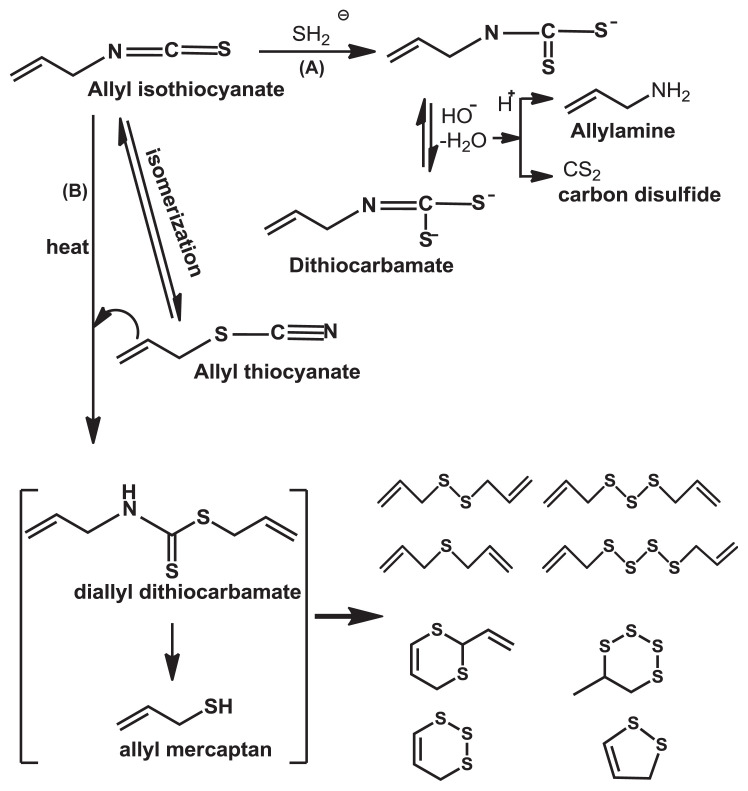
Because the carbon atom in isothiocyanic functional group, N==C==S, is extremely electrophilic, it can easily undergo nucleophilic additions with amino, hydroxyl, and thiol groups; β-dicarbonyl; and carboxylic acids to give thiocarbamoyl derivatives and several kinds of degradation products [24,27–31]. The rearrangement of isothiocyanate, R—N==C≡S, to thiocyanate, R—S—C≡N, isomerization of AITC, is more favorable in neutral conditions than in acidic and basic solutions, and its isomerization is stable in neutral and acidic environments. However, the decomposition through hydrolysis and oxidation of AITC readily occurs under an alkaline condition and at higher temperatures [25,32]. This can be easily explained by the fact that there was no stronger nucleophilic agent in neutral and acidic conditions [22,23]. Hydrolysis of AITC under an alkaline condition at 27°C generated thiourea (SC(NH2)2). The reaction mechanism is that the hydroxyl ion reacts directly with AITC to form an unstable intermediate, which then immediately captures a proton from water and becomes the monothiocarbamate shown in Fig. 3 [22,23].
Fig. 3.
Hydrolysis of allyl isothiocyanate under alkaline condition at 27°C.
The degradation rate of AITC alone is controlled by pseudo-first-order reaction kinetics [33]. Pechácek et al [22] studied AITC hydrolysis in an alkaline solution at a high temperature, 80°C, and concluded that, in addition to allylamine, diallylthiourea, and carbon disulfides, the newly identified major products were allyl dithiocarbamate and diallyl sulfides. Diallyl thiocarbamate is unstable and can be further hydrolyzed under an acidic or alkaline condition, leading to allylamine and carbon disulfide (Fig. 4A) [22]. Chen and Ho [25] found that, regardless of pH, the main products from thermal degradation at 100°C were aliphatic and cyclic sulfides, and not thiocarbamate, and indicated that allyl dithiocarbamate was heat labile, which later decomposed to the unstable compound allyl mercaptan, known as an important intermediate for generating volatile sulfide compounds through thermal degradation. The mechanism for thermal degradation is shown in Fig. 4.
Fig. 4.
Scheme for hydrolysis (A) and thermal degradation (B) of allyl isothiocyanate.
The interaction of AITC with amino acids such as cysteine and proteins including insulin, bovine serum albumin (BSA), ovalbumin, and lysozyme had been studied [31,29]. Reactions of either proteins or amino acids and AITC not only decrease the availability of AITC itself, but also change the nutritional values, such as digestibility, of proteins. Many studies reveal that the centered carbon atom in the isothiocyanate of AITC reacts with proteins by cleaving the disulfide bond in cysteine followed by polymer formation, and also attacks the free amino group in lysine and arginine residues in protein to produce a thiourea derivative. Cejpek et al [24] studied the reaction of AITC with alanine, glycine, and several di- and tripeptides at various pH values at room temperature. The reaction of AITC with α-NH2 groups of amino acids and peptides involves the addition of AITC and cleavage of 2-thiohydantoin, for which the reaction rate rises in proportion to pH within a pH range of 6–10. The reaction rates depend on the amount of unprotonated forms of amino compounds that vary with pH. Due to a lower pKa value, the alanine and/or glycine containing di- and tripeptides reacts more rapidly with AITC than free amino acid at a higher pH. The final products from the reaction are ATC-amino acids, ATC-peptides, 2-thiohydantoins, allylamine, and allylthiourea [24].
Many studies proposed the decomposition mechanism of AITC through hydrolysis under an alkaline condition following nucleophilic addition with the amine group, resulting in dialkyl thiourea formation [22–24,26]. Besides the reaction with α-NH2 groups of amino acids and proteins, AITC reacts with thiol and disulfide [22,29]. The centered carbon atom in isothiocyanic functional reacts with the thiol anion of amino acid side chain, producing thiocarbamate, which is a labile compound, easily oxidized by air generating dialkyl thiuram disulfides, or decomposed under mild acidic, neutral, and weakly alkaline solution yielding allylamine and carbon disulfide [22].
2.3. Compounds 2,5-dimethyl-4-hydroxy-3(2H)-furanone and 4-hydroxy-2 (or 5)-ethyl-5 (or 2)-methyl-3(2H)-furanone
Among 4-hydroxy-3(2H)-furanone derivatives such as 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol of DMHF), 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone, (homofuraneol), and 4-hydroxy-5-methyl-3(2H)-furanone, (norfuraneol), furaneol has the most considerable impact on food aroma due to its very low flavor threshold in water, which is 0.04 μg/kg, compared to those of norfuraneol and homofuraneol, which are 23,000 μg/kg and 20 μg/kg, and three of them give a caramel-like sweet flavor [34–38]. In addition, Buttery et al [39] found that odor thresholds of furaneol and their related odor compounds varied depending on pH of solution: the more acidic the condition, the lower the odor threshold.
Although homofuraneol was first identified as a character impact factor of Japanese style soy sauce, it has since been reported, on the basis of sensory experiment, to be of major importance for the overall flavor of soy sauce [40–44]. Furaneol or 2,5-dimethyl-4-hydroxy-3(2H)-furanone was identified for the first time as a natural aroma component in pineapple, and it has since been detected in many fruits including raspberry, strawberry, mango, kiwi, grape, and tomato [39,45,46]. Furaneol is the most important compound in strawberry and pineapple, also known as strawberry furanone and pineapple furanone, respectively [45,47]. In nature, furaneol can be rapidly converted into methoxyfuraneol by enzyme O-methyltransferase during fruit maturation [45]. Besides being biologically found in certain fruits, furaneol is also the key flavor component generated in heat-processed food such as wheat bread crust and popcorn because it can be formed by thermal degradation of fructose, pyrolysis of d-glucose or 1-deoxy-1-piperidino-d-fructose, and heating of amino acids with rhamnose [48–50].
So far, homofuraneol studies have focused on its generation, and not on its stability compared to furaneol. The keto–enol tautomerization of furaneol is pH dependent [35]. Furaneol is unstable both in the presence of air and in aqueous solutions [51–54]. The result of a stability study of furaneol in an aqueous buffer solution revealed that furaneol degradation was pH dependent, with optimum stability being at pH 4, and the rate of decomposition follows first-order kinetics [53]. From a study on the stability of naturally occurring furaneol derivatives at pH range 2.0–8.0 at 23°C [54], the greatest stability of furaneol in aqueous solutions was found to be at pH 3.5 and addition of saccharose and ethyl alcohol over a concentration range (0 ± 20%) has no effect on the stability of furaneol [53]. Furaneol is heat labile, which can go through thermal degradation to produce a variety of carbonyl and hydroxyl carbonyl compounds such as 2-hydroxy-3-butanone and 2-hydroxy-3-pentanone, aliphatic aldehydes, or alcohols [49]. High temperatures (130°C and 160°C) cause thermal degradation of furaneol. Amino acids and hydrogen sulfide are also the most important reactants that have an impact on the stability of furaneol, particularly at higher temperatures. Shu et al [50] studied the thermal degradation of furaneol in a closed system at 160°C at various pH (2.2, 5.1, and 7.1), and found that most of the degradation products were cyclic carbonyls as well as furanones, and the degradation was preferred at a lower pH. The initial stage of the degradation mechanism involved ring structure opening. The resulting open ring intermediate underwent a retroaldolization reaction to generate a group of primary products, including acetaldehyde, hydroxyacetone, 1-hydroxy-2-butanone, 3-hydroxy-2-butanone, and 2,3-butanedione, which reacted in intermolecular ways to generate secondary products. In this study, the important secondary products, 3-hydroxy-2-pentanedione and 2-hydroxy-3-pentanone, were formed by aldol condensation of the primary products acetaldehyde and hydroxyl acetone [50].
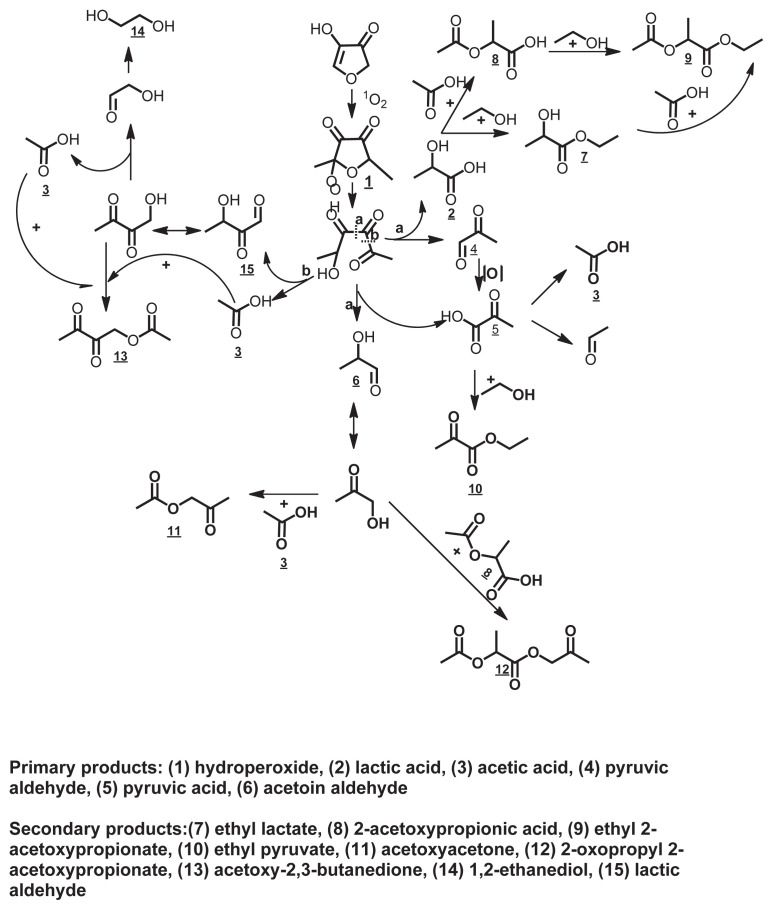
Photo-oxidative stability study of furaneol by Chen et al [52] showed that the initial state for DMHF photo-oxidation began with the ring structure opening as in thermal degradation mechanism, and the primary products of photosensitized oxidation of furaneol were generated, which then reacted with alcohols or acids, leading to acyclic ester formation and secondary products. The possible mechanism for photo-oxidation of furaneol is summarized in Fig. 5. It starts with exposure of furaneol to light in the presence of a photosensitizer, chlorophyll, and the singlet oxygen (1O2) is generated, which then attacks the double bond at the C-5 position, producing hydroperoxide. Later, this open-ring hydroperoxide forms 5-hydroxy-2,3,4-hexanetrione intermediate, which is hydrolyzed at either position a or position b to form the primary products or intermediate products including lactic acid, pyruvic aldehyde, pyruvic acid, lactic aldehyde, acetic acid, and acetoin aldehyde. The lactic acid then goes through a series of esterification processes with acetic acid or alcohol to form the secondary products including 2-acetoxypropionate, ethyl lactate, and ethyl 2-acetoxypropionate. The pyruvate aldehyde is oxidized to unstable pyruvic acid, which then easily undergoes an esterification reaction with ethanol to form ethyl pyruvate and proceeds through a degradation reaction to generate acetic acid as a photo-oxidation product. The acetoin aldehyde undergoes a keto–enol tautomerization process to form keto alcohols, which are able to react with acids to form esters including 2-oxopropyl 2-acetoxypropionate and acetoxyacetone. The intermediates from b part, such as lactic aldehyde, go through the same mechanism as acetoin aldehyde, leading to acetoxy-2,3-butanedione, and 1,2-ethanediol [52]. The results from this study in terms of intermediate, primary, and secondary products can be used to explain the prooxidant effect of both homofuraneol and furaneol [49].
Fig. 5.
Possible mechanism for photo-oxidation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone.
The DMHF thermal degradation mechanism in the presence of sulfur-containing compounds is different from DMHF thermal degradation itself. The reaction of furaneol with hydrogen sulfide or amino acid, such as cysteine, produces a thiophene derivative [50,55,56]. The ring oxygen in furaneol is readily exchangeable with a sulfur atom, so it is reactive to sodium sulfide, hydrogen sulfide, and sulfur-containing amino acids such as cysteine and glutathione [49,55,56]. It is well accepted that hydrogen sulfide (H2S) is a product of thermal degradation of sulfur-containing compounds such as cysteine, cystine, and glutathione. The chemical reaction involves thermal degradation of both furaneol and amino acid, and the interactions among degradation products include Maillard reaction and Strecker degradation. Van den Ouweland and Peer [55] obtained mercaptothiopene derivative from heating of furaneol with hydrogen sulfide in an aqueous solution at 100°C. Shu and Ho [56] studied reactivity between DMHF and cysteine at various pH at 160°C, and indicated that the products formed significantly depended on the pH of the solution. More thiopene derivatives were generated at a lower pH (2.2) than at a higher pH (5.1). Pyrazines were only formed in pH solution higher than pI of cysteine, 7.1 [56]. Zheng et al [49] investigated the possible reaction mechanism via Maillard reaction and Strecker degradation using the reactions of furaneol with cysteine, glutathione, sodium sulfide, and alanine at 130°C. The results indicated that the availability of hydrogen sulfide might be the limiting factor in types and amount of sulfur-containing compounds formed in the reaction [49]. Kunert-Kirchhoff and Baltes [57] reported that, even in the presence of sulfur-containing amino acid, furaneol reacted with phenylalanine in an autoclave, generating alkylpyrazine and alkyldihydrofuropyrazine.
2.4. Vanillin
Vanillin, 4-hydroxy-3-methoxybenzaldehyde, commercially called p-vanillin, is a major constituent of vanilla flavor, and is a well-known flavoring agent used in various food industries such as bakery, confectionary, ice cream, fragrance, cosmetics, and drug manufacture [58–63]. Since vanillin has three reactive functional groups, aldehydic group, phenolic hydroxyl, and aromatic nucleus, it can easily undergo different types of reactions. The aldehyde functional group can cause certain condensation reactions, leading to various substitutions. If its hydroxyl group is protected, vanillin can be oxidized to generate vanillic acid. Phenol is converted to esters and ethers, while the nucleus is readily substituted by halogen and nitro groups [59,64,65]. Oxidation of vanillin can occur both under alkaline condition and by enzymes including milk enzymes such as xanthine oxidase and peroxidase. Fargues et al [66] found that heating birch syrup at 100°C decreased the aroma intensity of vanillin. Vanillin is highly oxidized when reacted with oxygen in an alkaline solution through various pathways, and the reaction is favored at higher temperatures, < 100°C, as well as under elevated alkaline conditions. The rate of vanillin oxidation, which depends on the concentration of vanillin, follows first order at pH > 12 and second order at pH < 12. Oxygen concentration has an impact on the rate of vanillin oxidation at a higher pH (>12), but not at a lower pH (<12) [67,68]. The major product of air oxidation at high levels of alkalinity is vanillic acid, and the degradative pathway includes a Dakin-type reaction through methoxy hydroquinone and a demethoxylation reaction through p-hydroxybenzaldehyde [58,68]. A Dakin-type reaction is the reaction of o- and p-hydroxybenzaldehyde with hydrogen peroxide under acidic and basic conditions, with various substitutions on benzaldehyde. Anklam et al [60] and Baumgartner and Neukom [68] indicated that vanillin could also be oxidized by thermolabile enzymes, peroxidase/H2O2, and xanthine oxidase, yielding dimeric divanillin and vanillic acid in milk products, but xanthine oxidase was not the driving force of vanillin oxidation to vanillic acid, as shown in Fig. 6 [68,69]. Oxidation of vanillin in milk products to vanillic acid is pH dependent, which is shown to be favored at a higher pH, > 4 [60].
Fig. 6.
Enzymatic oxidation of vanillin and structure of Schiff base vanillin.
It is accepted that under a liquid or high-moisture food environment, flavor compounds having an aldehydic function can bind covalently to the amino groups of proteins via Schiff base formation [69,70]. Dikusar [69] found that the reaction of vanillin with biphenyl-4-amine in methanol gave a Schiff base-containing compound. Vanillin also reacts with amino acid and proteins via Schiff base formation, hydrogen bonding, hydrophobic interaction, and electrostatic interaction [71–75].
Interactions of vanillin with proteins influence the flavor perception, decrease the intensity of vanillin flavor, and are likely to influence the release of flavor compounds during consumption. Different types of proteins, protein conformation, pH, temperatures, and concentration of proteins have impacts on vanillin protein binding or the types of chemical interactions [71–77]. Mikheeva et al [70] reported that vanillin interacted with proteins including β-lactoglobulin, bovine serum albumin, and ovalbumin mainly through electrostatic interactions, since certain physical factors such as temperature, pH, and ionic strength had effects on the binding. Besides the electrostatic interaction, hydrophobic interaction is a major force for vanillin bovine serum albumin, while hydrogen bonding plays a key role for vanillin–casein interaction, as indicated by Chobpattana et al [78]. Li et al [72] studied the interaction of vanillin with different proteins, soy, whey, and casein proteins, and showed that whey protein could bind more vanillin or had a higher affinity, than soy and casein proteins. The binding of vanillin to protein were also increased by decreasing the temperature from 12°C to 4°C, which may be explained by the conformational change in tertiary and quaternary structures of protein at lower temperatures [79,80]. Owing to the interaction between carbonyl and hydroxyl groups, binding of vanillin with dairy proteins, casein, and whey proteins are governed by enthalpy. By contrast, the conformational change of soy protein has an impact on the binding of vanillin causing the interaction to be highly driven by entropy [74]. Heat treatments of sodium caseinate at 85°C/10 min, 68°C/30 min, and 75°C/15 min had no effect on vanillin flavor intensity [73,76]. On the contrary, McNeill and Schmidt [75] showed that vanillin flavor intensity in a heated whey protein isolate (85°C for 10 minutes) was higher than that in an unheated whey protein isolate. This can be explained by the unfolding of whey protein resulting in enhanced binding to vanillin because it is accepted that casein is more heat resistant to denaturation than whey protein [73,74]. There is little information about the reaction of vanillin with amino acids or proteins via Schiff base formation due to aldehydic group of vanillin and free NH2 groups of proteins. Schiff base formation through aldehyde–amine condensation was inhibited by the model system in presence of water [81]. Chobpattana et al [74] investigated the reaction kinetics of vanillin with different types of amino acids, lysine, cysteine, and phenylalanine, or peptides, pentalysine, glutathione, and aspartame, in the model system at elevated reaction temperatures of 55°C, 65°C, and 75°C. The result suggested that the interaction of vanillin and amino acid followed first-order kinetics, and the reaction rate depended on time and temperature, being higher at elevated temperatures. Schiff base formation is not a major reaction between vanillin and amino acid or protein [74]. Although many studies have shown that vanillin interacts with protein and amino acid, only a few studies have paid attention to the result of the reaction, including the off-flavors produced from the reaction and the perception of vanillin after reaction. Reiners et al [73] showed that the interaction between 1% milk protein, β-lactoglobulin, and vanillin at 8–200 mg/L for sensory evaluation test had no effect on the odor perception of vanillin [73].
2.5. MFT and furfurylthiol
While MFT was first identified in 1988 in heated canned tuna fish, 2-furfurylthiol (FFT), also called 2-furfuryl mercaptan, was known for the first time as a food component in roasted coffee [82]. Besides, they are known to possess an intense roasted coffee-like odor, the key aromas in heated processed foods and beverages such as cooked beef and wheat bread. FFT is also an important odorant in freshly popped corn and roasted white sesame [82–85]. The MFT odor threshold values are as low as 0.007 μg/kg in water and 0.0025 ng/L in air, whereas FFT has an odor threshold of 0.01 μg/kg in water of 0.01 ng/kg in air [82,85,86]. Many studies showed that both key coffee odors can be generated from the Maillard reaction of amino acid, cysteine, pentose, ribose/rhamnose or hexose, and glucose [82,84,85,87]. Besides the Maillard reaction, the minor pathways for MFT generation are thermal degradation of thiamine (vitamin B1), widely found in processed animal and plant food products, and reaction of norfuraneol [4-hydroxy-5-methyl-3(2H)-furanone], pentose degradation product, and cysteine or hydrogen sulfide in the model system [82,85]. FFT can be formed by heating glucose with hydrogen sulfide and ammonia [82]. Although, MFT represents the pleasant characteristic odor of certain foods, in some products such as orange juice, it is an off-flavor compound generated during storage [85,88]. The hydrogen atom of thiol group of MFT and FFT can be readily abstracted, reflecting their antioxidative capability [84]. It is well known that thiols are readily oxidized to disulfides, so MFT and FFT are unstable compounds and can be oxidized to disulfide derivatives [83–85,87]. Many qualitative and quantitative studies were conducted to support the instability of sulfur aroma through several reactions such as thermal degradation, reaction with food proteins, radical reaction, and ionic reaction in both food systems and model studies.
Oxidation of MFT and FFT in diethyl ether occurs even within 1 day at 6°C and the oxidative products generated are the corresponding disulfides or mixed disulfides. The oxidation rate of MFT was higher than that of FFT, which can be attributed to the high antioxidant activity of the former because of the easy abstraction of a hydrogen atom from the thiol group of MFT compared with that from FFT. Higher temperatures attained during heat treatment increased the MFT oxidation rate [84].
Flavors containing thiol groups, including FFT and MFT, have the ability to bind with food proteins. Mottram et al [83] found that heating a flavor compound containing thiol and disulfide groups in an aqueous solution at 100°C with egg albumin causes a decrease in the concentration of flavor. This could be attributed to the interchange (redox reaction) of thiol and disulfide groups of the flavor compound with those of proteins. This reaction depends on the structure of protein and the number and position of sulfhydryl groups.
FFT and MFT are key contributors to coffee aroma and are very reactive to other chemical compounds that are normally added to coffee brew; therefore, milk added to coffee also has a role in decreasing the quality of the sulfur aroma in coffee. The study showed that all types of milk and milk products such as UHT milk, condensed milk, skimmed milk, coffee cream, and whipped cream, and vegetable fat reduced the intensity of coffee-like attributes, such as roasty flavor, because their lipid, protein, and carbohydrate components influence the release of aroma substances in coffee brew [89]. Heat processing and duration of storage have a significant impact on the stability of sulfur aroma compounds in coffee, particularly FFT. It was reported that coffee flavor changed during sterilization at 121°C for 15 minutes or at 134°C for 3 minutes. There were support studies showing that heating caused substantial degradation of FFT and MFT through oxidation and hydrolysis [90,91]. Kumazawa and Masuda [91] investigated the influence of heating processes on the change in the flavors of coffee drinks. The sensory study showed that sterilization of a coffee drink at 121°C for 10 minutes in a can significantly decreased the potent roasty flavor of the fresh coffee drink [91]. The study also focused on the effects of pH, in the range of 3–7, on FFT concentration change during heat processing. Higher pH, particularly in the range of 5.0–7.0, and higher temperatures significantly reduced the FFT concentration. The major volatile product obtained from thermal degradation of FFT in an aqueous solution was difurfuryl disulfide, followed by furfural and furfuryl alcohol. Nonvolatile degradation products were presumably produced through a Fenton-type reaction [86,91]. The results indicated that various reactions, including thiol binding to coffee melanoidins, Fenton reaction, and pH-dependent degradation, caused the change in coffee flavor [91].
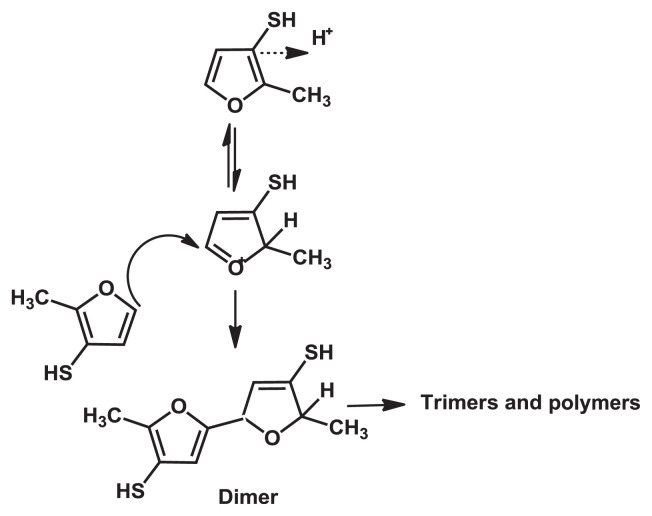
The stability of MFT and FFT in an aqueous process flavoring was investigated through cysteine and ribose model system under 50°C accelerated storage condition. Both in air and without air, MFT and FFT content did decrease with time, and MFT was found to be less stable than FFT [86,87]. This study concluded that the instability of MFT is not because of the oxidative pathway of MFT to form disulfide, but seem to be the result of the greater ability of MFT to undergo oligomerization/polymerization. MFT electrophilic species, formed from the protonation at 2-position, react with nucleophiles, which are the thiol group and furan ring of MFT. The result supported that the reactivity of 5-position of MFT is responsible for its rapid degradation. Besides reacting with itself, MFT can react with other thiols such as cysteine [87]. The proposed mechanism for polymerization of MFT is shown in Fig. 7.
Fig. 7.
Proposed degradation mechanism for polymerization of 2-methyl-3-furanthiol.
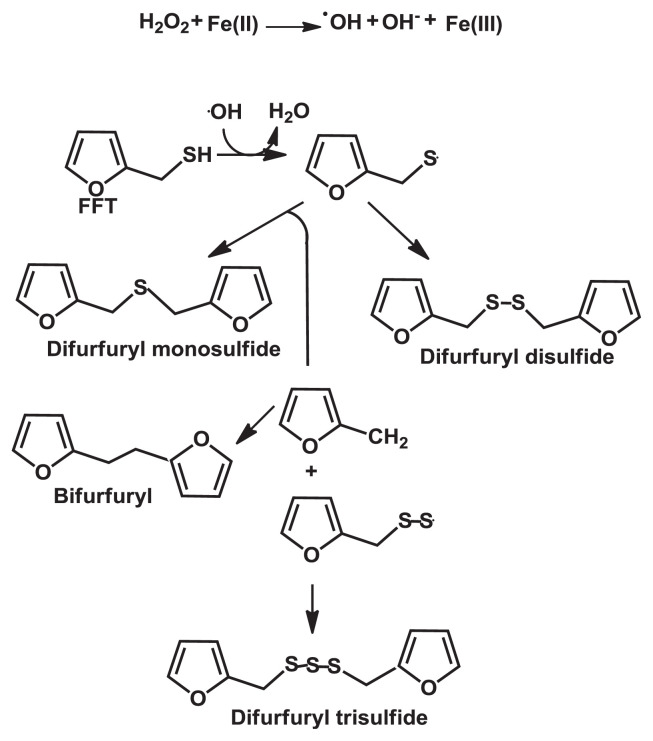
Oxidation and radical reaction also affects the stability of thiol flavor compounds, which appear to be a crucial problem of altering the coffee aroma. Reactive oxygen species, such as singlet oxygen (1O2), superoxide ( ), hydroperoxyl radical (•OOH), hydroxyl radical (•OH), and hydrogen peroxide (H2O2), were generated from oxygen (3O2) at ground state in the presence of water, light, energy, or catalytic activity. Among them, the most reactive form of oxygen is hydroxyl radical (•OH) [86,92,93]. In the presence of a transition metal in low oxidation states, oxidative processes can be initiated by H2O2 to generate hydroxyl radicals (•OH) via a Fenton reaction. Blank et al [86] investigated the stability of key coffee aroma compound FFT in the model Fenton-type reaction system and found 90% degradation of FFT within 1 hour at 37°C. The FFT degradation products were both volatile and nonvolatile substances with molecular weights in the range of 124–262 Da. The major FFT degradation product was difurfuryl disulfide, followed by bifurfuryl, and difurfuryl monosulfide, which all gave a burnt, rubbery, and sulfuryl roasty smell-like flavor. The degradation is temperature dependent, which caused 20% loss of FFT at room temperature compared to 90% loss at 37°C after 1 hour. The result clearly indicated that Fe (II) catalyzed the reductive cleavage of the O–O bond in H2O2 to generate •OH radical, so iron (Fe) and hydrogen peroxide (H2O2) play a key role in FFT degradation. The type of transition metal has an impact on the FFT oxidation in which Fe/Fe(II) is more effective than Mn or Cu due to easier redox cycling between the two oxidation states of Fe and Fe(II). Besides the generation of •OH by a Fenton-type reaction, the C-centered radical derived from the reaction of •OH with various organic molecules and the S-radical were also found, and these radicals are generated as intermediates during FFT degradation by the Fenton-type reaction, as shown in Fig. 8 [86].
Fig. 8.
Mechanism of hypothetical degradation of 2-furfurylthiol under Fenton-type reaction.
Apart from food proteins, FFT is capable of binding to other polymers such as brown macromolecules that are formed during thermal processing of food. Of particular concern is the covalent binding of FFT to melanoidins which are generated when carbohydrates react with amino compounds at higher temperatures during roasting of coffee bean. This type of binding may also have an impact on flavors containing thiols and brown colors in many food items including meat, bread crust, or roast sesame seeds, besides coffee.
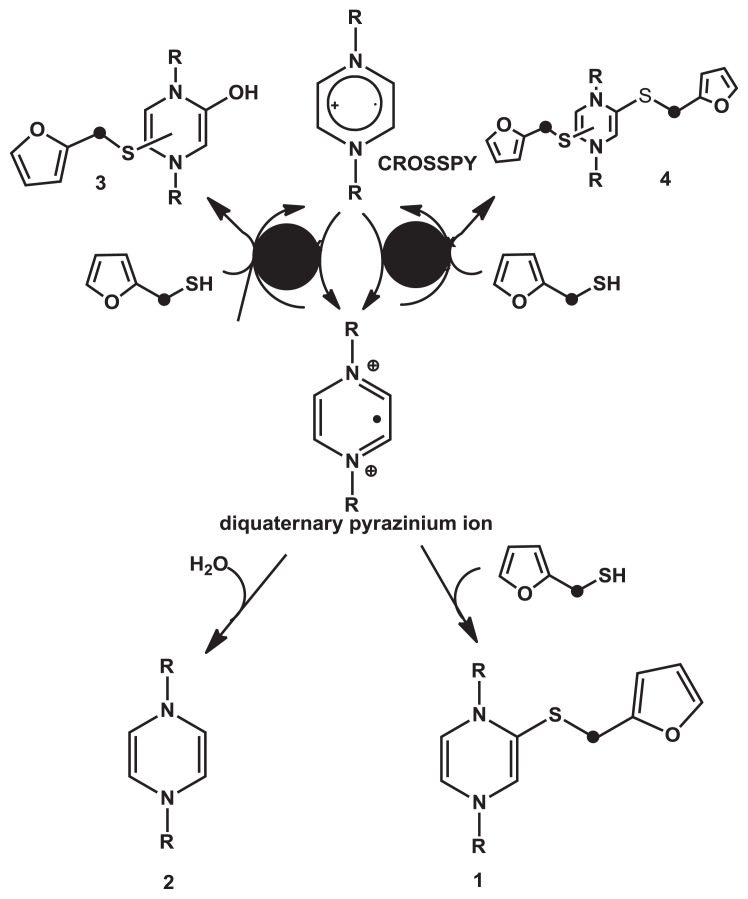
Hofmann and Schieberle [94] and Hofmann et al [95] found that in the model study, the sulfuryl-roasty aroma quality of coffee was reduced by adding coffee melanoidins, and the quantity of FFT was rapidly reduced (50% after 20 minutes and almost 100% after 30 minutes). However, it was still not clear whether melanoidins degraded the FFT only by covalent binding or through other degradation pathways such as oxidation. Hofmann and Schieberle [94] elucidated the chemical mechanism involved in coffee aroma change in the presence of coffee melanoidins by keeping coffee beverage warm. The outcome undoubtedly showed that in the presence of coffee melanoidins, the concentration of coffee thiols, including FFT and MFT, decreased drastically and FFT was most affected among other coffee thiols. Using [2H]-nuclear magnetic resonance and Liquid chromatography/mass spectrometry, they demonstrated that obviously both FFT and MFT were covalently bound to coffee melanoidins through Maillard-derived pyrazinium compounds formed as oxidation products of 1,4-bis-(5-amino-5-carboxy-1-pentyl)pyrazinium radical cations, called CROSSPY radicals. These radical cations, produced during melanoidin formation and found to be involved in a redox cycle of reaction intermediates, were oxidized, leading to diquaternary pyrazinium ions. These ions reacted with nucleophilic thiols such as FFT, generating the thioether 2-(2-furyl)methylthiol-1,4-dihydropyrazine, and in the absence of thiols, reacted with water to generate 2-hydroxy-1,4-dihydropyrazine [94]. The proposed chemical mechanism for binding of thiols to CROSSPY-related reaction intermediates is demonstrated in Fig. 9. Without oxygen, degradation of FFT was very slow, and the result suggested that either thiols reacted with oxidation products formed in the coffee matrix in the presence of oxygen or oxygen needed to create radical species caused thiol degradation. All these research works support that oxidation reduces the stability of FFT. This is well in line with the findings of Hofmann and Schieberle [94].
Fig. 9.
Proposed chemical pathway for covalent binding of thiols to CROSSPY-related reaction intermediates.
2.6. Methional
Methional, 3-(methylthio)propanal, having a cooked potato-like flavor, is formed by the Strecker degradation reaction between α-dicarbonyl compounds, the key intermediate products of the Maillard reaction, and methionine (Met) [96,97]. It is also found in wine, sardine, ham, and soy sauce [40,98,99]. In orange juice, it causes the off-flavor problem [88]. Potato processing causes the loss of a large amount of methional because it is heat labile and readily decomposes to methanethiol, which oxidizes to dimethyl disulfide [96]. Besides the thermal instability, methional is also unstable to light and can be converted to many sulfur compounds, particularly in light-exposed milk. Methional is decomposed to methanethiol and dimethyl sulfide by exposure to light. A report showed that the broth and potato flavors of methional changed to methanethiol-like flavors on additional light exposure [97]. Methional is reactive to oxygen-centered radicals such as hydroxyl radicals (•OH), superoxide anions (O−2), generating ethylene [100–102]. Using a pulse radiolysis study, the mechanism of oxidation by •OH was found to be more complicated than a simple fragmentation reaction [102]. Beauchamp and Fridovich [101] pointed out that methional reacts with hydroxyl radical rather than with superoxide anions to generate ethylene. Data on the mechanism of methional degradation are still lacking.
2.7. Compounds 2-AP, 6-acetyl-1,2,3,4-tetrahydropyridine, 2-acetyl-2-thiazoline, and 5-acetyl-2, 3-dihydro-4H-thiazine
The compound 2-AP (5-acetyl-3,4-dihydro-2H-pyrrole) has extremely low odor thresholds, 0.1 μg/L in water and 0.02 ng/L in air. It was first identified as the key flavor compound of cooked rice [103]. This compound is generated by thermal cooking and processing, through nonenzymatic Maillard reaction, of various foods, especially rice. Besides in cooked rice, 2-AP has also been identified, mostly in a low concentration, as a volatile flavor in various cooked cereals and cereal products including bread crust, toasted bread, corn tortillas, popcorn, and cooked sweet corn products; extrusion cooked maize flour; rice cakes; and puff pastries. In addition, it is shown to be the potent odorant of boiled potatoes, roasted wild mango seeds, roasted sesame seeds, pan-fired green teas, and taro [103–105]. It has been shown to be formed by acylation of 1-pyrroline by the respective 2-oxoaldehyde, with the elimination of formaldehyde.
The compound 6-acetyl-1,2,3,4-tetrahydropyridine (6-ATHP) is responsible for a typical roasty aroma similar to 2-AP, and it is a potent keynote flavor of crackers and popcorn. It also contributes to the aroma of several baked products such as potato chips, bread crust, corn tortilla, and toast rice cakes. Compared with 2-AP, the odor threshold of 6-ATHP is higher, which is about 1.0 μg/kg in water and 0.06 ng/L in air. Although both 2-AP and 6-ATHP are formed from the same precursors, 1-pyrroline and carbohydrate fragment, and significantly contribute to flavor of bread crust, 2-AP has the highest odor unit in wheat bread crust, while 6-ATHP dominates in rye bread crust [103].
Although not many studies have focused on the stability of these two compounds, a few of them focused on the loss of 2-AP in rice as a keynote flavor. These two flavor compounds are sensitive to light, oxygen, and heat, and the important factors that cause loss of rice flavor are the temperature of drying, storage moisture condition, and storage duration and condition during postharvest treatments [103,105–107].
Wongpornchai et al [106] investigated the impact of drying methods and storage time on the stability of 2-AP in Thai rice KhaoDawk Mali 105, the most popular aromatic rice variety of Thailand, in the stage of fresh paddy. The condition of drying was varied in six different drying methods: in modified air at 30°C and 40°C; in hot air at 40°C, 50°C, and 70°C; and by sun drying, under 15% moisture content for 10 months. The results showed that 2-AP content in all cases decreased more than four times after 10-month storage and off-flavor compounds, n-hexanal and 2-pentylfuran, were increasingly generated [106]. Schieberle [107] found that the flavor compounds of fresh popcorn, both 2-AP and 6-ATHP, are not stable. The concentration of four roast flavor compounds in fresh and stored hot air popped corn and in fresh pan-popped corn showed that 2-AP and 6-ATPH decreased 75 and 69%, respectively, after storage in a polyethylene bag for 1 week.
Both 2-acetyl-2-thiazoline (2-AT) and 5-acetyl-2,3-dihydro-4H-1,4-thiazine (5-ADHT) are sulfur-containing analogs of 2-AP and 6-ATHP, respectively, and are known as sulfur-containing popcorn-like flavor compounds that possess characteristic popcorn-like odor. The compound 5-ADHT was first identified from a model ribose–cysteine reaction system and has not been identified in food systems, whereas 2-AT was first identified in beef broth and is one of the characteristic roasted beef odor compounds. The odor threshold of 2-AT is 1 μg/L in water and of 5-ADHT 0.05 ng/L in air. It is clear that 2-AT is the product of a Maillard reaction in the presence of cysteine [108,109]. The compound 2-AT can be generated by the reaction of amino acid including cysteine with methylglyoxal in a model system [110]. It is a potent odorant of several processed meat products such as chicken broth, cooked meat patties, cooked chicken, and stew beef juice and found in several food items such as cooked mussels, cooked clams, cheddar cheese, pan-fired green tea, roasted white sesame seeds, sweet corn products, and heated yeast extracts [103].
The compound 2-AT is unstable during heat treatment in the presence of water. Fat-containing food systems can stabilize 2-AT. Hofmann and Schieberle [109] refluxed 2-AT in tap water (100°C) and found that the degradation was 96% after 60 minutes. By contrast, heating 2-AT in sunflower oil at 100°C caused only 2.5% 2-AT degradation compared to 99% under phosphate buffer (pH 5) in an autoclave at 145°C.
3. Conclusion
Many chemical reactions and numerous factors, including temperature, pH, storage period, enzymes, and oxygen, influenced the stability of flavor compounds. An understanding of flavor stability and the knowledge of an effective approach or technique to retard flavor degradation and improve the stability of flavor compounds are important to obtain desirable flavor for maximizing food product qualities.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1.Fisher C, Scott TR. Introduction—problems in flavour research. London, UK: Royal Society of Chemistry; 1997. [Google Scholar]
- 2. Liang CP, Wang M, Simon JE, Ho CT. Antioxidant activity of plant extracts on the inhibition of citral off-odor formation. Mol Nutr Food Res. 2004;48:308–17. doi: 10.1002/mnfr.200400027. [DOI] [PubMed] [Google Scholar]
- 3. Pihlasalo J, Klika KD, Murzin DY, Nieminen V. Conformational equilibria of citral. J Mol Struct. 2007;814:33–41. [Google Scholar]
- 4. Kimura K, Nishimura H, Iwata I, Mizutani J. Deterioration mechanism of lemon flavor. 2. Formation mechanism of off-odor substances arising from citral. J Agric Food Chem. 1983;31:801–4. [Google Scholar]
- 5. Hideki M. Deterioration of citral in acidic solution by light irradiation. Jpn Sci Technol A. 2000;44:285–7. [Google Scholar]
- 6. Kimura K, Iwata I, Nishimura H. Relationship between acid-catalyzed cyclization of citral and deterioration of lemon flavor. Agric Biol Chem. 1982;46:1387–9. [Google Scholar]
- 7. Schieberle P, Grosch W. Identification of potent flavor compounds formed in an aqueous lemon oil/citric acid emulsion. J Agric Food Chem. 1988;36:797–800. [Google Scholar]
- 8. Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA. Stability of citral in protein- and gum arabic-stabilized oil-in-water emulsions. Food Chem. 2008;106:698–705. doi: 10.1111/j.1750-3841.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- 9. Peacock VE, Kuneman DW. Inhibition of the formation of alpha.-p-dimethylstyrene and p-cymen-8-ol in a carbonated citral-containing beverage system. J Agric Food Chem. 1985;33:330–5. [Google Scholar]
- 10. Clark BC, Jr, Powell CC, Radford T. The acid catalyzed cyclization of citral. Tetrahedron. 1977;33:2187–91. [Google Scholar]
- 11. Ueno T, Masuda H, Ho CT. Formation mechanism of p-methylacetophenone from citral via a tert-alkoxy radical intermediate. J Agric Food Chem. 2004;52:5677–84. doi: 10.1021/jf035517j. [DOI] [PubMed] [Google Scholar]
- 12. Schieberle P, Ehrmeier H, Grosch W. Aroma compounds resulting from the acid catalyzed breakdown from citral. Lebensm Unters Forsh. 1988;187:35–49. [Google Scholar]
- 13. Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA. Chemical and physical stability of citral and limonene in sodium dodecyl sulfate–chitosan and gum arabic-stabilized oil-in-water emulsions. J Agric Food Chem. 2007;55:3585–91. doi: 10.1021/jf063472r. [DOI] [PubMed] [Google Scholar]
- 14. Yang X, Tian H, Ho CT, Huang Q. Inhibition of citral degradation by oil-in-water nanoemulsions combined with antioxidants. J Agric Food Chem. 2011;59:6113–9. doi: 10.1021/jf2012375. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Q, Ho C-T, Huang Q. Effect of ubiquinol-10 on citral stability and off-flavor formation in oil-in-water (O/W) nanoemulsions. J Agric Food Chem. 2013;61:7462–9. doi: 10.1021/jf4017527. [DOI] [PubMed] [Google Scholar]
- 16. Sosa N, Zamora MC, Chirife J, Schebor C. Spray-drying encapsulation of citral in sucrose or trehalose matrices: physicochemical and sensory characteristics. Int J Food Sci Technol. 2011;46:2096–102. [Google Scholar]
- 17. Choi SJ, Decker EA, Henson L, Popplewell LM, McClements DJ. Stability of citral in oil-in-water emulsions prepared with medium-chain triacylglycerols and triacetin. J Agric Food Chem. 2009;57:11349–53. doi: 10.1021/jf902761h. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Tian H, Ho CT, Huang Q. Stability of citral in emulsions coated with cationic biopolymer layers. J Agric Food Chem. 2011;60:402–9. doi: 10.1021/jf203847b. [DOI] [PubMed] [Google Scholar]
- 19. Choi SJ, Decker EA, Henson L, Popplewell LM, McClements DJ. Influence of droplet charge on the chemical stability of citral in oil-in-water emulsions. J Food Sci. 2010;75:C536–40. doi: 10.1111/j.1750-3841.2010.01693.x. [DOI] [PubMed] [Google Scholar]
- 20. Mei L, Choi SJ, Alamed J, Henson L, Popplewell M, McClements DJ, Decker EA. Citral stability in oil-in-water emulsions with solid or liquid octadecane. J Agric Food Chem. 2010;58:533–6. doi: 10.1021/jf902665b. [DOI] [PubMed] [Google Scholar]
- 21. Decker E, McClements DJ. Transition metal and hydroperoxide interactions: an important determinant in the oxidative stability of lipid dispersions. Inform. 2001;12:251–5. [Google Scholar]
- 22. Pecháček R, Velíšek J, Hrabcová H. Decomposition products of allyl isothiocyanate in aqueous solutions. J Agric Food Chem. 1997;45:4584–8. [Google Scholar]
- 23. Jiang ZT, Zhang QF, Tian HL, Li R. The reaction of allyl isothiocyanate with hydroxyl/water and β-cyclodextrin using ultraviolet spectrometry. Food Technol Biotechnol. 2006;44:423–7. [Google Scholar]
- 24. Cejpek K, Valusek J, Velisek J. Reactions of allyl isothiocyanate with alanine, glycine, and several peptides in model systems. J Agric Food Chem. 2000;48:3560–5. doi: 10.1021/jf991019s. [DOI] [PubMed] [Google Scholar]
- 25. Chen CW, Ho CT. Thermal degradation of allyl isothiocyanate in aqueous solution. J Agric Food Chem. 1998;46:220–3. doi: 10.1021/jf970488w. [DOI] [PubMed] [Google Scholar]
- 26. Kawakishi S, Namiki M. Decomposition of allyl isothiocyanate in aqueous solution. Agric Biol Chem. 1969;33:452–9. [Google Scholar]
- 27. Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–7. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 28. Zhang QF, Jiang ZT, Li R. Complexation of allyl isothiocyanate with β-cyclodextrin and its derivatives and molecular microcapsule of allyl isothiocyanate in β-cyclodextrin. Eur Food Res Technol. 2007;225:407–13. [Google Scholar]
- 29. Cejpek K, Urban J, Velíšek J, Hrabcová H. Effect of sulphite treatment on allyl isothiocyanate in mustard paste. Food Chem. 1998;62:53–7. [Google Scholar]
- 30. Kawakishi S, Kaneko T. Interaction of proteins with allyl isothiocyanate. J Agric Food Chem. 1987;35:85–8. [Google Scholar]
- 31. Todrick A, Walker E. A note on the combination of cysteine with allyl isothiocyanate. Biochem J. 1937;31:297–8. doi: 10.1042/bj0310297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ina KSA, Nobukuni M, Kishima I, Nakajima M. Studies on the volatile components of wasabi and horse radish.2. Degradation of alkyl isothiocyanates in methanol solution. J Jap Soc Food Sci Technol. 1981;28:371–5. [Google Scholar]
- 33. Ohta Y, Takatani K, Kawakishi S. Decomposition rate of allyl isothiocyanate in aqueous solution. Biosci Biotechnol Biochem. 1995;59:102–3. [Google Scholar]
- 34. Zabetakis I, Gramshaw JW, Robinson DS. 2,5-Dimethyl-4-hydroxy-2H-furan-3-one and its derivatives: analysis, synthesis and biosynthesis—a review. Food Chem. 1999;65:139–51. [Google Scholar]
- 35. Koga T, Moro K, Matsudo T. Antioxidative behaviors of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone against lipid peroxidation. J Agric Food Chem. 1998;46:946–51. [Google Scholar]
- 36. Sugawara E, Ohata M, Kanazawa T, Kubota K, Sakurai Y. Effects of the amino-carbonyl reaction of ribose and glycine on the formation of the 2(or 5)-Ethyl-5(or 2)-methyl-4-hydroxy-3(2H)-furanone aroma component specific to miso by Halo-tolerant yeast. Biosci Biotechnol Biochem. 2007;71:1761–3. doi: 10.1271/bbb.60715. [DOI] [PubMed] [Google Scholar]
- 37. Buttery RG, Takeoka GR, Krammer GE, Ling LC. Identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol) and 5-methyl-4-hydroxy-3(2H)-furanone in fresh and processed tomato. LWT Food Sci Technol. 1994;27:592–4. [Google Scholar]
- 38.Blank I, Devaud S, Fay LB. New aspects of the formation of 3(2H)-furanones through the Maillard reaction. In: Taylor AJ, Mottram D, editors. Flavor science: recent developments. Cambridge, UK: Royal Society of Chemistry; 1996. pp. 188–93. [Google Scholar]
- 39. Buttery RG, Takeoka GR, Ling LC. Furaneol: odor threshold and importance to tomato aroma. J Agric Food Chem. 1995;43:1638–40. [Google Scholar]
- 40. Steinhaus P, Schieberle P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J Agric Food Chem. 2007;55:6262–9. doi: 10.1021/jf0709092. [DOI] [PubMed] [Google Scholar]
- 41. Aishima T. Classification of soy sauce on principal components in GC profiles. Agric Biol Chem. 1979;43:1905–10. [Google Scholar]
- 42. Nunomura N, Sasaki M, Asao Y, Yokotsuka T. Isolation and identification of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone as a flavor component in shoyu (soy sauce) Agric Biol Chem. 1976;40:491–5. [Google Scholar]
- 43. Baek HH, Kim HJ. Solid phase microextraction–gas chromatography–olfactometry of soy sauce based on sample dilution analysis. Food Sci Biotechnol. 2004;13:90–5. [Google Scholar]
- 44. Lee SM, Seo BC, Kim YS. Volatile compounds in fermented and acid-hydrolyzed soy sauces. J Food Sci. 2006;71:C146–56. [Google Scholar]
- 45. Lavid N, Schwab W, Kafkas E, Koch-Dean M, Bar E, Larkov O, Ravid U, Lewinsohn E. Aroma biosynthesis in strawberry: S-adenosylmethionine: furaneol O-methyltransferase activity in ripening fruits. J Agric Food Chem. 2002;50:4025–30. doi: 10.1021/jf011409q. [DOI] [PubMed] [Google Scholar]
- 46. Raab T, Hauck T, Knecht A, Schmitt U, Holzgrabe U, Schwab W. Tautomerism of 4-hydroxy-2,5-dimethyl-3(2H)-furanone: evidence for its enantioselective biosynthesis. Chirality. 2003;15:573–8. doi: 10.1002/chir.10247. [DOI] [PubMed] [Google Scholar]
- 47. Murakami K, Haneda M, Makino T, Yoshino M. Prooxidant action of furanone compounds: implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Food Chem Toxicol. 2007;45:1258–62. doi: 10.1016/j.fct.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Schieberle P. Formation of furaneol in heat-processed foods. In: Teranishi R, Takeoka GR, Guntert M, editors. Flavor precursors. Washington, DC: American Chemical Society; 1992. pp. 164–74. [Google Scholar]
- 49. Zheng Y, Brown S, Ledig WO, Mussinan C, Ho CT. Formation of sulfur-containing flavor compounds from reactions of furaneol and cysteine, glutathione, hydrogen sulfide, and alanine/hydrogen sulfide. J Agric Food Chem. 1997;45:894–7. [Google Scholar]
- 50. Shu CK, Mookherjee BD, Ho CT. Volatile components of the thermal degradation of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J Agric Food Chem. 1985;33:446–8. [Google Scholar]
- 51. Liu TT, Yang TS. Effects of water-soluble natural antioxidants on photosensitized oxidation of conjugated linoleic acid in an oil-in-water emulsion system. J Food Sci. 2008;73:C256–61. doi: 10.1111/j.1750-3841.2008.00717.x. [DOI] [PubMed] [Google Scholar]
- 52. Chen CW, Shu CK, Ho CT. Photosensitized oxidative reaction of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J Agric Food Chem. 1996;44:2361–5. [Google Scholar]
- 53. Hirvi T, Honkanen E, Pyysalo T. Stability of 2,5-dimethyl-4-hydroxy-3(2H)-furanone and 2,5-dimethyl-4-methoxy-3(2H)-furanone in aqueous buffer solutions. Lebens Wissen u-Technol. 1980;13:324–5. [Google Scholar]
- 54. Roscher R, Schwab W, Schreier P. Stability of naturally occurring 2,5-dimethyl-4-hydroxy-3[2H]-furanone derivatives. Z Lebensm Unters Forsch. 1997;204:438–41. [Google Scholar]
- 55. Van den Ouweland GAM, Peer HG. Components contributing to beef flavor. Volatile compounds produced by the reaction of 4-hydroxy-5-methyl-3(2H)-furanone and its thio analog with hydrogen sulfide. J Agric Food Chem. 1975;2:501–5. [Google Scholar]
- 56. Shu CK, Ho CT. Effect of pH on the volatile formation from the reaction between cysteine and 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J Agric Food Chem. 1988;36:801–3. [Google Scholar]
- 57. Kunert-Kirchhoff J, Baltes W. Model reactions on roast aroma formation. Z Lebensm Unters Forch. 1990;190:9–13. [Google Scholar]
- 58.Northey RAH.The air oxidation of vanillin at high level of alkalinity. 215th ACS National Meeting; March 29–April 2, 1998; Dallas, TX. [Google Scholar]
- 59. Jose T, Nandibewoor S, Tuwar S. Kinetics and mechanism of the oxidation of vanillin by hexacyanoferrate(III) in aqueous alkaline medium. J Solution Chem. 2006;35:51–62. [Google Scholar]
- 60. Anklam E, Gaglione S, Müller A. Oxidation behaviour of vanillin in dairy products. Food Chem. 1997;60:43–51. [Google Scholar]
- 61. Munavalli DS, Chimatadar SA, Nandibewoor ST. Oxidation of vanillin by a new oxidant diperiodatoargentate(III) in aqueous alkaline medium. Ind Eng Chem Res. 2007;46:1459–64. [Google Scholar]
- 62. Walton NJ, Mayer MJ, Narbad A. Vanillin. Phytochemistry. 2003;63:505–15. doi: 10.1016/s0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 63. Kamat J, Ghosh A, Devasagayam TA. Vanillin as an antioxidant in rat liver mitochondria: Inhibition of protein oxidation and lipid peroxidation induced by photosensitization. Mol Cell Biochem. 2000;209:47–53. doi: 10.1023/a:1007048313556. [DOI] [PubMed] [Google Scholar]
- 64. Gusak KN, Kozlov NG. Reactions of vanillin and vanillal esters with 6-quinolylamine and phenidone. Russ J Gen Chem. 2005;75:1562–5. [Google Scholar]
- 65. Cestari AR, Vieira EFS, Mattos CRS. Thermodynamics of the Cu(II) adsorption on thin vanillin-modified chitosan membranes. J Chem Thermodyn. 2006;38:1092–9. [Google Scholar]
- 66. Fargues C, Mathias Á, Silva J, Rodrigues A. Kinetics of vanillin oxidation. Chem Eng Technol. 1996;19:127–36. [Google Scholar]
- 67.Northey RAZ.Effect of system variables on the kinetics and products of the alkaline oxidative degradation of vanillin and related compounds. 221st ACS National Meeting; April 1–5, 2001; San Diego. [Google Scholar]
- 68. Baumgartner J, Neukom H. Enzymatic oxidation of vanillin. Technol Hochsch. 1972;26:366–8. [Google Scholar]
- 69. Dikusar EA. Synthesis of Schiff bases from biphenyl-4-amine and vanillin, vanillal, and their esters. Russ J Org Chem. 2006;42:1293–8. [Google Scholar]
- 70. Mikheeva LM, Grinberg NV, Grinberg V, Tolstoguzov VB. Effect of thermal denaturation on vanillin binding to some food proteins. Nahrung. 1998;42:185–6. doi: 10.1002/(sici)1521-3803(199808)42:03/04<185::aid-food185>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 71. Ng PKW, Hoehn E, Bushuk W. Binding of vanillin by fababean proteins. J Food Sci. 1989;54:105–7. [Google Scholar]
- 72. Li Z, Grün IU, Fernando LN. Interaction of vanillin with soy and dairy proteins in aqueous model systems: a thermodynamic study. J Food Sci. 2000;65:997–1001. [Google Scholar]
- 73. Reiners J, Nicklaus S, Guichard E. Interactions between β-lactoglobulin and flavour compounds of different chemical classes. Impact of the protein on the odour perception of vanillin and eugenol. Lait. 2000;80:347–60. [Google Scholar]
- 74. Chobpattana W, Jeon IJ, Smith JS. Kinetics of interaction of vanillin with amino acids and peptides in model systems. J Agric Food Chem. 2000;48:3885–9. doi: 10.1021/jf9912102. [DOI] [PubMed] [Google Scholar]
- 75. McNeill VL, Schmidt KA. Vanillin interaction with milk protein isolates in sweetened drinks. J Food Sci. 1993;58:1142–4. [Google Scholar]
- 76. Hussein MM, D’Amelia RP, Manz AL, Jacin H, Chen WTC. Determination of reactivity of aspartame with flavor aldehydes by gas chromatography, HPLC and GPC. J Food Sci. 1984;49:520–4. [Google Scholar]
- 77.Ho CT, Huang TC, Cha AS, Sotirhos N. Studies on the stability of aspartame in the presence of glucose and vanillin. In: Charalambous G, editor. Frontiers of flavor. Amsterdam, The Netherlands: Elsevier Science Publishers; 1988. pp. 233–40. [Google Scholar]
- 78. Chobpattana W, Jeon IJ, Smith JS, Loughin TM. Mechanisms of interaction between vanillin and milk proteins in model systems. J Food Sci. 2002;67:973–7. [Google Scholar]
- 79. Kallio H, Rine S, Pangborn RM, Jennings W. Effect of heating on the headspace volatiles of Finnish birch syrup. Food Chem. 1987;24:287–99. [Google Scholar]
- 80.Damodaran S, editor. Fennema’s food chemistry. New York: Marcel Dekker Inc; 1996. [Google Scholar]
- 81. Damodaran S, Kinsella JE. Interaction of carbonyls with soy protein: conformational effects. J Agric Food Chem. 1981;29:1253–7. doi: 10.1021/jf60229a019. [DOI] [PubMed] [Google Scholar]
- 82. Hofmann T, Schieberle P. Quantitative model studies on the effectiveness of different precursor systems in the formation of the intense food odorants 2-furfurylthiol and 2-methyl-3-furanthiol. J Agric Food Chem. 1998;46:235–41. doi: 10.1021/jf9705983. [DOI] [PubMed] [Google Scholar]
- 83. Mottram DS, Szauman-Szumski C, Dodson A. Interaction of thiol and disulfide flavor compounds with food components. J Agric Food Chem. 1996;44:2349–51. [Google Scholar]
- 84. Hofmann T, Schieberle P, Grosch W. Model studies on the oxidative stability of odor-active thiols occurring in food flavors. J Agric Food Chem. 1996;44:251–5. [Google Scholar]
- 85. Kerscher R, Grosch W. Quantification of 2-methyl-3-furanthiol, 2-furfurylthiol, 3-mercapto-2-pentanone, and 2-mercapto-3-pentanone in heated meat. J Agric Food Chem. 1998;46:1954–8. [Google Scholar]
- 86. Blank I, Pascual EC, Devaud S, Fay LB, Stadler RH, Yeretzian C, Goodman BA. Degradation of the coffee flavor compound furfuryl mercaptan in model Fenton-type reaction systems. J Agric Food Chem. 2002;50:2356–64. doi: 10.1021/jf011329m. [DOI] [PubMed] [Google Scholar]
- 87. van Seeventer PB, Weenen H, Winkel C, Kerler J. Stability of thiols in an aqueous process flavoring. J Agric Food Chem. 2001;49:4292–5. doi: 10.1021/jf010348t. [DOI] [PubMed] [Google Scholar]
- 88. Bezman Y, Rouseff RL, Naim M. 2-Methyl-3-furanthiol and methional are possible off-flavors in stored orange juice: aroma-similarity, NIF/SNIF GC–O, and GC analyses. J Agric Food Chem. 2001;49:5425–32. doi: 10.1021/jf010724+. [DOI] [PubMed] [Google Scholar]
- 89. Bücking M, Steinhart H. Headspace GC and sensory analysis characterization of the influence of different milk additives on the flavor release of coffee beverages. J Agric Food Chem. 2002;50:1529–34. doi: 10.1021/jf011117p. [DOI] [PubMed] [Google Scholar]
- 90. Kumazawa K, Masuda H, Nishimura O, Hiraishi S. Change in flavor of coffee drink during heating. Nippon Shokuhin Kagaku Kogaku Kaishi. 1998;45:108–13. [Google Scholar]
- 91. Kumazawa K, Masuda H. Investigation of the change in the flavor of a coffee drink during Heat processing. J Agric Food Chem. 2003;51:2674–8. doi: 10.1021/jf021025f. [DOI] [PubMed] [Google Scholar]
- 92. De Schutter DP, Saison D, Delvaux F, Derdelinckx G, Delvaux FR. The chemistry of aging beer. Beer Health Dis Prevent. 2008;1:375–88. [Google Scholar]
- 93. Charles-Bernard M, Roberts DD, Kraehenbuehl K. Interactions between volatile and nonvolatile coffee components. 2. Mechanistic study focused on volatile thiols. J Agric Food Chem. 2005;53:4426–33. doi: 10.1021/jf048020y. [DOI] [PubMed] [Google Scholar]
- 94. Hofmann T, Schieberle P. Chemical interactions between odor-active thiols and melanoidins involved in the aroma staling of coffee beverages. J Agric Food Chem. 2001;50:319–26. doi: 10.1021/jf010823n. [DOI] [PubMed] [Google Scholar]
- 95. Hofmann T, Czerny M, Calligaris S, Schieberle P. Model studies on the influence of coffee melanoidins on flavor volatiles of coffee beverages. J Agric Food Chem. 2001;49:2382–6. doi: 10.1021/jf0012042. [DOI] [PubMed] [Google Scholar]
- 96. Di R, Kim J, Martin MN, Leustek T, Jhoo J, Ho C-T, Tumer NE. Enhancement of the primary flavor compound methional in potato by increasing the level of soluble methionine. J Agric Food Chem. 2003;51:5695–702. doi: 10.1021/jf030148c. [DOI] [PubMed] [Google Scholar]
- 97. Jung MY, Yoon SH, Lee HO, Min DB. Singlet oxygen and ascorbic acid effects on dimethyl disulfide and off-flavor in skim milk exposed to light. J Food Sci. 1998;63:408–12. [Google Scholar]
- 98. Ganeko N, Shoda M, Hirohara I, Bhadra A, Ishida T, Matsuda H, Takamura H, Matoba T. Analysis of volatile flavor compounds of sardine (Sardinops melanostica) by solid phase microextraction. J Food Sci. 2008;73:S83–8. doi: 10.1111/j.1750-3841.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 99. Campo E, Cacho J, Ferreira V. The chemical characterization of the aroma of dessert and sparkling white wines (Pedro Ximénez, Fino, Sauternes, and Cava) by gas chromatography–olfactometry and chemical quantitative analysis. J Agric Food Chem. 2008;56:2477–84. doi: 10.1021/jf072968l. [DOI] [PubMed] [Google Scholar]
- 100. Pryor WA, Tang RH. Ethylene formation from methional. Biochem Biophys Res Commun. 1978;81:498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- 101. Beauchamp C, Fridovich I. A mechanism for the production of ethylene from methional: the generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970;245:4641–6. [PubMed] [Google Scholar]
- 102. Bors W, Lengfelder E, Saran M, Fuchs C, Michel C. Reactions of oxygen radical species with methional: a pulse radiolysis study. Biochem Biophys Res Commun. 1976;70:81–7. doi: 10.1016/0006-291x(76)91111-6. [DOI] [PubMed] [Google Scholar]
- 103. Adams A, De Kimpe N. Chemistry of 2-acetyl-1-pyrroline, 6-acetyl-1,2,3,4-tetrahydropyridine, 2-acetyl-2-thiazoline, and 5-Acetyl-2,3-dihydro-4H-thiazine: extraordinary Maillard flavor compounds. Chem Rev. 2006;106:2299–319. doi: 10.1021/cr040097y. [DOI] [PubMed] [Google Scholar]
- 104. Favino TF, Fronza G, Fuganti C, Fuganti D, Grasselli P, Mele A. Penicillin acylase-mediated synthesis of 2-acetyl-1-pyrroline and of 2-propionyl-1-pyrroline, key roast-smelling odorants in food. Inclusion complexes with β-cyclodextrin and their NMR and MS characterization. J Org Chem. 1996;61:8975–9. doi: 10.1021/jo961474c. [DOI] [PubMed] [Google Scholar]
- 105. Mahatheeranont S, Keawsa-ard S, Dumri K. Quantification of the rice aroma compound, 2-acetyl-1-pyrroline, in uncooked Khao Dawk Mali 105 brown rice. J Agric Food Chem. 2000;49:773–9. doi: 10.1021/jf000885y. [DOI] [PubMed] [Google Scholar]
- 106. Wongpornchai S, Dumri K, Jongkaewwattana S, Siri B. Effects of drying methods and storage time on the aroma and milling quality of rice (Oryza sativa L.) cv. Khao Dawk Mali 105. Food Chem. 2004;87:407–14. [Google Scholar]
- 107. Schieberle P. Quantitation of important roast-smelling odorants in popcorn by stable isotope dilution assays and model studies on flavor formation during popping. J Agric Food Chem. 1995;43:2442–8. [Google Scholar]
- 108. Meynier A, Mottram DS. The effect of pH on the formation of volatile compounds in meat-related model systems. Food Chem. 1995;52:361–6. [Google Scholar]
- 109. Hofmann T, Schieberle P. Studies on the formation and stability of the roast-flavor compound 2-acetyl-2-thiazoline. J Agric Food Chem. 1995;43:2946–50. [Google Scholar]
- 110. Pripis-Nicolau L, de Revel G, Bertrand A, Maujean A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J Agric Food Chem. 2000;48:3761–6. doi: 10.1021/jf991024w. [DOI] [PubMed] [Google Scholar]