Abstract
In this study, leaves and stems of Talinum triangulare were sequentially extracted with phosphate buffer solution to obtain PTL and PTS (phosphate buffered extracts of T. triangulare leaves and stems), with 75% ethanol to obtained ETL and ETS (ethanol extracts of T. triangulare leaves and stems), or with 90°C boiling water to obtain WTL and WTS (water extracts of T. triangulare leaves and stems). We investigated the antioxidant activities of various T. triangulare extracts, analyzed the extracts’ stimulations on human mononuclear cell (MNC) growth and secretion of cytokines (interleukin-1 beta, interferon-γ, and tumor necrosis factor-α) and nitric oxide, and then assayed their subsequent inhibitions on human leukemic U937 cell growth. Results indicated that extracts of T. triangulare showed significant antioxidant activities. Among these extracts, WTS showed the highest stimulatory effect on human MNC growth. The secretion levels of interleukin-1 beta, interferon-γ, and tumor necrosis factor-α in the conditioned medium, wherein human MNC was treated with 500 μg/mL WTS for 72 hours, were 1275, 859, and 2222 pg/mL, respectively. All conditioned media obtained from human MNCs cultured with various T. triangulare extracts showed significant inhibition against U937 cell growth of over 40%. These results suggest that T. triangulare extracts may be used in health foods for their immunomodulatory potential.
Keywords: antioxidant activities, human leukemic U937 cells, human mononuclear cells, immunomodulatory activity, Talinum triangulare extracts
1. Introduction
Talinum triangulare (Jacq.) is also called Talinum fruticosum (L.) Juss. It is native plant in Mexico, the Caribbean, the Central America, and much of South America. Its common names include Waterleaf, Cariru, Surinam purslane, Philippine spinach, Ceylon spinach, Florida spinach, Potherb fame flower, Lagos bologi, and Sweetheart [1–3]. T. triangulare is widely grown in tropical regions as a leaf vegetable. Originating from the tropical Africa as a terrestrial herbaceous plant, it is now widely cultivated as a medicinal and food crop in India, South America, other parts of Asia, and Nigeria [4–6]. Several articles have shown that T. triangulare can increase stamina and function as an immunostimulant [7,8]. In Taiwan, T. triangulare has been used in the treatment and prevention of hepatic ailments and cancer in folk medicine.
Some vegetable extracts have been found to exhibit functional uses; in certain cases, they function as antioxidants [5,9–12], angiotensin I-converting enzyme inhibitors [13,14], antibiotics [15], and cancer cells’ inhibitors [16–18]. Many human diseases are known to be related to free radicals. The free radical scavenging medicines are antioxidants in nature. Hence, a relationship exists between human health and minor nutrients exhibiting antioxidant activities, such as vitamin C, vitamin E, β-carotene, flavonoids, and other antioxidants [19]. Furthermore, numerous studies (epidemiological, case-control, or prospective and retrospective cohort) related to dietary antioxidant intake have been linked to reducing cancer risks [17,20].
In investigating antioxidant activities, studies on vegetables’ effects in immunomodulation and cancer-cell-growth inhibition may also yield a deeper insight into their functionality. Vegetable extracts with high antioxidant activities can also be used for food preservation. Therefore, in this study, we investigated the extracts of T. triangulare for their antioxidant activities as well as their inhibitory effects on the growth of human leukemic U937 cells, by using an indirect model and studying their immunomodulatory activities.
2. Materials and methods
2.1. Materials
2.1.1. T. triangulare samples
Fresh T. triangulare was obtained from Chungliao (Nantou, Taiwan). T. triangulare samples were separated into leaf and stem, cleaned and immediately stored at −20°C overnight, then lyophilized for 48 hours. Following lyophilization, the dried samples were crushed to 30 mesh powders. The dried powders were stored at −20°C in darkness.
The extracts were prepared using the methods described by Nagai and Inoue [21] and Guo et al [22] with modifications. Three grams of leaf and stem powders was suspended and extracted with 30 volumes of 10 mM of sodium phosphate buffer (pH 7.0) then shaken at 4°C for 24 hours. Extracts were centrifuged at 4000g for 30 minutes, and the supernatants were pooled, lyophilized, and denoted as PTL (phosphate buffered extract of T. triangulare leaf) or PTS (phosphate buffered extract of T. triangulare stem). The residues were extracted with 30 mL 75% ethanol at 4°C for 24 hours. Extracts were centrifuged at 4000g for 30 minutes, and the supernatants were pooled, lyophilized, and denoted as ETL (ethanol extract of T. triangulare leaves) and ETS (ethanol extract of T. triangulare stems). Three grams of leaf and stem powders was extracted with distilled water at 90°C for 2 hours. The extracts were centrifuged at 4000g for 30 minutes, and the supernatants were pooled, lyophilized, and denoted as WTL and WTS (water extracts of T. triangulare leaves and stems, respectively). All samples were stored at −80°C until use.
2.1.2. Reagents
Roswell Park Memorial Institute (RPMI) 1640 and fetal bovine serum (FBS) were purchased from Hyclone (Logan, UT, USA). Sodium bicarbonate, HEPES, l-glutamine, and sodium pyruvate were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Trypan blue was obtained from e-Bioscience (San Diego, CA, USA). Potassium ferricyanide (K3Fe(CN)6), Trolox, α,α-diphenyl-β-picrylhydrazyl(DPPH), 2,2-azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS), EDTA, and α-tocopherol were also purchased from Sigma Chemical Co.
2.1.3. Flavonoids and phenolic acid contents in different T. triangulare extracts
T. triangulare extracts were analyzed for flavonoids and phenolic acid compositions via high-performance liquid chromatography (L-2130; Hitachi Co., katsuda, Japan) using an RP-18GP250 column (L, 250 mm; ID, 4.6 mm) and UV/Vis detectors. Flavonoids and phenolic acid were detected at 280 nm and separated using a linear gradient elution program with a mobile phase containing solvent A (10% methanol with 0.05% formic acid) and solvent B (70% methanol with 0.05% formic acid). The gradient program is shown in Table S1. The flow rate used was set at 1.0 mL/min throughout the gradient. Identification and quantification were accomplished by comparing the retention time of peaks in the methanol-containing solutions to that of the standard compounds.
2.2. Antioxidant activities
2.2.1. Reducing power
A method developed by Chiang and Chang [19] for a reducing power test was used. In brief, sample solutions, α-tocopherol, and butylated hydroxyanisole methanolic solutions (positive control group) were spiked with 2.5 mL of phosphate buffer and 2.5 mL of 1% potassium ferricyanide. Mixtures were kept in a 50°C water bath for 20 minutes, cooled by placing it in 20°C water bath for 5 minutes, spiked with 2.5 mL of 10% trichloroacetic acid, and then centrifuged at 800g for 10 minutes. The supernatant (5 mL) was mixed with 5 mL of distilled water and 1 mL of 0.1% ferric chloride. The absorbance at 700 nm was then detected with a spectrophotometer after reaction for 10 minutes; higher absorbance (A700) represents stronger reducing power.
2.2.2. DPPH radical scavenging activity
The scavenging effect on DPPH free radical was measured following the method described by Shimada et al [23] with some modifications. Sample solutions (5 mL) and α-tocopherol methanolic solutions (positive control group) were added to 1 mL of 1 mM DPPH in methanolic solution. The mixture was shaken and left to stand for 30 minutes at room temperature. The absorbance rates of the resulting solution and the positive control group was measured at 517 nm. The lower absorbance (A517) represents a higher DPPH scavenging activity, which is expressed as [1 – (test sample absorbance/blank sample absorbance)] × 100%.
2.2.3. Ferrous ion chelating ability
The method described by Decker and Welch [24] was adopted. Five milliliters of the test solutions, including sample and EDTA solutions, was spiked with 0.1 mL of 2mM FeCl2 and 0.2 mL of 5mM ferrozine solutions. After reaction for 10 minutes, the absorbance at 562 nm of the resulting solutions was recorded. The higher ferrous ion chelating ability of the test sample gave a lower absorbance (A562). The percentage of ferrous ion chelating ability is expressed as [1 – (test sample absorbance/blank sample absorbance)] × 100.
2.2.4. Trolox equivalent antioxidant capacity
The ABTS scavenging activity was determined using the Trolox equivalent antioxidant capacity (TEAC) method according to Yeh and Yen [25]. The TEAC assay is based on the capacity to quench ABTS·+ radical formation relative to Trolox. Briefly, 0.1 mL of extracts representing 1.0–10.0 mg/mL dry weight of plants was incubated for 45 seconds with 0.9 mL ABTS solution, and ABTS absorbance was measured at 734 nm. Data are expressed as Trolox equivalents, based on standard curves of 1.0–3.5 mmol Trolox.
2.3. Cell assays
2.3.1. Cell survival of human mononuclear cells by T. triangulare extracts
Human peripheral blood was obtained (in the laboratory) from healthy adult volunteers between the ages of 22 and 25 years. Human mononuclear cells (MNCs) were recovered by adding blood to Ficoll–Hypaque solution (1.077 g/mL; Sigma Chemical Co.) and centrifuging at 400g for 30 minutes. A suspension of 5 × 105 cells/mL in an RPMI 1640 medium was prepared. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assays for determining the growth of MNCs were modified from those of Liao et al [26] and Chen et al [27]. MNCs were incubated with various concentrations of different T. triangulare extracts in medium, medium only, and medium with same extract volume of phosphate buffer saline (PBS; control) at 37°C in a humidified atmosphere containing 5% CO2 for 24 and 72 hours, respectively. Following PBS washing, 25 μL of MTT reagent was added in FBS-free medium, and cells were incubated for 4 hours. One hundred microliters of MTT lysis buffer (25 mL of N,N-dimethyl formamide and 0.67 g/mL sodium dodecyl sulfate) was added, and then cells were incubated for 16 hours. After centrifugation, supernatants were assayed for absorbance at 570 nm. The growth index was calculated using the following equation:
2.3.2. Evaluation of cytokine productions in T. triangulare extracts-treated MNCs’ condition media
The cytokine test for the T. triangulare extracts was determined according to Chen et al[27]. In brief, a suspension of 1 × 106 cells/mL in an RPMI 1640 medium was prepared. MNCs were stimulated with different T. triangulare extracts at various concentrations for 24 and 72 hours, respectively. Cell media were filtered to obtain condition media (CMs). The concentrations of cytokines including interleukin-1 beta (IL-1β), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) were determined using commercial enzyme-linked immunoassay kits (Bender MedSystem, Inc., Burlingame, CA, USA).
2.4. Measurement of NO productions in MNC-CMs
NO productions in T. triangulare extracts-treated MNC-CMs were determined [28]. After an incubation of 24 and 72 hours, 100 μL of the MNC-CMs was mixed with an equal volume of Griess reagent (1% sulfanilamide/0.1% NED/2.5% H3PO4) in a 96-well flat-bottomed microtiter plate and incubated at room temperature for 15 minutes. The absorbance was read at 540 nm by a microplate reader (μQuant; BIO-TEK Instruments, Inc., Winooski, Vermont, USA). The nitrite concentrations were determined by comparison with the sodium nitrite standard curve.
2.4.1. T. triangulare extract-treated MNC-CM affecting human leukemia cell growth
The human leukemia U937 cell line was obtained from the Bioresource Collection and Research Center in Taiwan. Cells were cultured in RPMI 1640, supplemented with 10% heat-inactivated FBS, penicillin (100 units/mL), streptomycin (100 μg/mL), HEPES (10mM), sodium pyruvate (1mM), and sodium bicarbonate (10mM). Cells were subsequently incubated at 37°C in a humidified atmosphere containing 5% CO2. Then, 1 × 105 cells/mL U937 cells were seeded in a 12-well microtiter plate and treated with different T. triangulare extracts-treated MNC-CMs (fresh cell medium/CM = 4:1, v/v) for 5 days. The number of viable cells was counted using a trypan blue dye exclusion test [29]. Growth inhibition (%) was calculated with the following equation:
2.5. Statistical analysis
The data were subjected to analysis of variance using Statistic Analytical System (SAS, SAS Institute Inc., Cary, NC, USA) [30]. Duncan’s range tests were used for paired comparison of means. Results were considered significant if the associated p value is < 0.05.
3. Results
3.1. Flavonoids and phenolic acid contents in different T. triangulare extracts
Flavonoids are the most common and widely distributed group of plant phenolic compounds. In this study, the naringin, hesperisin, diosmin, quercetin, and hesperetin contents of different T. triangulare extracts were analyzed, and the results are shown in Table 1. The naringin content of WTS was the highest (263.7 ± 0.59 μg/100 mg) and that of PTL was the lowest (not detectable (ND)). The decreasing sequence of naringin contents of the leaf extracts was ETL > WTL > PTL; for the stem extracts, the decreasing sequence was WTS > ETS > PTS. The hesperisin contents of ETL, WTL, and WTS were 0.12 ± 0.01, 0.14 ± 0.01, and 0.12 ± 0.01 mg/100 mg, respectively. The diosmin contents of ETS, WTL, and WTS were 0.12 ± 0.01, 0.05 ± 0.01, and 0.12 ± 0.01 mg/100 mg, respectively. The quercetin content of WTL was the highest (2.72 ± 0.06 mg/100 mg) and that of ETS was the lowest (0.51 ± 0.05 mg/100 mg). The decreasing sequence of quercetin contents of the leaf extracts was WTL > PTL > ETL; for the stem extracts, the decreasing sequence was the same as that of the leaf extracts. However, hesperetin was only detectable in ETS (31.6 ± 0.32 μg/100 mg).
Table 1.
Contents of various flavonoids in different extracts of Talinum triangulare.
| Naringin | Hesperisin | Diosmin | Quercetin | Hesperetin | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| (μg/100 mg) | (mg/100 mg) | (mg/100 mg) | (mg/100 mg) | (μg/100 mg) | |
| ETL | 144.9 ± 0.68c | 0.12 ± 0.01b | ND | 0.53 ± 0.02e | ND |
| ETS | 150.5 ± 0.62b | ND | 0.12 ± 0.01a | 0.51 ± 0.05e | 31.6 ± 0.32a |
| WTL | 121.5 ± 1.90d | 0.14 ± 0.01a | 0.05 ± 0.01b | 2.72 ± 0.06a | ND |
| WTS | 263.7 ± 0.59a | 0.12 ± 0.01b | 0.12 ± 0.01a | 0.64 ± 0.01c | ND |
| PTL | ND | ND | ND | 1.43 ± 0.01b | ND |
| PTS | 42.6 ± 1.37e | ND | ND | 0.56 ± 0.02d | ND |
All values are presented as mean ± SD; within the same column values not sharing a common superscript letter are significantly different from one another by Duncan’s range test (p < 0.05; n = 3).
ETL = ethanol extract of T. triangulare leaves; ETS = ethanol extract of T. triangulare stems; ND = not detectable; SD = standard deviation; WTL = water extract of T. triangulare leaves; WTS = ethanol extract of T. triangulare stems.
It is well known that phenolic compounds belong to the bioactive components of plant products and have good health-promoting activities. In this study, contents of gallic acid, protocatechuic acid, catechin, vanillic acid, chlorogenic acid, caffeic acid, and ferulic acid were detected in different T. triangulare extracts, and the results are shown in Table 2. WTS had the highest gallic acid content (334.80 ± 1.52 μg/100 mg), whereas PTL had the lowest level (0.75 ± 0.02 μg/100 mg). The decreasing sequence of gallic acid contents of the leaf extracts was WTL > ETL > PTL; for the stem extracts, the decreasing sequence was WTS > PTS > ETS. Both protocatechuic acid and vanillic acid were not detected in all T. triangulare extracts. The highest levels of catechin (17.13 ± 0.01 mg/100 mg), chlorogenic acid (55.33 ± 0.53 mg/100 mg), and caffeic acid (8.32 ± 0.22 μg/100 mg) were all found in WTL, and the lowest in PTL (ND). WTS showed the highest ferulic acid content (70.84 ± 0.35 μg/100 mg) and PTL the lowest (ND). The decreasing sequence of all catechin, chlorogenic acid, caffeic acid, and ferulic acid contents of the leaf extracts was WTL > ETL > PTL. For the stem extracts, the decreasing sequence of catechin, caffeic acid, and ferulic acid contents was WTS > ETS > PTS; but for chlorogenic acid content, it was ETS > WTS > PTS.
Table 2.
Contents of various phenolic acids in different Talinum triangulare extracts.
| Hydroxybenzoic acids | Hydroxycinnamic acids | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Gallic acid | Protocatechuic acid | Catechin | Vanillic acid | Chlorogenic acid | Caffeic acid | Ferulic acid | |
|
|
|
|
|
|
|
|
|
| (μg/100 mg) | (mg/100 mg) | (mg/100 mg) | (mg/100 mg) | (mg/100 mg) | (μg/100 mg) | (μg/100 mg) | |
| ETL | 13.84 ± 0.33d | ND | 13.15 ± 0.01c | ND | 17.49 ± 0.73d | 3.79 ± 0.29c | 15.03 ± 0.11d |
| ETS | 12.63 ± 0.42e | ND | 12.43 ± 0.29d | ND | 22.83 ± 0.39b | 2.44 ± 0.09d | 29.54 ± 0.50b |
| WTL | 64.48 ± 0.55b | ND | 17.13 ± 0.01a | ND | 55.33 ± 0.53a | 8.32 ± 0.22a | 17.90 ± 0.22c |
| WTS | 334.80 ± 1.52a | ND | 14.23 ± 0.11b | ND | 18.67 ± 0.40c | 5.57 ± 0.15b | 70.84 ± 0.35a |
| PTL | 0.75 ± 0.02f | ND | ND | ND | ND | ND | ND |
| PTS | 30.42 ± 0.25c | ND | 11.01 ± 0.02e | ND | 4.01 ± 0.02e | 0.96 ± 0.02e | 11.01 ± 0.02e |
All values are means ± SD. Values not sharing a common letter (superscript) within the same column are significantly different from one another by Duncan’s range test (p < 0.05; n = 3).
ETL = ethanol extract of T. triangulare leaves; ETS = ethanol extract of T. triangulare stems; ND = not detectable; SD = standard deviation; WTL = water extract of T. triangulare leaves; WTS = ethanol extract of T. triangulare stems.
3.2. Antioxidant activities of different T. triangulare extracts
As these T. triangulare extracts contained various and significant amounts of flavonoids and phenolic acids, we examined their antioxidant activities. The yields of different extracts (WTL, WTS, PTL, PTS, ETL, and ETS) were 14.45%, 16.38%, 9.51%, 11.87%, 12.26%, and 13.48%, respectively. The WTS and ETS of T. triangulare extracts were shown to have the greatest DPPH and ferrous ion chelating ability relative to other T. triangulare extracts (Table 3). As for the reducing power of extracts, absorbance at 700 nm ranged between 0.19 and 0.49 at a sample concentration of 1 mg/mL. The highest activity was in ETL (0.49), and the lowest was in PTL (0.24; Table 3). For the stem extracts, PTS showed the highest activity (0.38), and WTS showed the lowest (0.19) (Table 3). The DPPH radical scavenging activities ranged between 5.71% and 60.93%. The decreasing sequence of DPPH radical scavenging activities of the leaf and stem extracts was ETS and WTS > ETL and WTL > PTS and PTL (Table 3). The extracts’ ferrous ion chelating ability ranged between 19.49% and 69.30%. The decreasing sequence of the ferrous ion chelating ability of the leaf and stem extracts was similar to DPPH radical scavenging activities (Table 3). Meanwhile, the TEAC value ranged between 2.05 and 2.18 mmol Trolox. None of the extracts showed any significant differences.
Table 3.
Antioxidant activities of different extracts of Talinum triangulare.
| Samples (1 mg/mL) | DPPH scavenging (%) | Reducing power (absorbance at 700 nm) | Ferrous ion chelating ability (%) | TEAC value (mM Trolox) |
|---|---|---|---|---|
| ETL | 43.57 ± 0.05b | 0.49a | 40.93 ± 0.37b | 2.15 ± 0.007a |
| ETS | 60.93 ± 0.87a | 0.27c | 69.30 ± 1.70a | 2.05 ± 0.000b |
| WTL | 41.20 ± 0.86c | 0.28c | 47.26 ± 0.47b | 2.05 ± 0.000b |
| WTS | 60.10 ± 0.50a | 0.19d | 63.94 ± 1.25a | 2.17 ± 0.003a |
| PTL | 5.71 ± 0.04e | 0.24c | 19.49 ± 0.11c | 2.18 ± 0.002a |
| PTS | 8.34 ± 0.01d | 0.38b | 25.03 ± 0.64c | 2.15 ± 0.004a |
Data with different letters (superscript) within the same column are significantly different (p < 0.05).
DPPH = α,α-diphenyl-β-picrylhydrazyl; ETL = ethanol extract of T. triangulare leaves; ETS = ethanol extract of T. triangulare stems; SD = standard deviation; TEAC = Trolox equivalent antioxidant capacity; WTL = water extract of T. triangulare leaves; WTS = ethanol extract of T. triangulare stems.
From the results shown in Table 3, we may deduce that the stem extracts of T. triangulare have greater antioxidant activities than its leaf extracts. Furthermore, extracts obtained from 90°C boiling water and 75% ethanol showed stronger antioxidant activities than those from PBS.
3.3. Effects of various concentrations of WTL and WTS on growth of MNC
Because T. triangulare extracts with 90°C boiling water (WTL and WTS) showed almost the highest concentrations of flavonoids and phenolic acids, and the highest antioxidant activities, we used WTL and WTS as samples to examine the immunomodulatory activities of T. triangulare extracts via an indirect mode. First, we tested whether WTL or WTS affected growth of MNCs. MNCs are cells existing in the peripheral blood of healthy adults. It would be beneficial to human health if MNCs could be stimulated, given that they can secrete cytokines to enhance human immunity. MNCs were treated with various concentrations of WTL and WTS for 24 and 72 hours, respectively. The growth indexes of MNCs were calculated using treatments with medium only as 1.00. Table 4 illustrates that both WTL and WTS stimulated MNC growth. The MNC stimulations increased with time and dosage. WTS showed stronger MNC stimulating effects than WTS. Thus, the highest growth index (1.79) was achieved by a treatment using WTL at 1000 μg/mL for 72 hours. However, the same volume of PBS treatment showed no effect on MNC growth.
Table 4.
Growth index of MNC after incubation with indicated concentrations of Talinum triangulare extracts for 24 and 72 hours.
| Growth index of MNC | |||||
|---|---|---|---|---|---|
|
| |||||
| Sample concentration (μg/mL) | |||||
|
| |||||
| Sample | 100 | 200 | 500 | 800 | 1000 |
| WTL (24 h) | 1.24 ± 0.01b | 1.23 ± 0.04b | 1.33 ± 0.04a | 1.35 ± 0.05a | 1.36 ± 0.03a |
| WTS (24 h) | 1.33 ± 0.01b | 1.31 ± 0.03b | 1.35 ± 0.02ab | 1.41 ± 0.02a | 1.43 ± 0.04a |
| Medium (24 h): 1.00 ± 0.00 | |||||
| PBS (24 h): 1.02 ± 0.01 | |||||
| WTL (72 h) | 1.32 ± 0.04b | 1.32 ± 0.06b | 1.35 ± 0.02ab | 1.40 ± 0.02a | 1.41 ± 0.07a |
| WTS (72 h) | 1.67 ± 0.01b | 1.65 ± 0.01b | 1.74 ± 0.02a | 1.70 ± 0.00a | 1.79 ± 0.02a |
| Medium (72 h): 1.00 ± 0.00 | |||||
| PBS (72 h): 0.99 ± 0.03 | |||||
Data with different letters (superscript) within the same row are significantly different (p < 0.05).
MNC = mononuclear cell; PBS = phosphate buffer saline; WTL = water extract of T. triangulare leaves; WTS = ethanol extract of T. triangulare stems.
3.4. Inhibitions of human leukemic U937 cell growth by WTL- and WTS-treated MNC-CMs
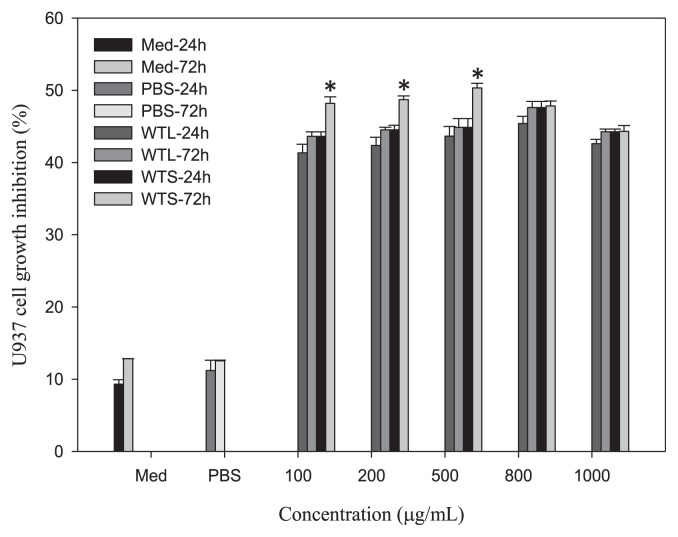
From previous results, we showed that both WTL and WTS stimulated MNC growth. We next tested whether WTL- and WTS-treated MNC-CMs can inhibit human leukemic U937 cell growth. MNCs were treated with various concentrations of WTL and WTS for 24 and 72 hours, respectively, to obtain different CMs. The inhibitory effects on the growth of human leukemic U937 cells were studied following different CMs’ treatments. The experimental results shown in Fig. 1 indicate that MNC-CMs prepared from both WTL- and WTS-treated CMs significantly inhibited the growth of U937 cells when compared with the PBS-treated control group. All WTL- and WTS-treated MNC-CMs showed over 40% growth inhibition ratio of U937 cells. The results of U937 growth inhibitions showed time-related—but not dosage-related—effect because growth inhibitions increased with treatment time but not with extract dosage. The MNC-CMs with 500 μg/mL WTS and 800 μg/mL WTL treatments for 72 hours showed the highest U937 cell growth inhibitions of 50.33% and 48.35%, respectively.
Fig. 1.
Growth inhibitions of U937 cells treated with MNC-CMs prepared from different Talinum triangulare extracts for 24 and 72 hours. Values are expressed as mean ± SD (n = 3). *p < 0.01, statistically significant difference within each group. MNC-CM = mononuclear cell-condition medium; SD = standard deviation.
3.5. Cytokine concentrations in WTL- and WTS-treated MNC-CMs
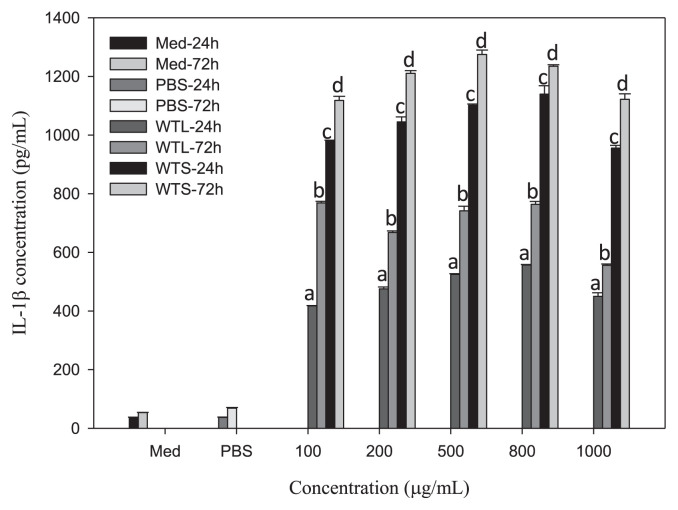
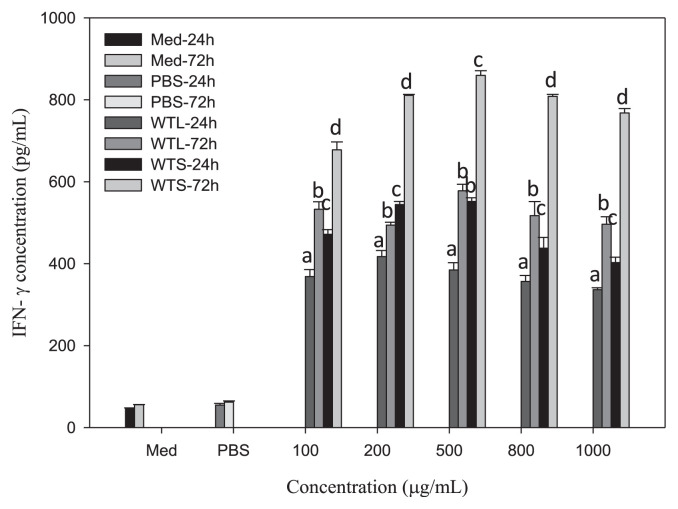
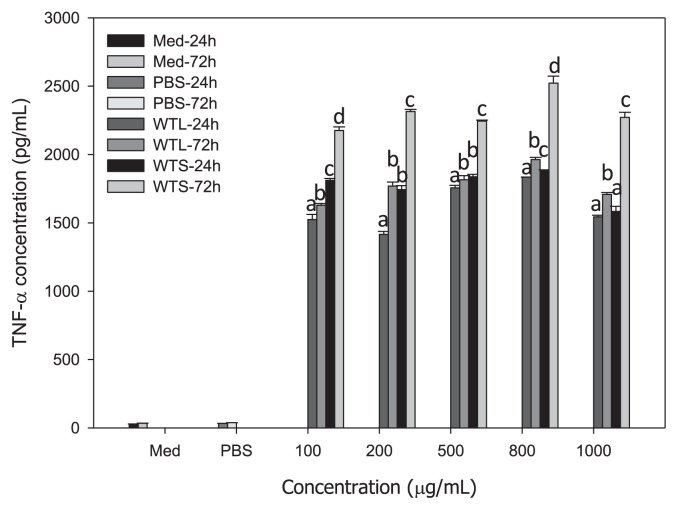
To explain why WTL- and WTS-treated MNC-CMs can significantly inhibit leukemic U937 cell growth, we investigated the concentrations of three cytokines (IL-1β, IFN-γ, and TNF-α) in WTL- and WTS-treated MNC-CMs, because these three cytokines secreted by activated MNCs have been shown to have antiproliferative and differentiating effects on human leukemic U937 cells [31,32]. MNCs were treated with various concentrations of WTL and WTS for 24 and 72 hours, respectively. The concentrations of cytokines in the supernatants of cultured media were then determined. All IL-1β (Fig. 2), IFN-γ (Fig. 3), and TNF-α (Fig. 4) concentrations in WTL- and WTS-treated MNC media were significantly increased compared with those in PBS-treated control group. The concentrations of cytokines in these media also showed time-related—but not dosage-related—effects (as shown in Fig. 1), because concentrations of cytokines increased with treatment time but not with extract dosage. The IL-1β concentrations in WTL- and WTS-treated MNC-CMs (at 500 μg/mL, for 72 hours) were 741.47 and 1275.01 pg/mL, respectively (Fig. 2); the IFN-γ concentrations were 577.89 and 859.48 pg/mL, respectively (Fig. 3); the TNF-α concentrations were 1862.85 and 2222.29, respectively (Fig. 4). At the same concentration and treatment time, all cytokine concentrations of MNCs treated with WTS were higher than those of WTL treatments. From these results, it can be concluded that T. triangulare extracts of WTS and WTL are capable of stimulating MNCs to secrete cytokines. Therefore, WTL- and WTS-treated MNC-CMs can significantly inhibit leukemic U937 cell growth.
Fig. 2.
Concentrations of IL-1β in various MNC-CMs treated with different Talinum triangulare extracts for 24 and 72 hours. Values are expressed as mean ± SD (n = 3). Each letter indicates a statistically significant difference within each group, p < 0.01. IL-1β = interleukin-1 beta; MNC-CM = mononuclear cell-condition medium; SD = standard deviation.
Fig. 3.
Concentrations of IFN-γ in various MNC-CMs treated with different Talinum triangulare extracts for 24 and 72 hours. Values are expressed as mean ± SD (n = 3). Each letter indicates a statistically significant difference within each group, p < 0.01. IFN-γ = interferon-gamma; MNC-CM = mononuclear cell-condition medium; SD = standard deviation.
Fig. 4.
Concentrations of TNF-α in various MNC-CMs treated with different Talinum triangulare extracts for 24 and 72 hours. Values are expressed as mean ± SD (n = 3). Each letter indicates a statistically significant difference within each group, p < 0.01. MNC-CM = mononuclear cell-condition medium; SD = standard deviation; TNF-α = tumor necrosis factor-alpha.
3.6. Effect on nitric oxide productions of WTL- and WTS-treated MNCs
As nitric oxide (NO) is an important cellular messenger for many physiological responses including inflammation, we were interested in whether WTL and WTS treatment induced NO productions in MNCs. As shown in Table 5, all NO concentrations in WTL- and WTS-treated MNC media were significantly increased compared with those in the PBS-treated control group. The NO concentrations in WTL-treated groups also showed time-related, but not dosage-related, effect because NO concentration increased with treatment time but not with extract dosage. However, the NO concentrations in WTS-treated groups showed reversed time-related (except for 1000 μg/mL), but not dosage-related, effect because NO concentrations decreased with treatment time (except for the dosage of 1000 μg/mL) but not with extract dosage. MNCs (200 and 500 μg/mL) treated with WTS for 24 hours showed the highest NO concentrations (2.79 μM). For WTL, all (200, 500, and 800 μg/mL) WTL-treated MNCs treated for 72 hours showed the highest NO concentrations (1.87–1.92 μM). Because NO could induce the death of leukemic U937 cells [27], we showed that WTS and WTL can stimulate the growth of MNCs (Table 4) and enhance cytokine secretions (Figs. 2–4) and NO productions (Table 5) of MNCs. Therefore, WTS- and WTL-treated MNC-CMs can significantly inhibit the growth of leukemic U937 cells (Fig. 1).
Table 5.
Concentrations of nitric oxide in media of MNC incubated with indicated concentrations of Talnum triangulare extracts for 24 and 72 hours.
| Concentrations of nitric oxide (μM) | |||||
|---|---|---|---|---|---|
|
| |||||
| Sample concentrations (μg/mL) | |||||
|
| |||||
| Sample | 100 | 200 | 500 | 800 | 1000 |
| WTL (24 h) | 0.98 ± 0.05ab | 0.85 ± 0.08b | 1.05 ± 0.13a | 0.85 ± 0.02b | 0.85 ± 0.11b |
| WTS (24 h) | 2.59 ± 0.05bc | 2.79 ± 0.04a | 2.79 ± 0.04a | 2.46 ± 0.07c | 0.78 ± 0.04d |
| Medium (24 h): 0.33 ± 0.03 | |||||
| PBS (24 h): 0.42 ± 0.01 | |||||
| WTL (72 h) | 1.75 ± 0.03b | 1.88 ± 0.10a | 1.87 ± 0.08a | 1.92 ± 0.08a | 1.79 ± 0.04b |
| WTS (72 h) | 1.67 ± 0.01c | 1.65 ± 0.01c | 1.74 ± 0.02ab | 1.70 ± 0.00b | 1.79 ± 0.02a |
| Medium (24 h): 0.41 ± 0.01 | |||||
| PBS (72 h): 0.44 ± 0.04 | |||||
Data with different letters within the same row are significantly different (p < 0.05).
MNC = mononuclear cell; PBS = phosphate buffer saline; WTL = water extract of T. triangulare leaves; WTS = ethanol extract of T. triangulare stems.
4. Discussion
Oxidative stress is responsible for more than 100 human diseases [5] including cancers worldwide. Flavonoids are polyphenols widely distributed in fruits and vegetables, and have been shown to be good antioxidants. Phenolic compounds are bioactive components of plants and show health-promoting activities. In this study, we reported that extracts from T. triangulare showed significant contents of flavonoids (Table 1) and phenolic acids (Table 2), and that T. triangulare extracts also showed significant antioxidant activities (Table 3). Because WTL and WTS could significantly stimulate MNC growth (Table 4), secretions of cytokines (Figs. 2–4), and productions of NO (Table 5), both WTL- and WTS-treated MNC-CMs showed significant inhibitory effects on human leukemic U937 cell growth (Fig. 1). Thus, T. triangulare extracts showed significant immunomodulatory activities that can inhibit leukemic U937 cancer cells. Based on these results, we suggest that T. triangulare extracts can be used as healthy foods because of their antioxidant activities and immunomodulatory potential.
Stem extracts of T. triangulare (such as WTS) have higher concentrations of flavonoids and phenolic acids than leaf extracts of T. triangulare (such as WTL) (Tables 1 and 2), and also show stronger antioxidant activities (Table 3). These results are reliable. Developing a new extraction method to obtain more flavonoids and phenolic acids from T. triangulare should increase the extract’s antioxidant activities. Furthermore, WTS also showed stronger stimulations of MNCs than WTL at any same dosage and time interval (Table 4), induction of more cytokine secretions (Figs. 2–4), and higher production of NO at 24 hours (Table 5); in addition, WTS-treated MNC-CMs showed stronger inhibitory effects than WTL-treated MNC-CMs on the cell growth of U937 cells (Fig. 1). These results are also reliable. They show that antioxidant activities are significantly related to growth inhibition of U937 cells, because the stronger antioxidant activities of T. triangulare extracts showed better U937 cell growth inhibitions. The secreted cytokines and NO are significantly related to growth inhibition of U937 cell, because U937 cell growth inhibition increases with increasing concentrations of cytokines and NO. Although the NO concentrations in WTS (72 hours) groups were lower than those in WTS (24 hours) groups, except for the sample concentration of 1000 μg/mL (Table 5), the concentrations of the three examined cytokines secreted by MNCs in WTS (72 hours) groups were all higher than those in the WTS (24 hours) groups (Figs. 2–4). In combination with the effects of NO and three cytokines, the inhibitory effects on U937 cells in the WTS (72 hours) groups were higher than those in the WTS (24 hours) groups at sample concentrations of 100–500 μg/mL, although no difference was found at 800–1000 μg/mL (Fig. 1).
Because the extracts of T. triangulare with stronger antioxidant activities show stronger immunomodulatory activities, it is implied that the contents of flavonoids and phenolic acids in T. triangulare are responsible not only for its antioxidant activities but also for its immunomodulatory activities. In addition, the stem of T. triangulare shows more promise compared with its leaf as a potential healthy food because it exhibits higher antioxidant and immunomodulatory activities. Further studies will examine whether T. triangulare extracts show antioxidant activities and determine the mechanism that induces cytokine secretion and NO production in MNCs.
The MNC-CMs treated with 500 μg/mL WTS for 72 hours showed the highest U937 cell growth inhibition (at 50.33%). Furthermore, the growth inhibition of U937 cells treated with MNC-CMs prepared with the cold-water extract of Flammulina velutipes at 800 μg/mL was 60% [31]; with the water extract of rice at 500 mg/mL, growth inhibition was calculated as 35% [26]; with bovine skimmed colostrums and their hydrolysates collected on the 2nd day postpartum at 500 μg/mL, it was 50% [27]; and with semipurified polysaccharides of Antrodia camphorate at 100 μg/mL, it was 55% [29]. Collectively, these results indicate a similar potential of WTS as immunomodulatory extracts.
The results of this study show that T. triangulare extracts exhibit immunomodulatory effects through the stimulation of MNCs for secretions of cytokines (IL-1β, IFN-γ, and TNF-α) and induction of NO into the MNC-CMs. The cytokines (IL-1β, IFN-γ, and TNF-α) are able to induce the differentiation and death of U937 cells [31,32]. NO is recognized as one of the most versatile players in the immune system. It is involved in the pathogenesis and control of infectious diseases, tumors, autoimmune processes, and chronic degenerative diseases [33]. Yang and Park [28] reported that antioxidant enzyme inhibitors enhance NO-induced cell death in U937 cells. It may be stated from these results that T. triangulare extracts not only inhibit the growth of U937 cells and exhibit their immunomodulatory activities, but also stimulate the growth of MNCs.
Supplementary Information
Table S1.
HPLC solvent gradient elution program.
| Time (min) | Eluent solvent (%) | |
|---|---|---|
|
| ||
| A | B | |
| 0 | 100 | 0 |
| 5 | 80 | 20 |
| 15 | 70 | 30 |
| 20 | 70 | 30 |
| 30 | 65 | 35 |
| 35 | 65 | 35 |
| 45 | 50 | 50 |
| 55 | 50 | 50 |
| 65 | 30 | 70 |
| 70 | 30 | 70 |
| 75 | 0 | 100 |
| 80 | 0 | 100 |
| 85 | 0 | 100 |
A = 10% methanol and 0.05% formic acid; B = 70% methanol and 0.05% formic acid.
HPLC = high-performance liquid chromatography.
Acknowledgments
This work was supported in part by the National Science Council Taiwan, R.O.C. (grant number: NSC 99-2314-B-212-001-MY3).
Funding Statement
This work was supported in part by the National Science Council Taiwan, R.O.C. (grant number: NSC 99-2314-B-212-001-MY3).
Footnotes
Conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1. Fasuyi AO. Nutritional potentials of some tropical vegetable leaf meals: chemical characterization and functional properties. Afr J Biotechnol. 2005;5:049–53. [Google Scholar]
- 2. Fasuyi AO. Bio-nutritional evaluations of three tropical leaf vegetables (Telfairia occidentalis, Amaranthus cruentus and Talinum triangulare) as sole dietary protein sources in rat assay. Food Chem. 2007;103:757–65. [Google Scholar]
- 3. Uwah EI, Ndahi NP, Ogugbuaja VO. Study of the levels of some agricultural pollutants in soils and water leaf (Talinum triangulare) obtained in maiduguri. Nigeria J Appl Sci Environ. 2009;4:71–8. [Google Scholar]
- 4. Kumar A, Prasa MNV, Sytar O. Lead toxicity, defense and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere. 2012;89:1056–65. doi: 10.1016/j.chemosphere.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 5. Liang D, Zhou Q, Gong W, Wang Y, Nie Z, He H, Li J, Wu J, Wu C, Zhang J. Studies on the antioxidant and hepatoprotective activities of polysaccharides from Talinum triangulare. J Ethnopharmacol. 2011;136:316–21. doi: 10.1016/j.jep.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 6. Nyffeler R, Eggli U. Disintegrating Portulacaceae: a new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. Taxon. 2010;59:227–40. [Google Scholar]
- 7. Agbonon A, Eklu-Gadegbeku K, Aklikokou K, Gbeassor M, Akpagana K, Tam TW, Arnason JT, Foster BC. In vitro inhibitory effect of West African medicinal and food plants on human cytochrome P450 3A subfamily. J Ethnopharmacol. 2009;128:390–4. doi: 10.1016/j.jep.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Fenny KL, Andreanus AS, Immaculata M. Uji aktivitas imunostimulan daun ginseng Sumatera (Talinum triangulare Willd) leaves and Korea ginseng (Panax ginseng C.A. Mayer) leaves. Bandung, Indonesia: Skripsi, Department Farmasi, Institute Teknologi Bandung; 1996. [Google Scholar]
- 9. Moyo M, Amoo SO, Ncube B, Ndhlala AR, Finnie JF, Van Staden J. Phytochemical and antioxidant properties of unconventional leafy vegetables consumed in southern Africa. S Afr J Bot. 2013;84:65–71. [Google Scholar]
- 10. Uusiku NP, Oelofse A, Duodu KG, Bester MJ, Faber M. Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: a review. J Food Compost Anal. 2010;23:499–509. [Google Scholar]
- 11.Yahia EM. The contribution of fruit and vegetable consumption to human health. In: De la Rosa LA, Alvarez-Parrilla E, González-Aguilar GA, editors. Fruit and vegetable phytochemicals: chemistry, nutritional value and stability. New Delhi, India: Wiley-Blackwell; 2010. pp. 3–51. [Google Scholar]
- 12. Andarwulan N, Batari R, Sandrasari DA, Bolling B, Wijaya H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010;121:1231–5. doi: 10.1016/j.foodchem.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oboha G, Ademiluyia AO, Akinyemia AJ, Henleb T, Jamiyu A, Saliua JA, Uwe Schwarzenbolzb U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012;4:450–8. [Google Scholar]
- 14. McCarty MF. Scavenging of peroxynitrite-derived radicals by flavonoids may support endothelial NO synthase activity, contributing to the vascular protection associated with high fruit and vegetable intakes. Med Hypotheses. 2008;70:170–81. doi: 10.1016/j.mehy.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 15. Packiavathy IASV, Agilandeswari P, Musthafa KS, Pandian SK, Ravi AV. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res Int. 2012;45:85–92. [Google Scholar]
- 16. Sun J, Liu RH. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006;241:124–34. doi: 10.1016/j.canlet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 17. Jayaprakasha GK, Murthy KMC, Etlinger M, Shivappa M, Mantur SM, Patil BS. Radical scavenging capacities and inhibition of human prostate (LNCaP) cell proliferation by Fortunella margarita. Food Chem. 2012;134:184–91. [Google Scholar]
- 18. Kim J, Jayaprakasha GK, Uckoo RM, Patil BS. Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem Toxicol. 2012;50:423–30. doi: 10.1016/j.fct.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 19. Chiang SH, Chang CY. Antioxidant properties of caseins and whey proteins from colostrums. J Food Drug Anal. 2005;13:57–63. [Google Scholar]
- 20. Becker EM, Nissen LR, Skibsted LH. Antioxidant evaluation protocols: food quality or health effects. Eur J Food Res Technol. 2004;219:561–71. [Google Scholar]
- 21. Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004;84:181–6. [Google Scholar]
- 22. Guo H, Ekusa A, Iwai K, Yonekura M, Takahata Y, Morimatsu F. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol (Tokyo) 2008;54:191–5. doi: 10.3177/jnsv.54.191. [DOI] [PubMed] [Google Scholar]
- 23. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthane on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 24. Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–7. [Google Scholar]
- 25. Yeh CT, Yen GC. Effects of phenolic acids on human phenol sulfotransferases in relation to their antioxidant activity. J Agric Food Chem. 2003;51:1474–9. doi: 10.1021/jf0208132. [DOI] [PubMed] [Google Scholar]
- 26. Liao HF, Chen YY, Yang YC, Wang CS, Chen YJ. Rice (Oryza sativa L.) inhibits growth and induces differentiation of human leukemic U937 cells through activation of peripheral blood mononuclear cells. Food Chem Toxicol. 2006;44:1724–9. doi: 10.1016/j.fct.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 27. Chen CW, Chiang SH, Wang SY, Lin YT, Chang CY. Growth inhibition and differentiation effects of protein hydrolysates from bovine colostrums on human leukemic U937 cells. J Food Biochem. 2013;37:8–17. [Google Scholar]
- 28. Yang ES, Park JW. Antioxidant enzyme inhibitors enhance nitric oxide-induced cell death in U937 cells. Biochimie. 2006;88:869–78. doi: 10.1016/j.biochi.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29. Liu JJ, Huang TS, Hsu ML, Chen CC, Lin WS, Lu FJ, Chang WH. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol Appl Pharmacol. 2004;201:186–93. doi: 10.1016/j.taap.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Institute SAS. SAS/IML user’s guide. Version 9.1. Cary, NC: SAS Institute Inc; 2001. [Google Scholar]
- 31. Ou HT, Shieh CJ, Chen YJ, Chang MH. The antiproliferative and differentiating effects of human leukemic U937 cells mediated by cytokines from activated mononuclear cells by dietary mushrooms. J Sci Food Agric. 2005;53:300–5. doi: 10.1021/jf0493425. [DOI] [PubMed] [Google Scholar]
- 32. Chen YJ, Shiao MS, Lee SS, Wang SY. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997;60:2349–59. doi: 10.1016/s0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- 33. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
HPLC solvent gradient elution program.
| Time (min) | Eluent solvent (%) | |
|---|---|---|
|
| ||
| A | B | |
| 0 | 100 | 0 |
| 5 | 80 | 20 |
| 15 | 70 | 30 |
| 20 | 70 | 30 |
| 30 | 65 | 35 |
| 35 | 65 | 35 |
| 45 | 50 | 50 |
| 55 | 50 | 50 |
| 65 | 30 | 70 |
| 70 | 30 | 70 |
| 75 | 0 | 100 |
| 80 | 0 | 100 |
| 85 | 0 | 100 |
A = 10% methanol and 0.05% formic acid; B = 70% methanol and 0.05% formic acid.
HPLC = high-performance liquid chromatography.