Abstract
Response inhibition has been a core issue in addictive behavior. Many previous studies have found that response inhibition abilities are damaged in those with drug dependence. However, whether heroin addicts who are treated with methadone maintenance have an abnormal response inhibition ability is not clear. In order to investigate the response inhibition functions in heroin addicts who were treated with methadone maintenance, electroencephalography (EEG) was used to examine 14 heroin addicts treated with methadone maintenance (HDM), 17 heroin addicts (HD), and 18 healthy controls (HC) in an equiprobability Go\NoGo task. The reaction times (RTs) for the Go stimuli in the HD group were slower than those in the HDM and HC groups. Event-related potential (ERP) measurements showed that NoGo stimuli elicited larger N2 amplitudes than Go stimuli in the HDM and HC groups. However, for the HD group, the N2 amplitudes were similar for the two conditions. In addition, the HDM and HD groups were associated with longer P3 latencies. Our results demonstrated that methadone maintenance treatment might ease the deficits in response inhibition that result from long-term drug abuse. However, compared to normal people, HDM patients have serious problems evaluating and inhibiting inappropriate behaviors.
Keywords: event-related potential (ERP), heroin addiction, methadone maintenance treatment, N2, P3, response inhibition
1. Introduction
To date, there have been many theories of addiction that assume that executive function plays an important role in generating drug dependence and addictive behavior [1–2], and that response inhibition is the core of executive function [3]. Response inhibition refers to the conscious inhibition of a response that is unrelated to the current task and that is automatically activated [4]. Response inhibition, which is a core component of executive function, plays an important role in the inhibition of an inappropriate response in an individual in order to adapt to survive. Response inhibition is a prerequisite for appropriate behavior, and response inhibition damage or abnormalities will lead to inappropriate or illegal behavior [5]. Some researchers have found that response inhibition and addictive behaviors are highly correlated, which means that individuals with weaker response inhibition are more prone to addictive behavior. Thus, individuals with high impulsivity and low response inhibition are more likely to use and be dependent on drugs [6]. Therefore, response inhibition is a core issue in addictive behavior.
Heroin addiction is a type of addictive behavior, and the study of heroin addicts’ response inhibition abilities have found that long-term heroin use can damage brain structures, resulting in damage to the response inhibition ability. At the behavioral level, there have been many studies that have used Stroop, Go/NoGo, and Stop Signal tests to examine the response inhibition of heroin addicts by using their reaction time (RT) and percentage correct as indexes, and these studies have found that heroin addicts have much longer RTs and less accuracy in response inhibition tasks [7–8]. Neuroimaging studies that examine brain function at the structural level have further confirmed that the structure that is associated with executive control in the brains of heroin addicts is damaged. Fu et al [9] have used event-related potential (ERP) technology to determine the obstacles that heroin addicts face during the conflict-monitoring stage. Yang et al [10] have used functional magnetic resonance imaging technology and have found that heroin addicts have some deficits in response inhibition, even after the drug is withdrawn. These studies have used different methods and techniques to confirm that the inhibition of heroin addicts’ control function is due to varying extents of defects or damage and that these defects or damage to the function are the main reason that leads to their drug addiction or relapse.
Methadone maintenance treatment is one of the main alternative therapies used to treat patients with opiate addiction worldwide, and more and more heroin addicts are participating in methadone maintenance treatment in China. Some researchers found that methadone maintenance treatment can significantly reduce the patient’s withdrawal symptoms, but there are no significant improvements in the abnormalities in the neural mechanisms that are associated with heroin dependence. Long-term heroin consumption causes adaptive changes in brain systems that may persist for a long time [11]. The research of Verdejo et al [12] has discovered that methadone itself produces significant cognitive impairments and increases the already present cognitive impairments in addicts who take it. Some researchers have found that rehabilitation can effectively improve the cognitive function damage that is caused by buprenorphine, placebo, and methadone [13]. The effects of methadone maintenance treatment on the heroin addicts’ neural mechanisms underlying response inhibition need further discussion. In China, a large number of people take part in methadone maintenance treatment. However, relapse and furtive inhalation phenomenon often occur. Thus, an investigation of the effects of methadone maintenance treatment on heroin addicts’ response inhibition has important practical significance.
Most previous studies on response inhibition have examined ERPs with a high time resolution, and they have displayed the time course of the information processing and provided electrophysiological indicators of cognitive function. Most of these studies have used classical paradigms, such as Go/NoGo, Stop Signal, oddball, and some others, that have been adapted for these studies. Because the stop signal and stimulation that evoke electroencephalography (EEG) components in the Stop Signal paradigm can generate some interferences, and in the oddball paradigm there are some novel stimulus effects on brain electrical components other than the stimulation-evoked EEG components, this study adopted the Go/NoGo paradigm. The classic Go/NoGo paradigm asks participants to react to the high probability of a Go stimulus and to inhibit the NoGo stimulation with a small probability. Stimulus probability may affect the amplitude of EEG components, and the low probability usually produces a larger component of P3 [14]. In order to eliminate the probability of interference in the experiment, this study employed the equiprobability Go/NoGo paradigm.
The Go/NoGo task induced two ERP components that reflect response inhibition processing under NoGo conditions [15]. The first ERP component is the NoGo-N2, which is the largest negative component that appears in the frontal scalp when the stimulus is presented for 200 milliseconds. Compared to the Go condition, the NoGo condition results in N2 with a more negative amplitude. This phenomenon is called the NoGo-N2 effect, and N2d (the amplitude of NoGo-N2 with the amplitude of Go-N2 subtracted) indicates this effect [16–17]. It has been argued that the NoGo-N2 effect reflects response inhibition, which is a top-down mechanism that suppresses the incorrect tendency to respond and operates at a processing stage prior to motor execution [18]. A study by Yin and Liu [19] has found that the relationship between response inhibition and the effect of NoGo-N2 is that NoGo-N2 reflects the process of response inhibition. The second ERP component is NoGo-P3, which is the largest positive component that appears in the central area when the stimulus is presented for 300–500 milliseconds, and Go-P3 reaches the maximum in the parietal position [20]. NoGo-P3 has a larger positive amplitude than Go-P3 does at the central scalp—this phenomenon is called the NoGo-P3 effect. NoGo-P3 is the electrophysiological reflection of response inhibition. A previous study has found that a reduction in NoGo-P3 that may be related to alterations in successful inhibition is the dominant reaction [21], and it has no connection with the process of response inhibition.
Consequently, the participants in the present study were heroin addicts who participated in methadone maintenance treatment, and we employed the equiprobability Go/NoGo paradigm; we used ERP technology to investigate the neural mechanisms of response inhibition in the heroin addicts who participated in methadone maintenance.
2. Methods
2.1. Participants
Fourteen heroin addicts (9 males and 5 females) receiving methadone maintenance treatment (HDM) were selected from the methadone maintenance treatment center in the Qi Lihe District of Lanzhou City, Gansu Province. The age [mean ± standard deviation (SD)] was 38.210 ± 9.133 years. Among the participants, four people did not work, two participants reported working part-time, seven people had a permanent job, and one was retired. Three of them were unmarried, 10 were married, and one was divorced. Participants reported the average time of using methadone as 2.396 ± 1.330 years. The criteria for inclusion in the HDM group were as follows: (1) took heroin for more than two years before taking methadone; (2) not taking heroin or other illicit drugs except methadone during methadone maintenance treatment; (3) no mental illness history, neurological history, or serious head injury history.
Seventeen heroin addicts who did not receive methadone maintenance treatment (HD) were recruited from the Health Center of the Second Re-education School of Gansu. All participants were male. The average age was 34.400 ± 9.956 years. Among these participants, all people did not work. Eight of them were unmarried, eight were married, and one was divorced. Participants reported the average time of being in prison as 1.273 ± 0.582 years. The average length of education was 9.267 ± 3.882 years. The criteria for inclusion in the HD group were as follows: (1) took heroin for more than one years; (2) no mental illness history, neurological history, or serious head injury history.
Eighteen healthy controls (HC), matched in age (35.89 ± 10.035 years) and education level (10.833 ± 4.656 years), were recruited from the local community. The group consisted of 14 men and four women. Among these participants, one did not have work, three had part-time jobs, 14 had permanent jobs; seven people were unmarried and 11 people were married. The criteria for inclusion in the HD group were as follows: (1) no heroin or other illegal drug use history; (2) no mental illness history, neurological history, or serious head injury history.
There were no differences in age and length of education across the three groups, and all of the participants were right-handed, native-Chinese speakers without achromatopsia and hypochromatopsia, and had normal or corrected-to-normal vision. All participants gave written informed consent. After the experiment, all participants were given a payment.
2.2. Materials and procedures
In the experiment, the visual stimuli were two squares (one red and one green) and the visual angle of stimulus was 2° × 2°. The stimuli were randomly presented one by one at the center of a blank gray screen by the Eprime-2.0 system (Psychology Software Tools, Inc.). Participants were seated in a quiet room in a chair approximately 85 cm away from the screen center. The stimuli were presented at intervals of 200–400 milliseconds, 100 milliseconds, and 1000 milliseconds. The participants were instructed to respond by pressing a button using their thumbs as quickly as possible after the Go stimuli appeared and to withhold the response when the NoGo stimuli appeared. For half of the participants in each group, the red square was used as the Go stimulus, and for the other half of the participants in each group, the green square was used as the Go stimuli. In total, the task consisted of 60 Go stimuli and 60 NoGo stimuli. During the experiment, participants were instructed to try their best to avoid moving or blinking their eyes when the stimulus (red or green square) was presented.
Before the beginning of the formal experiment, participants were required to do some exercises, with 20 trails of red squares and 20 trails of green squares. When the participants had mastered the requirement of pressing the key to control their eye blink well (when the rate of correct reached 90%), they could enter the formal experiment.
2.3. EEG recording and analysis
EEG was continuously recorded from scalp electrodes using the 256-channel HydroCel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR, USA). The impedance for all electrodes was kept below 50 kΩ, and all recordings were referenced to Cz. Signals were amplified with a 0.1–100 Hz elliptical bandpass filter and digitized at a 250-Hz sampling rate. EEG data were segmented to epochs of 800 milliseconds after stimulate onset with a 200-millisecond prestimulus baseline. For each trial, channels were marked as artifacts if signals exceeded 200 μV. Trials with more than 10 channels marked as artifacts were excluded. For trials with less than 10 channels marked as artifacts, an algorithm that derived values from neighboring channels via spherical spline interpolation was used to replace bad channels. Eye movements were monitored by recording the horizontal and vertical electrooculogram (HEOG and VEOG) using bipolar electrode placements at the outer canthi of both eyes (HEOG). Trials were excluded if the signal variation of horizontal electrooculography and vertical electrooculography exceeded 140 μV and 55 μV, respectively. Prior to analysis, EEG data was digitally filtered with 0.1-Hz high-pass and 45-Hz low-pass filters and re-referenced off-line to an average reference value. Epochs of EEG data in the same condition were corrected to the 200-millisecond prestimulus baseline.
2.4. Data analysis
Responses were scored if the appropriate key was pressed within a 300–2000 millisecond period after the adjective phrase onset. To analyze the behavioral data statistically, participants’ RTs for the Go task, the accuracy of the Go task, and the error rate of the NoGo task were subjected to separate one-way analysis of variance (ANOVA) with group (HC, HD, HDM) as the between-participant variable.
According to the previous studies [16,22–23] and the topographic distribution of the components in our study, the amplitude of N2 was measured as the mean amplitude across 80 milliseconds centered around the individual peak latency (group, condition, and location) between 120 milliseconds and 260 milliseconds, and the amplitude of P3 was measured as the mean amplitude across 100 milliseconds centered around the individual peak latency between 260 milliseconds and 500 milliseconds. For N2, we selected 10 electrodes at the frontal (Fz, F1, F2, F3, F4) and central-frontal (FCz, FC1, FC2, FC3, FC4) areas where the N2 were most pronounced. For P3, we selected 15 electrodes at the central (Cz, C1, C2, C3, C4), central-parietal (CPz, CP1, CP2, CP3, CP4) and parietal (Pz, P1, P2, P3, P4) areas where the P3 were most pronounced. We also calculated the difference waves of N2 (N2d) and P3 (P3d) from NoGo minus Go at specified time windows. In addition, the latency of P3 was also measured. The amplitude of N2 and P3 and the latency for P3 were analyzed by three-way mixed repeated measures ANOVA (group × condition × location), and the N2d and P3d were analyzed by two-way mixed repeated measures ANOVA (group × location). The amplitude and latency of ERPs used for each location in the analysis were the mean amplitude of the five electrodes in the same area. We adopted SPSS 13.0 (SPSS Inc., Chicago, IL, USA) for ANOVA, and if necessary we used the Greenhouse–Geisser method to correct p values. For multiple comparisons, the Bonferroni correction was used.
3. Results
3.1. Behavioral results
The correction rates and RTs to the stimulus are shown in Table 1. Statistical results showed that the difference of RTs in the GO condition were significant (p < 0.05). Post-hoc analysis showed that the RTs of HD in the Go condition were longer than HDM and HC (p < 0.05); there were no significant differences between HDM and HC (p > 0.05). There were no significant differences in the error rate to the NoGo stimulus and the correction rate to the Go stimulus between the groups (p > 0.05).
Table 1.
Behavioral results for the HDM, HD, and HC groups.
| HDM | HD | HC | F | p | |
|---|---|---|---|---|---|
| RTs to Go stimulus (ms) | 343.733 ± 53.018 | 421.799 ± 1.328 | 297.017 ± 61.923 | 15.896 | < 0.001 |
| Error rate to NoGo stimulus | 0.023 ± 0.050 | 0.056 ± 0.118 | 0.005 ± 0.008 | 1.733 | 0.189 |
| Correction rate to Go stimulus | 0.918 ± 0.163 | 0.949 ± 0.166 | 0.998 ± 0.003 | 1.303 | 0.283 |
Data are presented as mean ± standard deviation (SD).
HC = healthy controls; HD = heroin addicts; HDM = methadone maintenance treatment of heroin addicts; RT = reaction time.
3.2. Statistical analysis of ERPs
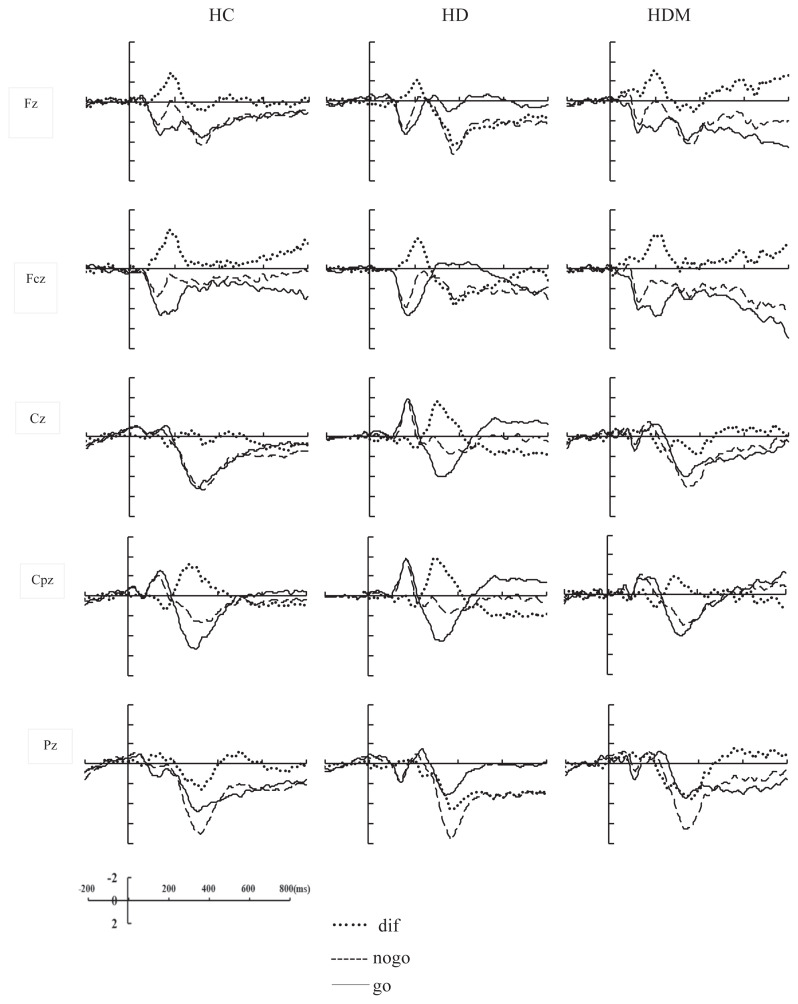
Fig. 1 shows the total average waveforms of ERP and the difference waves for each group at Fz, Fcz, Cz, Cpz, and Pz.
Fig. 1.
The total average waveforms of ERPs for the HC, HD, and HDM groups. ERP =event-related potential; HC =healthy controls; HD =heroin addicts; HDM =methadone maintenance treatment of heroin addicts.
3.2.1. Amplitude of N2
The amplitudes of N2 were analyzed by a three-way mixed repeated measures ANOVA with condition (Go and NoGo) and location (frontal and central-frontal) as the within-participant variables and group (HC, HD, HDM) as the between-participant variable. The results showed a significant main effect of location: F (1, 46) = 32.572, p < 0.001, η2 = 0.415; the amplitude of N2 at central-frontal was significantly larger than at frontal. The main effects of the condition were significant: F (1, 46) = 22.337, p < 0.001, η2 = 0.327; the NoGo stimulus induced greater negative amplitude than the Go stimulus at this time window. Importantly, a significant interaction effect of the factors group × condition was observed: F (2, 46) = 3.246, p < 0.05, η2 = 0.124. Further analysis revealed that the amplitudes of N2 were larger in the NoGo condition than in the Go condition for the HC and HDM groups (p < 0.01), whereas there were no differences between the Go and NoGo condition for the HD group (p > 0.05). No other effects or interactions reached the significance p > 0.05.
3.2.2. Amplitude of N2d
The amplitudes of N2d were analyzed by a two-way mixed repeated measures ANOVA with location (frontal and central-frontal) as the within-participant variables and group (HC, HD, HDM) as the between-participant variable. The main effect of group was significant: F (2, 46) = 3.246, p < 0.05, η2 = 0.124; post-hoc t tests indicated that the amplitudes of N2d were larger for HC as compared to HD (p < 0.05). No other effects or interactions reached the significance p > 0.05.
3.2.3. Amplitude of P3
The amplitudes of P3 were analyzed by a three-way mixed repeated measures ANOVA with condition (Go and NoGo), location (central, central-parietal, and parietal) as the within-participants variables and group (HC, HD, HDM) as the between-participant variable. The ANOVA showed a significant main effect of location: F (2, 92) = 9.199, p < 0.001, η2 = 0.167. Pairwise comparisons showed that the amplitudes at the central and central-parietal scalp were larger than those at the parietal scalp (p < 0.05). The main effects of the condition were significant: F (1, 46) = 4.292, p < 0.05, η2 = 0.085; the NoGo stimulus induced a greater P3 amplitude than the Go stimulus. The results also showed a significant interaction of the factors location × condition, F (2, 92) = 62.176, p < 0.001, η2 = 0.575. Simple effect analysis revealed that the amplitudes of P3 were larger in the NoGo condition than in the Go condition at the central and central-parietal scalp (p < 0.05), whereas the amplitudes of P3 were larger in the Go condition than in the NoGo condition at the parietal scalp (p < 0.05). No other main or interaction effects reached the significance p > 0.05.
3.2.4. Amplitude of P3d
The amplitudes of P3d were analyzed by a two-way mixed repeated measures ANOVA with location (central, central-parietal, and parietal) as the within-participant variables and group (HC, HD, HDM) as the between-participant variable. The results showed the main effects of the location: F (2, 92) = 62.176, p < 0.001, η2 = 0.575. Pairwise comparisons showed that the amplitude of P3d at the central and central-parietal scalp were significantly larger than those at the parietal scalp (p < 0.001). No other main effects or interactions reached the significance p > 0.05.
3.2.5. Latency of P3
The latencies of P3 were analyzed by three-way mixed repeated measures ANOVA with condition (Go and NoGo), location (central, central-parietal, and parietal) as the within-participant variables and group (HC, HD, HDM) as the between-participant variable. The results showed a significant main effect of location: F (2, 92) = 14.978, p < 0.001, η2 = 0.246; pairwise comparisons showed that the latencies of P3 at the parietal scalp were shorter than those at the central and central-parietal scalp (p < 0.05). The main effects of the condition were significant: F (1, 46) = 1.761, p < 0.01, η2 = 0.144; the NoGo condition was associated with longer P3 latency than the Go stimulus. The main effect of group was also significant: F (2, 46) = 6.573, p < 0.01, η2 = 0.222; post-hoc t tests indicated that the latency of P3 was longer for HD and HDM as compared to HC (p < 0.05). The results also showed a significant interaction of the factors location × condition: F (2, 94) = 10.418, p < 0.001, η2 = 0.185. Simple effect analysis revealed that the latencies of P3 were longer in the NoGo condition than in the Go condition at the central-parietal and parietal scalp (p < 0.05), whereas there was no difference between the Go condition and the NoGo condition at the central scalp (p > 0.05). No other main or interaction effects reached the significance p > 0.05.
4. Discussion
In the present study, we used ERPs to explore the neural mechanisms of response inhibition in a Go/NoGo task in heroin addicts who were treated with methadone maintenance and heroin addicts who were not treated with methadone maintenance. According to the behavioral results, we found that the RTs of heroin addicts (HD) and the heroin addicts who were treated with methadone maintenance (HDM) were slower than those of healthy controls (HC) in the Go condition, which suggested that the HD and HDM groups needed more time to identify the stimuli and to react. However, there were no differences in the percentages of errors between the NoGo condition and the Go condition among any of the groups, suggesting that the behavioral results cannot effectively reveal the differences in response inhibition between the groups. According to the ERP results, we found that the N2d was smaller in the HD group compared to the HC group and that the latency of P3 in the HD and HDM groups was longer than that in the HC group.
In the present study, the amplitude of N2 was larger in the NoGo condition than in the Go condition, and this was a reflection of the so-called NoGo-N2 effect. For the P3 component, similarly the amplitude of P3 was also larger in the NoGo condition than in the Go condition, and this was a reflection of the NoGo-P3 effect. These findings suggested that our experiments induced the response inhibition process successfully [22]. Further analyses of the N2 amplitudes suggested that only the HC and HDM groups showed the NoGo-N2 effect, and in the HD group no difference was found between the Go and NoGo condition (NoGo-N2 effect). In the Go/NoGo task, the participants need more cognitive resources to inhibit the reaction trend to the Go stimulus in the NoGo condition [18], thus leading to the amplitude of NoGo-N2 being larger than that in the Go-N2. Therefore, it may be said that the HC and HDM groups showed normal response inhibition, but the HD group had difficulties with response inhibition.
N2d is the index of the NoGo-N2 effect, and a larger N2d reflects a stronger response inhibition [15–16]. In the present study, we found that the N2d of the HD group was significantly smaller than that in the HC group, further confirming that the HD group had a serious deficit in response inhibition compared to the HC group. In general, the cognitive process in a NoGo task includes stimulus identification, response selection, and the inhibition of activation. In the present study, the HD group did not show any significant differences relative to the HC group in behavioral task performance (error rate to NoGo stimulus), suggesting that there were no significant differences between the two groups for stimulus identification or response selection. However, the amplitude of NoGo-N2 was smaller in the HD group than in the HC group—this may suggest that the ability to inhibit the underlying activation in the HD group was weaker than that in the HC group and that their inhibition ability was seriously deficient. However, we did not find any differences in N2d between the HDM and HC groups, which may indicate that the deficit in response inhibition resulting from long-term heroin abuse was relieved to some extent by the methadone maintenance treatment. In addition, the HDM and HD groups had no differences in N2d. This further showed that the deficit in inhibitory control in the HD group was not completely improved after the methadone maintenance treatment.
The latencies of ERPs usually reflect the efficiency of cognitive processing, and a large number of studies have found that the latency of P3 is associated with cognitive processing speed [23–24]. Usually, the more difficult the task is, the longer the P3 latency is. A previous study on response inhibition also found that the latencies of P3 were associated with inhibition function, and the stronger the inhibitory ability was, the shorter the latency of P3 was [25]. In our study, we found that the latency of P3 was longer in the NoGo condition than in the Go condition. This may have been due to a need for more cognitive resources to inhibit the reaction tendency in the Go condition, thus resulting in a longer latency of P3 under the NoGo condition. Importantly, in the present study, we found that the latency of P3 was longer in the HDM and HD groups compared to the HC group, and this result was consistent with the behavioral data in the present study that showed that the RTs for the Go stimulus in the HD and HDM groups were longer than those in the HC group. This suggested that the speeds of information processing in the HD and HDM groups were significantly slower than that in the HC group and that the HD and HDM groups needed more time to evaluate and inhibit the inappropriate behaviors.
In summary, our results demonstrated that methadone maintenance treatment might have eased the deficits in response inhibition that result from long-term drug abuse, but, compared to normal people, addicts have serious problems in evaluating and inhibiting inappropriate behaviors. It is necessary to note, however, that, in this study, the HDM participants were treated with methadone maintenance for only 1.5 years. Although methadone maintenance treatment improves the response inhibition ability in heroin-dependent patients, they are not able to attain a normal level. Thus, we should improve the coverage and quality of methadone maintenance treatment [26].
Acknowledgments
This work was supported by National Natural Science Foundation of China (Project 31360233) and National Science Fund for Distinguished Young Scholars of China (31300838). We thank Mingshun Wang and Huizhen Lu from the methadone clinics and Linglong Zhao and Xinla Li from the Centers for Disease Control and Prevention in Qilihe, Lanzhou, Gansu for their help in recruiting participants. In addition, we thank Aibao Zhou and Ruixue Xia for their help and guidance with the ERP technology and methods. Lastly, we thank our graduate students for their help with the data collection and processing.
Funding Statement
This work was supported by National Natural Science Foundation of China (Project 31360233) and National Science Fund for Distinguished Young Scholars of China (31300838).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Olmstead MC. Animal models of drug addiction: where do we go from here? Q J Exp Psychol. 2006;59:625–53. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- 2. Wiers RW, Bartholow BD, Wildenberg E, Thush C, Engels RC, Sher KJ, Grenard J, Ames SL, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. 2007;86:263–83. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 3. Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Schizophr Res. 2001;39:147–65. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 4. Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–93. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 5. Li CSR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–92. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav R. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7. Yang C, Zhou JX. A controlled study of executive function in heroin addicts. Chin Ment Health J. 2004;18:682–4. [In Chinese] [Google Scholar]
- 8.Lv Y. Unpublished Masters Dissertation. Yun Nan Normal University; 2006. The function of heroin abstinents’ behavior inhibition and dynamic characteristics. [In Chinese] [Google Scholar]
- 9. Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming-Fan, Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–6. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 10. Yang B, Yang SY, Zhao L, Yin LH, Liu X, An SS. Event-related potentials in a Go/Nogo task of abnormal response inhibition in heroin addicts. Sci China Ser C. 2009;52:780–8. doi: 10.1007/s11427-009-0106-4. [DOI] [PubMed] [Google Scholar]
- 11. Li Q, Wang Y, Li W, Yang WC, Zhu J, Chang HF, Wu N, Zheng Y, Wang W. fMRI study on craving and brain activity in response to heroin-related cues in patients with methadone maintenance treatment. J Pract Radiol. 2012;28:1–5. [In Chinese] [Google Scholar]
- 12. Verdejo A, Toribio I, Orozco C, Puente KL, Pérez-García M. Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug Alcohol Depen. 2005;78:283–8. doi: 10.1016/j.drugalcdep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13. Attou A, Figiel C, Timsit-Berthier M. Opioid addiction: P300 assessment in treatment by methadone substitution. Neurophysiol Clin. 2001;31:171. doi: 10.1016/s0987-7053(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 14. Bokura H, Yamaguchi S, Kobayashi S. Event-related potentials for response inhibition in Parkinson’s disease. Neuropsychologia. 2005;43:967–75. doi: 10.1016/j.neuropsychologia.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 15. Luijten M, Littel M, Franken IH. Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials. PLoS One. 2011;6:e18898. doi: 10.1371/journal.pone.0018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related ERP components: variation with modality, age, and time-on-task. J Psychophysiol. 2002;16:167. [Google Scholar]
- 17. Ruchsow M, Groen G, Kiefer M, Buchheim A, Walter H, Martius P, Reiter M, Hermle L, Spitzer M, Ebert D, Falkenstein M. Electrophysiological evidence for reduced inhibitory control in depressed patients in partial remission: a Go/Nogo study. Int J Psychophysiol. 2008;68:209–18. doi: 10.1016/j.ijpsycho.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 18. Kim MS, Kim YY, Yoo SY, Kwon JS. Electrophysiological correlates of behavioral response inhibition in patients with obsessive–compulsive disorder. Depress Anxiety. 2007;24:22–31. doi: 10.1002/da.20195. [DOI] [PubMed] [Google Scholar]
- 19. Yin G, Liu T. NoGo-N2, P3 effect analysis in Go/NoGo paradigm. J Univ Electronic Sci Technol China. 2011;40:465–9. [In Chinese] [Google Scholar]
- 20. Strik WK, Fallgatter AJ, Brandeism D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalogr Clin Neurophysiol. 1998;108:406–13. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 21. Donchin E, Coles MG. Context updating and the P300. Behav Brain Sci. 1998;21:152–4. [Google Scholar]
- 22. Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 24. Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cognition. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 25. Aotsuka A, Weate SJ, Drake JME, Drake ME, Jr, Paulson GW. Event-related potentials in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1996;36:215–20. [PubMed] [Google Scholar]
- 26. Li J, Li X. Current status of drug use and HIV/AIDS prevention in drug users in China[J] J Food Drug Anal. 2013;21:S37–41. doi: 10.1016/j.jfda.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]