Abstract
Volatile extracts from the seeds of Dendranthema nankingense Hand.-Mazz. and Borago officinalis L. were prepared using simultaneous distillation and extraction, and analyzed with gas chromatography–mass spectrometry on two capillary gas chromatography columns of different polarity. Ninety-five volatile compounds were identified in D. nankingense seeds, with hexanal, benzeneacetaldehyde, borneol, (−)-camphor, and 3-methyl-1-butanol being the predominant species. Sixty-five volatile compounds were identified in B. officinalis seeds, with 2-pentanone, 2,3-dihydro-benzofuran, 3-methyl butanal, and hexanal being the most abundant species. Thirty-three compounds, including short-chain aliphatic aldehydes, alcohols, and ketones, were common to both seeds. The volatile composition of both seeds varied significantly depending on their respective origins. The volatile terpenoids borneol and (−)-camphor could be key bioactive contributors to the characteristic flavor and cooling effects of D. nankingense. For the first time, coumaran was identified as an abundant species in plant seeds.
Keywords: borneol, camphor, coumaran, seeds, volatile compounds
1. Introduction
Dendranthema nankingense Hand.-Mazz. is a perennial herb of the family Compositae, which can be classified into the category of pungent and cool nature according to traditional Chinese medicine theories. It is harvested in the summer and used to relieve external syndromes induced by hot diseases [1]. Soup cooked with the leaves of D. nankingense is very popular in eastern China, as it has heat-clearing and detoxifying effects. People who have drunk the soup have reported that they clearly experienced a cooling and refreshing sensation throughout their mouths and throats.
Borago officinalis L. is an annual herbaceous plant of the Boraginaceae family, which is native to Europe and North Africa. This plant is currently cultivated throughout the world and is used for medicinal purposes, and in beverages and salads [2]. B. officinalis has been defined as a future “superfood” because of the high level of γ-linolenic acid present in its seeds [3]. It has received considerable attention because of the antioxidant and antiradical activities of its phenolic constituents [4].
Many plants with volatile smelling flavor have been used as traditional herbs or foods in many countries since ancient times [5,6]. The volatiles from plant seeds have also received considerable interest because of their chemical diversity and functional activities. For example, coriander seeds contain a volatile oil, used in a medicinal context for the treatment of indigestion, worms, rheumatism, and pain in the joints [7]. To date, there have been no reports in the literature regarding volatiles from the seeds of D. nankingense, although a number of papers have reported the volatile compositions of its leaves [8–10]. Research and development on the seeds of B. officinalis has progressed at a steady pace over the past 30 years. Previous studies have primarily focused on γ-linolenic acid and, to a lesser extent, on phenolic compounds. Despite a long history of use of B. officinalis seed oil in foods and medicine, there have been few published studies regarding the volatile compounds contained in the oil. To our knowledge, there has only been one paper reporting a very limited number of volatile compounds from B. officinalis seeds that were harvested in Tunisia [11]. Thus, nothing is known about the volatile composition of B. officinalis seeds from other areas, and how their origin might affect the composition of their volatile components.
This study provides a comprehensive investigation of the volatile components from the seeds of D. nankingense and B. officinalis harvested in China. Furthermore, the study compares the results with those in the literature for the same family, and discusses the functional applications of herbal seeds in food and medicine.
2. Materials and methods
2.1. Materials and chemicals
Seeds of D. nankingense and B. officinalis were provided by the Qiutian Seed Co., Ltd. (Nanjing, China) and the Winner Horticultural Seed Co., Ltd. (Shanghai, China), respectively.
Pentane and ether were purchased from CNW (Düsseldorf, Germany) and Sinopharm (Shanghai, China), respectively. Both solvents were distilled prior to use. Anhydrous sodium sulfate was obtained from Lingfeng (Shanghai, China). Methyl nonanoate was supplied by Fluka (Buchs, Switzerland). The n-alkanes (C7–C28) were purchased from Aladdin (Shanghai, China). Other standard volatile compounds were bought from Sigma-Aldrich China Co. (Shanghai, China).
2.2. Isolation of volatile oils
The volatile extracts were obtained from the seed using a simultaneous distillation and extraction (SDE) setup equipped with Likens–Nickerson apparatus connected to a vacuum pump. The seeds (50 g) were initially cooled to 4°C and then ground using a commercial blender for 10 seconds. The seed powder was then mixed with double-distilled water (500 mL), and a solution of methyl nonanoate (50 μg) in a 1:1.12 (v/v) pentane/ether mixture (1 mL) was added as an internal standard. The mixture was placed in a 2000-mL round-bottom flask, whereas the solvent mixture [50 mL of a 1:1.12 (v/v) pentane/ether] was added to a 250-mL round-bottom flask in the SDE equipment. The sample flask was heated to 52°C for distillation and extraction, respectively. After 1.5 hours of processing, the collected solvent distillate was dried over anhydrous sodium sulfate and concentrated at 42°C using a Vigreux column to a final volume of approximately 1 mL for gas chromatography–mass spectrometry (GC-MS) analysis. The extractions were performed in triplicate.
2.3. GC-MS and GC analysis
GC–MS analysis was carried out using a polar capillary DB-WAX column (polyethylene glycol, 30 m × 0.25 mm i.d. × 0.25 μm film thickness; Agilent, Folsom, CA, USA) and a nonpolar HP-1 column (dimethyl polysiloxane, 30 m × 0.25 mm i.d. × 0.25 μm film thickness; Agilent, USA) on a QP2010 GC-MS system (Shimadzu, Kyoto, Japan). The oven temperature was held at 40°C for 2 minutes, and then increased at a rate of 5°C /min to a final temperature of 250°C. The oven was then held at 250°C for 10 minutes. The temperatures of injector and ion source were set at 230°C and 200°C, respectively. GC–MS was operated at an ionization energy of 70 eV in the electron impact mode over a range of 33–650 amu. Helium was used as a carrier gas at a constant flow rate of 1.6 mL/min. The extract (0.3 μL) was injected with an autosampler in splitless mode.
Retention indices were calculated according to the Kovats method using n-alkanes (C7–C28) as external references. The experimental retention indices of the volatiles were compared with data from the literature [12–14]. To identify the peaks in the mass spectra, we compared our mass spectra with mass spectral libraries (NIST/EPA/NIH 2.0, Wiley 7, and LIBTX) and with literature data [12], and also with authentic reference standards when available. Analyses were performed twice.
GC analysis was performed on a Shimadzu GC2010 system equipped with a flame ionization detector. The column and conditions of the temperatures were identical to those used in the GC–MS analysis. The relative percentage amounts of each identified compounds were calculated with the peak area normalization. Analyses were carried out twice. Approximate concentrations of the volatile compounds were calculated according to the internal standard method using methyl nonanoate.
3. Results and discussion
3.1. Components of volatile oil from the seeds of D. nankingense
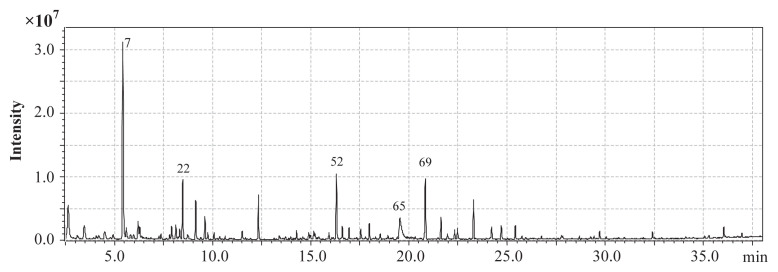
SDE of D. nankingense seeds yielded a yellow oil (2.3%, w/w), from which 95 compounds were isolated and identified. The components are listed in Table 1. In the majority of cases, the amounts of identified compounds were very low (< 1%), and aldehydes (43.5%), terpenoids (25.3%), and alcohols (17.6%) were identified as the predominant chemical classes (Fig. 1). The five most abundant compounds, representing approximately 43% of the total amount of volatile compounds in the raw seeds, were hexanal (19.8%), 3-methyl-1-butanol (5.2%), (−)-camphor (5.8%), benzeneacetaldehyde (6.4%), and borneol (6.2%). Similarly, hexanal, benzeneacetaldehyde, and 3-methyl-1-butanol were identified as the major volatile components in pine and grape seeds [15,16]. In comparison, hexanal and 3-methyl-1-butanol were not found in the leaf oils of D. nankingense [8–10]. The volatile compounds in the leaf harvested in Jiangsu province were borneol (26.9%), bornyl acetate (18.6%), germacrene D (6.06%), and α-sesqui-phellandrene (6.06%) [10]. The most abundant compounds in the leaf oil from Anhui province were camphor (11.6%), caryophyllene oxide (6.7 %), β-santalol (14.4%), and bisabolol oxide A (17.9%) [8].
Table 1.
Volatile extract composition of Dendranthema nankingense seeds.
| No. | Retention time on DB-WAX (min) | Compounds | RIs | Identification and MS ions (EI)a | Total area (%) | Approximate concentration (μg/mL extract)b | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| DB-WAX | HP-1 | ||||||
| 1 | 2.63 | 3-Methyl butanal | 924 | <700 | ++++; M+ 86 (16.67), 44 | 4.5 | B |
| 2 | 3.10 | 2-Ethylfuran | 962 | <700 | ++++; M+ 96 (32.46), 81 | 0.3 | C |
| 3 | 3.45 | Pentanal | 985 | <700 | ++++; M+ 86 (6.59), 44 | 1.7 | C |
| 4 | 4.06 | α-Pinene | 1021 | 921 | ++++; M+ 136 (7.67), 93 | 0.2 | D |
| 5 | 4.78 | 2-Methylbutanoic acid ethyl ester | 1059 | 841 | ++++; M+ 130 (2.03), 57 | 0.2 | D |
| 6 | 4.91 | Camphene | 1065 | 942 | +++; M+ 136 (15.98), 93 | 0.4 | C |
| 7 | 5.41 | Hexanal | 1086 | 779 | ++++; M+ 100 (1.21), 44 | 19.8 | A |
| 8 | 5.59 | 2-Methyl-1-propanol | 1093 | <700 | ++++; M+ 74 (15.32), 43 | 1.1 | C |
| 9 | 5.81 | β-Pinene | 1102 | 967 | ++++; M+ 136 (8.83), 93 | 0.6 | C |
| 10 | 5.96 | 3-Pentanol | 1110 | <700 | ++++; M+ 88 (0.65),59 | 0.4 | C |
| 11 | 6.19 | Sabinene | 1120 | 966 | +++; M+ 136 (12.19), 93 | 1.9 | C |
| 12 | 6.27 | 2-Pentanol | 1124 | <700 | ++++; M+ 88 (1.03), 45 | 1.0 | C |
| 13 | 6.61 | 2-Butylfuran | 1139 | 880 | +++; M+ 124 (13.46), 81 | 0.1 | D |
| 14 | 6.84 | 1-Butanol | 1148 | <700 | ++++; M+ 74 (1.86), 56 | 0.1 | D |
| 15 | 7.25 | 2-Ethyl-hexanal | 1165 | 937 | +++; M+ 128 (2.17), 57 | 0.2 | D |
| 16 | 7.36 | β-Myrcene | 1169 | 984 | +++; M+ 136 (5.24), 41 | 0.5 | C |
| 17 | 7.62 | α-Terpinene | 1178 | 1007 | ++++; M+ 136 (42.98), 121 | 0.1 | D |
| 18 | 7.80 | 2-Heptanone | 1185 | 872 | ++++; M+ 114 (6.60), 43 | 0.3 | C |
| 19 | 8.11 | D-limonene | 1195 | 1032 | ++++; M+ 136 (23.41), 68 | 1.4 | C |
| 20 | 8.22 | 3-Methyl-2-butenal | 1199 | 759 | +++; M+ 84 (100.00), 55 (77.16) | 0.1 | D |
| 21 | 8.30 | 1,8-Cineole | 1202 | 1020 | ++++; M+ 154 (37.13), 43 | 1.0 | C |
| 22 | 8.45 | 3-Methyl-1-butanol | 1208 | 721 | ++++; M+ 88 (0.61), 55 | 5.2 | B |
| 23 | 8.71 | (E)- 2-Hexenal | 1219 | 829 | +++; M+ 98 (22.10), 41 | 0.4 | C |
| 24 | 9.12 | 2-Pentyl-furan | 1236 | 981 | +++; M+ 138 (18.35), 81 | 3.4 | B |
| 25 | 9.38 | (Z)-Ocimene | 1246 | 1025 | +++; M+ 136 (5.87), 93 | 0.2 | D |
| 26 | 9.59 | 1-Pentanol | 1254 | 759 | ++++; M+ 88 (1.16), 42 | 1.8 | C |
| 27 | 9.74 | Styrene | 1259 | 875 | +++; M+ 104 (100.00), 103 (48.72) | 0.5 | C |
| 28 | 10.06 | β-Cymene | 1271 | 998 | +++; M+ 134 (26.37), 119 | 0.6 | C |
| 29 | 10.35 | Terpinolen | 1281 | 1078 | +++; M+ 136 (62.05), 93 | 0.4 | C |
| 30 | 10.62 | Octanal | 1290 | 980 | ++++; M+ 128 (1.20), 43 | 0.3 | C |
| 31 | 11.07 | 2-Ethenyl-2-butenal | 1307 | n.d. | ++; M+ 96 (43.07), 67 | 0.1 | D |
| 32 | 11.50 | (Z)-2-Heptenal | 1324 | 932 | ++++; M+ 112 (6.85), 41 | 0.8 | C |
| 33 | 11.66 | 2-Methyl-3-octanone | 1331 | 1068 | +++; M+ 142 (4.55), 43 | 0.1 | D |
| 34 | 11.92 | 6-Methyl-5-hepten-2-one | 1341 | n.d. | +++; M+ 126 (11.16), 43 | 0.1 | D |
| 35 | 12.32 | 1-Hexanol | 1356 | 862 | ++++; M+ 102 (0.58), 56 | 3.4 | B |
| 36 | 13.12 | (Z)- 3-Hexen-1-ol | 1385 | 835 | +++; M+ 100 (3.35), 67 | 0.1 | D |
| 37 | 13.38 | Nonanal | 1394 | 1083 | ++++; M+ 142 (1.01), 57 | 0.3 | C |
| 38 | 13.42 | 3-Octanol | 1395 | 988 | +++; M+ 130 (2.38), 59 | 0.1 | D |
| 39 | 13.70 | (E)-3-Octen-2-one | 1406 | 1014 | +++; M+ 126 (14.38), 43 | 0.2 | D |
| 40 | 13.96 | Thujone | 1417 | 1101 | +++; M+ 152 (6.57), 110 | 0.2 | D |
| 41 | 14.08 | 2-Octanol | 1422 | 995 | +++; M+ 130 (0.63), 45 | 0.1 | D |
EI = electron impact; GC–MS = gas chromatography–mass spectrometry; RI = retention index.
The reliability of the proposed identification has been indicated according to the following: ++++, mass spectrum and retention indices both on polar and nonpolar columns agreed with those of the standards, mass spectral libraries and literature; +++, mass spectrum and retention indices both on polar and nonpolar columns agreed with mass spectral libraries and the literature; ++, mass spectrum and retention index on a polar column agreed with the values from the mass spectral libraries and the literature. MS ions (EI) were given by GC-MS on a DB-WAX column. They consisted of molecular ion M+ (relative abundance) and the base peak ion, or the peak ion with the second-highest relative abundance if the molecular ion was the base peak.
A: 500–1000; B: 100–500; C: 10–100; and D: 1–10.
Fig. 1.
GC–MS chromatogram (DB-WAX) of the volatile extract from the seed of Dendranthema nankingense, highlighting the most abundant compounds. Peak number as in Table 1. GC–MS =gas chromatography–mass spectrometry.
Interestingly, the seeds contained significant quantities of volatile terpenoids such as camphor and borneol, which are commonly found in the leaves and flowers of D. nankingense [8–10]. It appears that these two volatiles could be present throughout the entire life cycle of D. nankingense, providing the characteristic flavor and functional components of the plant. The relative amount of borneol in the leaf oil of D. nankingense harvested in Jiangsu province [10] is 4.5 times greater than that in our study. The sum of the relative amounts of borneol and camphor in the leaf oil of D. nankingense harvested in Anhui province [8] is very similar to that in this study. Camphor and borneol are collectively regarded as the key bioactive components of a number of traditional Chinese medicines, including Amomum villosum Lour seeds and the two Compositae herbs, Chrysanthemum morifolium Ramat and Flos Chrysanthemi Indici [17,18]. Camphor and borneol have also been identified as the main components in Tanacetum vulgare, which is another Compositae plant that grows wild in Lithuania [19]. Furthermore, camphor was identified as a major volatile component in D. indicum, which is a Chinese herbal medicine [20]. The relative amount of camphor in the D. nankingense seeds was similar to that found in coriander (Coriandrum sativum L.) seeds harvested in Atlantic Canada [21]. Camphor is also a key compound in several commercial and potentially commercial essential oils from the eucalyptus species and other myrtaceae plants [22]. Borneol has a pungent, camphor-like odor and provides a burning taste sensation, somewhat reminiscent of mint. It can be synthesized by reduction of camphor or from pinene [23]. Natural borneol, however, only consists of d-borneol, whereas synthetic borneol contains both d-borneol and isoborneol. Borneol is a common ingredient in many traditional Chinese herbal formulas, where it is more commonly referred to by its Chinese name, bingpian. It occurs not only in the essential oils of numerous medicinal plants, but also in the volatile fractions of edible oil seeds [24]. Borneol induces a variety of physiological responses, including stimulation of the digestive system by increasing the production of gastric juices; toning the heart and improving circulation; treating bronchitis, coughs, and colds; reducing swelling; and relieving stress; it can also be used as a tonic to promote relaxation and reduce fatigue [25]. Furthermore, a number of studies have shown that borneol could improve nasal and oral bioavailability by enhancing the permeability of the blood–brain barrier and improving the distribution of drugs in the brain [26]. It is also widely used for the treatment of anxiety and pain, and as an anesthetic, and can be used in combination with Gardenia for the treatment of stroke in traditional Chinese medicines [27]. As a fragrance ingredient, borneol has been widely used in cosmetics, fine fragrances, and cleaners and detergents. If further studies elucidate new functions and bioactive mechanisms, demand for natural borneol and camphor may increase in the future. With this in mind, D. nankingense seeds or its essential oil may serve as an important source of natural camphor and borneol. Of the other terpenoids identified in the volatiles, 1,8-cineloe, linalool, thujone, chrysanthenone, spathulenol, ocimene, camphene, and caryophyllene were identified as the major constituents in other Compositae plants [28–30]. Caryophyllene, caryophyllene oxide, and 4-terpineol were also found in the leaf of D. nankingense [6].
3.2. Components of volatile oil from the seeds of B. officinalis
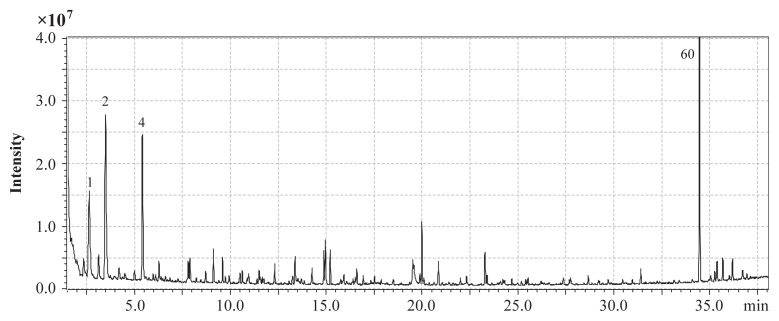
SDE of B. officinalis seeds afforded a pale, tawny-colored oil (yield 2.2%, w/w). The color of the volatile oil was very similar to that reported by Mhamdi et al [11]; however, the SDE yield was 22 times greater than that obtained with the hydro-distillation method used by Mhamdi et al [11]. Furthermore, the observed composition of the volatile mixture from B. officinalis seeds differed considerably between the two studies, and we identified 65 compounds, which is four times as many volatile compounds than Mhamdi et al [11] identified. The volatile compounds are listed in Table 2 (Fig. 2). Surprisingly, in comparison with the qualitative results reported by Mhamdi et al [11], only three compounds—hexanol, linalool, and phenol—coincided. Aldehydes (35.4%), heterocyclic compounds (25.2%), and ketones (22.8%) were identified as the major chemical classes, and 3-methyl butanal (10.8%), 2-pentanone (18.0%), hexanal (10.8%), and 2,3-dihydro-benzofuran (16.1%) were the most abundant compounds. These four main compounds represented approximately 55% of the total amount of volatile compounds in the raw seeds. By comparison, the most abundant volatiles in the borage seeds harvested in Tunisia were β-caryophyllene (26%), p-cymen-8-ol (19.7%), decanal (10.4%), and (Z)-3-hexenol (6.3%). Hexanal, a common plant volatile compound with a grassy odor, was not detected in the Tunisia seeds. These results suggest that the volatiles of B. officinalis seeds might vary significantly depending on their origin, harvest maturity, and storage time. The percentage of hexanal can increase with increasing harvest and storage time of the seeds, and is significantly affected by the amount of lipoxygenase present in the seeds. This phenomenon has been observed in both pine and rice seeds [15,31]. 2,3-Dihydro-benzofuran, also known as coumaran, has been observed as a minor component in several studies involving green tea, Rooibos tea, cotton honey, some fruits, and many plant leaves. It was also found to be a volatile marker compound in 10 honeys originating from unifloral Salvia officinalis L., which contained 4.1–6.8% of coumaran [32]. To the best of our knowledge, our study is the first to identify coumaran as a major (> 10%) volatile component in plants. Thus, B. officinalis seeds may serve as a potential source for the production of coumaran.
Table 2.
Volatile extract composition of Borago officinalis seeds.
| No. | Retention time on DB-WAX (min) | Compounds | RIs | Identification and MS ions (EI)a | Total area (%) | Approximate concentration (μg/mL extract)b | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| DB-WAX | HP-1 | ||||||
| 1 | 2.64 | 3-Methyl butanal | 925 | <700 | ++++; M+ 86 (16.67), 44 | 10.8 | B |
| 2 | 3.50 | 2-Pentanone | 987 | <700 | ++++; M+ 86 (20.02), 43 | 18.0 | A |
| 3 | 5.00 | 2,3-Pentanedione | 1069 | <700 | +++; M+ 100 (16.32), 43 | 0.7 | C |
| 4 | 5.41 | Hexanal | 1086 | 779 | ++++; M+ 100 (1.21), 44 | 10.8 | B |
| 5 | 5.97 | 3-Pentanol | 1110 | <700 | ++++; M+ 88 (0.65),59 | 0.4 | B |
| 6 | 6.28 | 2-Pentanol | 1124 | <700 | ++++; M+ 88 (1.03), 45 | 1.2 | C |
| 7 | 6.62 | 2-Butyl furan | 1139 | 880 | +++; M+ 124 (13.46), 81 | 0.3 | C |
| 8 | 6.85 | 1-Butanol | 1148 | <700 | ++++; M+ 74 (1.86), 56 | 0.1 | D |
| 9 | 7.81 | 2-Heptanone | 1185 | 872 | ++++; M+ 114 (6.60), 43 | 0.9 | C |
| 10 | 7.90 | Heptanal | 1188 | 881 | ++++; M+ 114 (2.01), 70 | 1.2 | C |
| 11 | 8.24 | Dodecane | 1200 | 1199 | ++++; M+ 170 (4.12), 57 | 0.2 | D |
| 12 | 8.71 | (E)-2-Hexenal | 1219 | 829 | +++; M+ 98 (22.10), 41 | 0.7 | C |
| 13 | 9.12 | 2-Pentyl-furan | 1236 | 981 | +++; M+ 138 (18.35), 81 | 1.9 | C |
| 14 | 9.60 | 1-Pentanol | 1254 | 759 | ++++; M+ 88 (1.16), 42 | 1.3 | C |
| 15 | 9.74 | Styrene | 1259 | 875 | +++; M+ 104 (100.00), 103 (48.72) | 0.3 | C |
| 16 | 9.93 | Methyl-pyrazine | 1267 | 796 | +++; M+ 94 (100.00), 67 (47.31) | 0.5 | C |
| 17 | 10.51 | 2-Octanone | 1287 | 964 | +++; M+ 128 (6.18), 43 | 0.7 | C |
| 18 | 10.63 | Octanal | 1290 | 980 | ++++; M+ 128 (1.20), 43 | 0.8 | C |
| 19 | 10.87 | 1-Hydroxy-2-propanone | 1299 | <700 | +++; M+ 74 (11.62), 43 | 0.3 | C |
| 20 | 10.96 | 1-Octen-3-one | 1302 | 959 | ++++; M+ 126 (0.96), 55 | 0.5 | C |
| 21 | 11.39 | 2,5-Dimethyl-pyrazine | 1320 | 885 | +++; M+ 108 (93.21), 42 | 0.3 | C |
| 22 | 11.50 | (Z)-2-Heptenal | 1325 | 932 | +++; M+ 112 (6.85), 41 | 0.8 | C |
| 23 | 11.57 | 2,6-Dimethyl-pyrazine | 1327 | 882 | ++++; M+ 108 (100.00), 42 (90.15) | 0.3 | C |
| 24 | 11.67 | 2-Methyl-3-octanone | 1331 | 1074 | +++; M+ 142 (4.55), 43 | 0.2 | D |
| 25 | 12.32 | 1-Hexanol | 1356 | 862 | ++++; M+ 102 (0.58), 56 | 0.9 | C |
| 26 | 13.07 | 2-Ethyl-6-methyl-pyrazine | 1383 | 976 | +++; M+ 122 (65.87), 121 | 0.2 | D |
| 27 | 13.25 | 2-Nonanone | 1390 | 1072 | +++; M+ 142 (8.94), 43 | 0.5 | C |
| 28 | 13.38 | Nonanal | 1394 | 1083 | ++++; M+ 142 (1.01), 57 | 1.6 | C |
| 29 | 13.70 | (E)-3-Octen-2-one | 1406 | 1014 | +++; M+ 126 (14.38), 43 | 0.3 | C |
| 30 | 14.26 | (E)-2-Octenal | 1430 | 1034 | +++; M+ 126 (3.29), 41 | 0.8 | C |
| 31 | 14.88 | 1-Octen-3-ol | 1454 | 981 | ++++; M+ 128 (0.60), 57 | 1.6 | C |
| 32 | 14.96 | Acetic acid | 1457 | <700 | ++++; M+ 60 (76.08), 43 | 2.7 | B |
| 33 | 15.21 | Furfural | 1467 | 802 | ++++; M+ 96 (100.00), 95 (99.23) | 1.8 | C |
| 34 | 15.76 | (Z)-6-Nonenal | 1488 | 1007 | +++; M+ 140 (0.85), 41 | 0.2 | D |
| 35 | 15.93 | 2-Decanone | 1494 | 1173 | +++; M+ 156 (5.11), 58 | 0.4 | C |
| 36 | 16.53 | Pyrrole | 1518 | 748 | ++++; M+ 67 (100.00), 39 (64.74) | 0.3 | C |
| 37 | 16.60 | Benzaldehyde | 1521 | 929 | ++++; M+ 106 (96.18), 77 | 0.8 | C |
| 38 | 16.93 | 2-Nonenal | 1535 | 1144 | +++; M+ 140 (3.96), 43 | 0.5 | C |
| 39 | 17.33 | Linalool | 1552 | 1088 | ++++; M+ 154 (1.24), 71 | 0.2 | D |
| 40 | 17.53 | 1-Octanol | 1560 | 1069 | ++++; M+ 130 (0.34), 56 | 0.4 | C |
| 41 | 17.88 | 5-Methyl-2-furancarboxaldehyde | 1574 | 939 | +++; M+ 110 (100.00), 109 (79.03) | 0.2 | D |
EI = electron impact; GC–MS = gas chromatography–mass spectrometry; RI = retention index.
The reliability of the proposed identification has been indicated according to the following: ++++, mass spectrum and retention indices both on polar and nonpolar columns agreed with those of the standards, mass spectral libraries and literature; +++, mass spectrum and retention indices both on polar and nonpolar columns agreed with mass spectral libraries and the literature; ++, mass spectrum and retention index on a polar column agreed with the mass spectral libraries and the literature. MS ions (EI) were given by GC-MS on a DB-WAX column. They consisted of molecular ion M+ (relative abundance) and the base peak ion, or the peak ion with the second-highest relative abundance if the molecular ion was the base peak.
A: 500–1000; B: 100–500; C: 10–100; and D: 1–10.
Fig. 2.
GC–MS chromatogram (DB-WAX) of the volatile extract from the seed of Borago officinalis, highlighting the most abundant compounds. Peak number as in Table 2. GC–MS =gas chromatography–mass spectrometry.
Thirteen heterocyclic compounds, including derivatives of pyrazine, pyrrole, and furan, were identified in the volatiles of B. officinalis seeds. Pyrazine, pyrrole, and furan derivatives are widely distributed in processed food items, such as roasted sorghum, wines, and roasted almond [33–35]. Four of the alkylpyrazines detected—methyl-pyrazine, 2,5-dimethyl-pyrazine, 2,6-dimethyl-pyrazine, and 2-ethyl-6-methyl-pyrazine—have also been found in roasted soybean oils, and may contribute to the characteristic toasted, nut- and peanut-like burnt aroma [36,37]. Of these four compounds, 2-methyl-pyrazine, 2,5-dimethyl-pyrazine, and 2-ethyl-6-methyl-pyrazine are synthetically accessible via the reaction between d-ascorbic acid and l-threonine or l-serine.
3.3. Similarities between volatile oils obtained from D. nankingense and B. officinalis seeds
The volatile oil yields from both types of seeds were very similar, and 33 of the volatile compounds were common to both seeds, with the majority of these compounds being aliphatic aldehydes and alcohols. Of these compounds, hexanal, 1-hexanol, nonanal, and 1-octen-3-ol have also been reported in poppy seeds and groats [38,39]. Hexanal is particularly abundant in both seeds. High levels of hexanal have been found in a number of plant seeds, including pine seeds, coriander seeds, rice seeds, and Trichosanthes kirilowii seeds [15,23,31,40]. All four of these volatiles play important roles as food flavor ingredients in baked goods, frozen dairy products, candies, and nonalcoholic beverages.
Both seeds contained seven C8 compounds with a mushroom flavor, and 1-octen-3-ol, (E)-3-octen-2-one, and octanal were common to them both. 1-Octen-3-ol, also known as “mushroom alcohol,” is a volatile alcohol produced by many edible fungi species [41]. Furthermore, it is a component of the volatile oil and plays an important role in the odor profiles of several plants, especially in members of the Lamiaceae family.
4. Conclusion
A detailed investigation of the volatile extracts of two seeds (D. nankingense and B. officinalis) prepared by SDE revealed a total of 127 compounds. The major volatiles found in the D. nankingense seeds were hexanal, benzeneacetaldehyde, borneol, (−)-camphor, and 3-methyl-1-butanol. These compounds, especially borneol and (−)-camphor, can be used as quality markers for D. nankingense. The major volatiles found in the B. officinalis seeds were 2-pentanone, coumaran, 3-methyl butanal, and hexanal. Coumaran was the most abundant volatile component in B. officinalis seeds.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31171704), and the System Construction Project for Green Vegetables from Shanghai Municipal Agricultural Commission, China.
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 31171704), and the System Construction Project for Green Vegetables from Shanghai Municipal Agricultural Commission, China.
Footnotes
Conflicts of interest
All authors have no conflicts of interest.
REFERENCES
- 1. Zhao CX, Zeng YX, Wan MZ, Li RX, Liang YZ, Li CY, Zeng ZD, Chau FT. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. J Sep Sci. 2009;32:660–70. doi: 10.1002/jssc.200800484. [DOI] [PubMed] [Google Scholar]
- 2. Branca F. Trials related to the cultivation of wild species utilized in Sicily as vegetables. Italy Hortus. 2001;8:22–6. [Google Scholar]
- 3. Del Río-Celestino M, Font RA, De Haro-Bailón A. Distribution of fatty acids in edible organs and seed fractions of borage (Borago officinalis L.) J Sci Food Agric. 2008;88:248–55. [Google Scholar]
- 4. Wettasinghe M, Shahidi F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem. 1999;67:399–414. [Google Scholar]
- 5. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PY, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 6. Chen HC, Peng LW, Sheu MJ, Lin LY, Chiang HM, Wu CT, Wu CS, Chen YC. Effects of hot water treatment on the essential oils of calamondin. J Food Drug Anal. 2013;21:363–8. [Google Scholar]
- 7. Wangensteen H, Samuelsen AB, Malterud KE. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–7. [Google Scholar]
- 8. Guan YL, Zou DS, Wang YJ. Analysis of chemical compositions of volatile oils from Dendranthema nankingense in Anhui province. Chin Traditional Patent Med. 2007;29:914–6. [Google Scholar]
- 9. Ji LL. Chemical compositions of the essential oil from leaves and stalks of Chrysanthemum nankingense Hand. Mazz and its anti-fungal activities. Shipin Kexue. 2005;26:91–4. [Google Scholar]
- 10.Lü L, Qin MJ, Wu G.Comparison of volatile constituents in leaf oils of five Dendranthema nankingense. Proceedings of the 7th Annual Academic Conference of China Society of Natural Resources; 2006; Fuzhou, China. pp. 317–22. [Google Scholar]
- 11. Mhamdi B, Wannes WA, Bourgou S, Marzouk B. Biochemical characterization of Borage (Borago officinalis L.) seeds. J Food Biochem. 2009;33:331–41. [Google Scholar]
- 12.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4th ed. Carol Stream, IL: Allured Publ. Corp; 2007. [Google Scholar]
- 13.Rychlik M, Schieberle P, Grosch W. Compilation of odor thresholds, odor qualities and retention indices of key food odorants. Deutsche Forschungsanstalt für Lebensmittelchemie and Instit für Lebensmittelchemie der Technischen Universitat München; 1998. [Google Scholar]
- 14.Kondjoyan N, Berdague JL. A compilation of relative retention indices for the analysis of aromatic compounds. 1st ed. Clermont Ferrand, France: Laboratoire Flaveur; 1996. [Google Scholar]
- 15. Tammela P, Nygren M, Laakso I, Hopia A, Vuorela H, Hiltunen R. Volatile compound analysis of ageing Pinus sylvestris L. (Scots pine) seeds. Flavour Fragr J. 2003;18:290–5. [Google Scholar]
- 16. Bail S, Stuebiger G, Krist S. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108:1122–32. doi: 10.1016/j.foodchem.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 17. Dong L, Wang JY, Deng CH, Shen XZ. Gas chromatography-mass spectrometry following pressurized hot water extraction and solid-phase microextraction for quantification of eucalyptol, camphor, and borneol in Chrysanthemum flowers. J Sep Sci. 2007;30:86–9. doi: 10.1002/jssc.200600207. [DOI] [PubMed] [Google Scholar]
- 18. Ye Q, Deng CH. Determination of camphor and borneol in traditional Chinese medicines by microwave-assisted extraction and gas chromatography with flame ionization detector. Anal Lett. 2008;41:2387–401. [Google Scholar]
- 19. Judzentiene A, Mockute D. The inflorescence and leaf essential oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochem Syst Ecol. 2005;33:487–98. [Google Scholar]
- 20. Wang CZ, Su Y, Li D, Cai B, Guo YL. Analysis of volatile organic compounds from Dendranthema indicum var. aromaticum by headspace gas chromatography-mass spectrometry and accurate mass measurement. Anal Lett. 2010;43:2297–310. [Google Scholar]
- 21. Zheljazkov VD, Pickett KM, Caldwell CD, Pincock JA, Roberts JC, Mapplebeck L. Cultivar and sowing date effects on seed yield and oil composition of coriander in Atlantic Canada. Ind Crops Prod. 2008;28:88–94. [Google Scholar]
- 22. Cornwell CP, Lassak EV. Camphor in commercial and potentially commercial oils of eucalyptus species and other myrtaceae. J Essent Oil Res. 2010;22:59–65. [Google Scholar]
- 23.Burdock GA. Fenaroli’s handbook of flavor ingredients. 4th ed. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 24. Cioni PL, Flamini G, Caponi C, Ceccarini L, Morelli I. Analysis of volatile fraction, fixed oil and tegumental waxes of the seeds of two different cultivars of Helianthus annuus. Food Chem. 2005;90:713–7. [Google Scholar]
- 25. Buchbauer G, Jager W, Jirovetz L, Meyer F, Dietrich H. Effects of valerian root oil, borneol, isoborneol, bornyl acetate and isobornyl acetate on the motility of laboratory animals (mice) after inhalation. Pharmazie. 1992;47:620–2. [PubMed] [Google Scholar]
- 26. Lu Y, Chen XL, Du SY, Wu Q, Yao ZL, Zhai YS. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of geniposide in rats. Arch Pharmacol Res. 2010;33:691–6. doi: 10.1007/s12272-010-0507-8. [DOI] [PubMed] [Google Scholar]
- 27. Quintans-Junior LJ, Guimaraes AG, Araujo BES, Oliveira GF, Santana MT, Moreira FV, Santos MRV, Cavalcanti SCH, Junior WDL, Boteiho MA, Ribeiro LAA, Nobrega FFF, Almeida RN. Carvacrol, (–)-borneol and citral reduce convulsant activity in rodents. Afr J Biotechnol. 2010;9:6566–72. [Google Scholar]
- 28. Saroglou V, Dorizas N, Kypriotakis Z, Skaltsa HD. Analysis of the essential oil composition of eight Anthemis species from Greece. J Chromatogr A. 2006;1104:313–22. doi: 10.1016/j.chroma.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 29. Salido S, Valenzuela LR, Altarejos J, Nogueras M, Sanchez A. Composition and infraspecific variability of Artemisia herbaalba from southern Spain. Biochem Syst Ecol. 2004;32:265–77. [Google Scholar]
- 30. Vernin G, Merad O, Vernin GMF, Zamkotsian RM, Parkanyi C. GC-MS analysis of Artemisia herba alba Asso essential oils from Algeria. Dev Food Sci. 1995;37:147–205. [Google Scholar]
- 31. Suzuki Y, Ise K, Li CY, Honda I, Iwai Y, Matsukura U. Volatile components in stored rice [Oryza sativa (L.)] of varieties with and without lipoxygenase-3 in seeds. J Agric Food Chem. 1999;47:1119–24. doi: 10.1021/jf980967a. [DOI] [PubMed] [Google Scholar]
- 32. Jerkovic I, Mastelic J, Marijanovic Z. A variety of volatile compounds as markers in unifloral honey from Dalmatian sage (Salvia officinalis L.) Chem Biodives. 2006;3:1307–16. doi: 10.1002/cbdv.200690134. [DOI] [PubMed] [Google Scholar]
- 33. Cullere L, Escudero A, Perez-Trujillo JP, Cacho J, Ferreria V. 2-Methyl-3-(methyldithio)furan: a new odorant identified in different monovarietal red wines from the Canary Islands and aromatic profile of these wines. J Food Compos Anal. 2008;21:708–15. [Google Scholar]
- 34. Lasekan O, Abbas K. Analysis of volatile flavour compounds and acrylamide in roasted Malaysia tropical almond (Terminalia catappa) nuts using supercritical fluid extraction. Food Chem Toxicol. 2010;48:2212–6. doi: 10.1016/j.fct.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 35. Miklósy E, Kerényi Z. Comparison of the volatile aroma components in noble rotted grape berries from two different locations of the Tokaj wine district in Hungary. Anal Chim Acta. 2004;513:177–81. [Google Scholar]
- 36. Jung MY, Bock JY, Back SO, Lee TK, Kim JH. Pyrazine contents and oxidative stabilities of roasted soybean oils. Food Chem. 1997;60:95–102. [Google Scholar]
- 37. Wilkens WF, Lin FM. Volatile flavor components of deep fat-fried soybeans. J Agric Food Chem. 1970;18:337–9. [Google Scholar]
- 38. Janes D, Prosen H, Kreft I, Kreft S. Aroma compounds in Buckwheat (Fagopyrum esculentum Moench) groats, flour, bran, and husk. Cereal Chem. 2010;87:141–3. [Google Scholar]
- 39. Krist S, Stuebiger G, Unterweger H. Analysis of volatile compounds and triglycerides of seed oils extracted from different poppy varieties (Papaver somniferum L.) J Agric Food Chem. 2005;53:8310–6. doi: 10.1021/jf0580869. [DOI] [PubMed] [Google Scholar]
- 40. Wu SM, Xu T, Casimir CA. Effect of roasting on the volatile constituents of Trichosanthes kirilowii seeds. J Food Drug Anal. 2014;22:310–7. doi: 10.1016/j.jfda.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu SM, Krings U, Zorn H, Berger RG. Volatile compounds from the fruiting bodies of beefsteak fungus Fistulina hepatica (Schaeffer: Fr.) Fr. Food Chem. 2005;92:221–6. [Google Scholar]