Abstract
Heating effect on total phenol, flavonoids, antioxidant activity, and sugar content of six onion varieties has been quantitatively investigated to explore the effect of different temperatures. The onion varieties comprised one red-skinned variety, two white-skinned varieties, and three yellow-skinned varieties. The heating temperature was scanned at 80°C, 100°C, 120°C, and 150°C for 30 minutes each, and quantitative analysis was performed relative to the powdered onion at ambient temperature. Quercetin, glucosides and sugar content were analyzed using high-performance liquid chromatography. The total phenolic and antioxidant content increased in all six varieties. The total flavonoid levels showed a considerable change. On heating the onion samples at 120°C for 30 minutes, the red-skinned variety showed the highest level of total phenolic content [13712.67 ± 1034.85 μg of gallic acid equivalent/g dry weight (μg GAE/g DW)] and total flavonoids [3456.00 ± 185.82 μg of quercetin equivalents/g dry weight (μg Q/g DW)], whereas the content of total phenolics and total flavonoids were 13611.83 ± 341.61 μg GAE/g DW and 3482.87 ± 117.17 μg Q/g DW, respectively, for the yellow-skinned (Sunpower) variety. Quercetin and its glucoside contents increased up to 120°C and then decreased at 150°C, whereas the sugar content continuously decreased with heating. All cultivars showed the same pattern in the heating effect, and the predominant flavonoids were destroyed at higher temperatures. Therefore, it is improper to expose onion powder to a temperature higher than 120°C.
Keywords: antioxidant activity, onion powder, sugar content, total flavonoids, total phenolics
1. Introduction
Onion (Allium cepa L.) is one of the most cultivated vegetables in the world and is a good source of flavonoids. Flavonoids are bioactive components that possess a distinct flavor and aroma, and have potential health benefits [1]. Heating vegetables during the cooking process causes the loss of heat-sensitive compounds and reduces the nutritional quality. Heating is accountable for the oxidation, thermal degradation, and leaching of bioactive compounds from fresh vegetables [2]. Depending on the morphology and nutritional properties of vegetables, positive and negative effects of heating have been reported [3]. Different heating conditions (e.g., heating duration and temperatures) have different effects on the antioxidant properties of vegetables [4]. To obtain maximum health benefits, raw onion should be used or moderately cooked. In onion, quercetin aglycone accounts for up to 10% of the total flavonoids, and the remaining amount is in the form of glucosides. The compositional variations of quercetin and its glucosides exist in the yellow, white, and red onion varieties; and various other flavonoids, flavonols, anthocyanins, and dihydroflavonols exist in different cultivars [5]. Compared to red onions, yellow onions contain a high level of quercetin, and white onions have the lowest concentration [6]. More interest has recently been focused on the sweet and less pungent onion cultivars because of their appealing sweetness and lower pungency. The shelf life of the sweet onion is shorter than that of nonsweet onions, which can be attributed to the high water content [7]. Before being marketed to large food and trade companies, onions are typically cured, dried, and held in special long-term stores. Onions with a short shelf life are used within a short time period or they are processed as a sauce, fried chips, onion powder, etc. However, heating is the best method to increase the storage potential of sweet onion cultivars with a low shelf life [8].
Dehydrated onion has great commercial value because of its culinary and medicinal properties. For instance, the food industry sells onion powder as a nutraceutical or as a dietary supplement [9]. The processing of onion powder involves several steps such as storage, pretreatment, drying, and boiling. These steps affect the composition of the bioactive components of the onion. The fate of phytochemicals during processing and their bioavailability after consumption has been investigated in different vegetables [10–12]. The evaluation of the bioactive components of onion has practical importance and the stability of antioxidants during the sautéing, baking, boiling, and heat processing of fruits and vegetables has been discussed previously [4,13,14]. The beneficial health effects of antioxidants attract the interest of consumers and the food industries. Therefore, it is important to study the content of antioxidants in foods during heating at various temperatures. In this paper, we aim to analyze the chemical composition of powdered onions before and after heating at different temperatures that are generally applied during processing such as those used for making ketchup, sauces, soups, chips, meat products, and crackers. For this analysis, we selected six different onion varieties, which include red onion, yellow onion, and white (i.e., sweet) onion.
2. Materials and methods
2.1. Chemicals and standard solutions
All solvents used in this study were of high-performance liquid chromatography (HPLC) grade. Water was obtained from J. T. Baker (Phillipsburg, NJ, USA); methanol, from Dunstan (Seoul, Korea); and acetonitrile, from Daejung (Gyonggi-do, Korea). Trifluoroacetic acid (TFA; extrapure grade) was supplied by Alfa Aesar (Ward Hill, MA, USA). Quercetin-3,4′-O-diglucoside and quercetin-4′-O-monoglucoside were supplied by Polyphenols Laboratories AS (Sandnes, Norway). The purity of the flavonol standards was controlled by HPLC and was >99%. Gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Tro-lox), 2,4,6-tris (1-pyridyl)-5-triazine (TPTZ), ferric chloride, and Folin–Ciocalteu reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA), and 2,2-diphenyl-1-picrylhydrazyl was purchased from Wako Pure Chemicals (Osaka, Japan) and were used for antioxidant assays. The saccharide standards sucrose (>99.5%), d-glucose (>99.5%), and d-fructose (guaranteed reagent grade) were obtained from Fluka (Buchs, Switzerland), Sigma-Aldrich (Buchs, Switzerland), and Junsei Chemical Co. (Koshigaya, Saitama, Japan), respectively.
The stock solutions of quercetin (1 mg/mL) and quercetin glucosides (4 mg/mL) were prepared in 75% ethanol. All solutions were stored at −20°C. Calibration of standards was obtained by appropriate dilution of the stock solutions.
2.2. Sample preparation
Onions were grown at the Bioenergy Crop Research Center at the National Institute of Crop Science of the Rural Development Administration (Muan, Republic of Korea). Six onion varieties selected for this study were harvested from April to May 2013. The onions were cured in the field for 10 days and transported to the laboratory. The onion varieties were redskinned (Colossal), yellow-skinned (Sunpower, Chairman, 110455), and white-skinned (110444, B-67); their dry matter contents are described in Table 1.
Table 1.
General information, dry matter percentage, and weight loss of six different onion varieties.
| Sample no. | Onion type | DM% | % Weight loss at 80°C, heated for 30 min | % Weight loss at 100°C, heated for 30 min | % Weight loss at 120°C, heated for 30 min | % Weight loss at 150°C, heated for 30 min | Bulb shape index | Cultivar type | Seed company |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Colossal (red) | 10.39 ± 0.9a | 0.71 ± 0.00a | 1.01 ± 0.04a | 2.2 ± 0.03a | 2.2 ± 0.05a | 92 | F1 | Asia |
| 2 | Sunpower (yellow) | 7.26 ± 0.53b | 0.83 ± 0.01a | 1.31 ± 0.05a | 3.2 ± 0.00d | 3.2 ± 0.04c | 98 | F1 | Koregon |
| 3 | 110455 (yellow) | 11.26 ± 0.52a | 0.91 ± 0.02b | 1.21 ± 0.03b | 2.8 ± 0.01a | 2.2 ± 0.02a | 90 | F1 | New Seoul |
| 4 | Chairman (yellow) | 9.71 ± 0.84b | 0.59 ± 0.00a | 2.11 ± 0.02d | 2.2 ± 0.02a | 3.2 ± 0.00a | 97 | F1 | Hungnong |
| 5 | 110444 (white) | 11.41 ± 0.92a | 0.81 ± 0.01b | 2.21 ± 0.03c | 2.7 ± 0.05b | 2.2 ± 0.01b | 88 | F1 | Asia |
| 6 | B-67 (white) | 12.93 ± 0.53a | 0.91 ± 0.04b | 2.20 ± 0.04c | 2.2 ± 0.04a | 2.2 ± 0.08b | 84 | F1 | Asia |
Column-wise values with the same superscripts indicate no significant difference (p < 0.05).
All values are expressed as the mean ± SD.
DM% = dry matter percentage; SD = standard deviation.
For each variety, replicate composite samples were prepared by mixing equal amounts of onion powder using 15 healthy onion bulbs in triplicate. To prepare the onion powder, approximately 800 g of onions were skinned, chopped, and freeze-dried. The resulting lyophilized onions were then ground into powder. The samples were stored in sealed plastic bottles at −20°C until analysis.
2.3. Dry matter percentage determination
The percentage of dry matter was determined before the sample was freeze-dried. For each variety, chopped samples of approximately 35 g were maintained in an oven with air circulation initially at 80°C for 24 hours, and then at 105°C for 2 hours. Each determination was performed in triplicate.
2.4. Heat treatment of the onion samples
Onion powder (10 g) was placed in a single layer in a Pyrex petri dish and heated in an oven at 80°C, 100°C, 120°C, and 150°C for 30 minutes each. After heating, the onion powder was allowed to cool at room temperature. After cooling a second time, the weight was measured to check the percentage of weight loss.
2.5. Extraction of phenolic compounds
The samples were extracted in triplicate, based on the method previously reported by Bonaccorsi et al [15], but with slight modifications. Approximately 1 g of onion powder was maintained overnight in 20 mL of 75% ethanol at 4°C. The supernatant was separated and the residue was again mixed in 20 mL of 75% ethanol, and then stirred by a magnetic stirrer for 1 hour. The slurry was centrifuged at 10,000 rpm for 20 minutes at 4°C. The supernatant was removed, and the residue powder was mixed with fresh 75% ethanol. The centrifugation process was repeated twice. The combined 75% ethanol fractions were evaporated on a rotary evaporator at 45°C to approximately 8 mL and 75% ethanol was added to up to 10 mL. The extracts were stored at −20°C.
2.6. Analysis of quercetin and its glucosidase content
The HPLC analysis of all extracts was performed using an Agilent 1100 chromatograph (Agilent, Palo Alto, CA, USA) that was equipped with a solvent delivery system, an autosampler, a DAD (Diode Array Detectors) detector set at 360 nm, and a ChemStation data acquisition system. Flavonoids were separated on a Zorbax Eclipse XDB C-18 column (250 mm × 4.6 mm) with a particle size of 5 μm (Agilent, Santa Clara CA, USA) and protected using a Phenomenex (Torrance, CA, USA) C18-type guard column. The column was maintained at 25°C. The mobile phase consisted of 0.1% TFA in water (solvent A) and methanol (solvent B). A gradient elution program was set as follows: 0–10 minutes, 20% B; 10–15 minutes, 20–80% B; 15–22 minutes, 80–20% B. The flow rate was 0.8 mL/min, and the injected volume was 10 μL. Quercetin flavonols were quantified by comparing them with their respective calibration curves. Chromatographic analysis of each replicate sample was repeated twice, and the average peak areas were used in calculations.
2.7. Analysis of total flavonoids
The total flavonoids were determined by the colorimetric method of Chang et al [16], but with some modification. Onion extracts (0.5 mL) were mixed with 1.5 mL of 75% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 0.1M potassium acetate, and 2.8 mL of distilled water. The reaction mixture was maintained for 30 minutes at room temperature. The absorbance was measured against a blank at 415 nm using a Shimadzu UV-1700 spectrophotometer (Shimadzu, Tokyo, Japan). Quercetin was used for the standard calibration curve and the total flavonoid content was expressed in μg of quercetin equivalents/g dry weight (μg Q/g DW).
2.8. Analysis of total phenolics
The content of total phenolics was analyzed spectrophoto-metrically at 790 nm using the Folin–Ciocalteau colorimetric method described by Dalamu et al [17], but with some modification. Onion extracts (100 μL) were mixed with 2.9 mL distilled water in a test tube. Dilute Folin-Ciocalteau reagent (0.5 mL) was then added. Samples were mixed properly and allowed to stand for 15 minutes. Two milliliters of 20% sodium carbonate aqueous solution was added. The reaction mixture was incubated at room temperature for 90 minutes, and the absorbance was measured at 790 nm against the blank using a Shimadzu UV-1700 spectrophotometer (Shimadzu). The standard curve was prepared from gallic acid, and the content of total phenolics were expressed as μg of gallic acid equivalents/g dry weight (μg GAE/g DW).
2.9. Analysis of antioxidant activity
2.9.1. Fluorescence recovery after photobleaching assay
The fluorescence recovery after photobleaching (FRAP) assay was performed, as described by Benzie and Strain [18]. It was performed with a Shimadzu UV-1700 spectrophotometer (Shimadzu). The experiment was conducted at 37°C under low pH (3.6) with a blank sample in parallel. The FRAP working reagent was freshly prepared by mixing 300mM acetate buffer (pH 3.6), 10mM TPTZ in 40mM hydrogen chloride (HCl), and 20mM iron(III) chloride (FeCl3·H2O) in the ratio of 10:1:1. For each assay, 2.90 mL of the FRAP reagent and 100 μL of the onion extracts were used. After 30 minutes, the absorbance of the reaction (incubated at 37°C) was measured at 593 nm. Calibration was performed using Trolox and the values were expressed in μmol Trolox equivalent/g of dry weight (μmol TE/g DW).
2.9.2. 2,2-Diphenyl-1-picrylhydrazyl assay
A 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was performed using the method of Brand-Williams et al [19], but with some modifications. The DPPH solution was prepared by dissolving 24 mg DPPH with 100 mL of 75% ethanol and stored at −20°C until use. The working solution was prepared by diluting the DPPH solution with 75% ethanol to obtain an absorbance in the range of 1.1 ± 0.02 units at 515 nm. The DPPH solution (950 μL) was mixed with 50 μL standards or with sample extracts, and diluted with 1 mL of 75% ethanol. It was then incubated for 20 minutes. A control was prepared by adding 1050 μL of 75% of ethanol to 950 μL of DPPH solution. The absorbance was measured against the blank using the Shimadzu UV-1700 spectrophotometer (Shimadzu). All results were expressed in μmol Trolox equivalent/g of dry weight (μmol TE/g DW).
2.10. Analysis of sugars
Sugars were extracted in triplicate, based on the method of Kahane et al [20], with slight modifications. Approximately 1 g of onion powder was mixed with 50 mL of 80% ethanol and refluxed for 1 hour. The samples were filtered through a Buchner filter and readjusted to 50 mL with 80% ethanol. Samples were then concentrated in a rotary evaporator under reduced pressure at <50°C. These concentrated extracts were diluted with 10 mL of water and stored at −20°C until used for analysis. The extract was filtered through 0.2-μm syringe filters immediately before analysis.
Twenty microliters of the extract was injected into a Zorbax carbohydrate (150 mm × 4.6 mm) column by Agilent (Palo Alto, CA, USA) protected with an Agilent NH2 precolumn. The sample was eluted with acetonitrile:water (75:25, v:v), as recommended by the manufacturer. The column temperature was maintained at 30°C and the flow rate was 1 mL/min. The analysis was performed using a Shimadzu 10A-VP series chromatograph (Shimadzu) with a Rheodyne 7725i manual injector (Rheodyne, Cotati, CA, USA) using a 20-μL sample loop and a refractive index detector calibrated against standard solutions (2–25 mg/mL) of respective sugars. Chromatograms were integrated using the Shimadzu Class-VP software. Each injection was repeated 2–3 times.
2.11. Statistical analysis
For each sample, the results are presented as the mean ± standard deviation for the three replicates. In chromatographic assays, each replicate solution was injected two or three times, and the averaged peak areas were used to calculate the analyte concentrations. Differences between mean values were assessed using the Student t test at a significance level of p < 0.05. All statistical calculations were performed using OriginPro 8.1 software (OriginLab; Northampton, MA, USA).
3. Results and discussion
3.1. Dry matter and weight loss
Table 1 presents the dry matter percentage and the percentage weight loss of the heated onion powder for the six studied varieties. Table 1 also shows the percentage weight loss for all onion varieties heated at 80°C, 100°C, 120°C, and 150°C for 30 minutes each, and presents the general information of the varieties.
3.2. Effect of heating on the major flavonoids
The four major flavonoids in onion are quercetin aglycone (Q), quercetin-4′-O-monoglucoside (QMG), quercetin-3,4′-O-diglucoside (QDG), and isorhamnetin-3-glucoside (IMG). These flavonoids were determined using HPLC. The corresponding changes on heating are presented in Table 2. In general, heating had a positive effect on all four flavonoids. For instance, the total flavonoid content in the red onion variety (Q + QMG + QDG + IMG) increased from 9.34 μmol/g DW to 9.70 μmol/g DW on heating at 120°C for 30 minutes and then decreased to 5.40 μmol/g DW at 150°C. In all the studied onion varieties, the total flavonoid content increased up to 120°C, and then decreased at 150°C. Quercetin was detected in all studied varieties, and some varieties contained components below the detection level. Usually pretreatment of onions includes sun drying, curing, boiling, and roasting. Most of these treatments can affect the content of flavonoids. For instance, after drying using different processes such as freezing, vacuum, and hot air, the dried onion powder had a different proportion of quercetin, QMG, and QDG [21]. The reason for the different proportion of QMG and QDG could be because of the conversion or release of glucosides or bound phenolics into free phenolic derivatives or because of the formation of complexes by reaction with metals [10]. In the present study, varieties such as Colossal, Sunpower, Chairman, and 110455 show fluctuation in both glucosides at different heating temperatures with a slight increment at 120°C, compared to the ambient temperature; however, 110444 and B-67 have substantially less QMG, and QDG was below the detection limit. The reason for the fluctuation could be because of the heating time required at a particular temperature for the breakdown of cellular constituents to release the individual flavonols. Makris and Rossiter [4] report that quercetin conjugates in the onion bulb are resistant to thermal degradation. However, during food processing autolytical changes can influence the flavonol composition in onions [22]. During the storage of heat-treated and nonheat-treated onions, there were no differences in the amount of discarded onions because of rot or mold [23]. Heating of the Hyred, Red Baron, and Recorra varieties at 36°C for 24 hours did not have any effect on the total flavonols content, whereas prolonged heating for 96 hours lowered the flavonoid content. Thus, we concluded that heating unfavorably affects the metabolism of onions [23]. The contradictory change of flavonoids in raw and processed onion has been reported in previous studies. For instance, depending on the frying time, onion losses 23–29% of quercetin conjugates [22]. A boiling time between 3 minutes and 60 minutes similarly resulted in 20.6–75% loss of quercetin in the boiled onion tissue [4,22,24–26]. However, flavonol loss in boiled tissues is not because of the chemical breakdown of quercetin conjugates, but because of the leaching of quercetin into the cooking water [4,22,24,25]. Regardless of the cooking treatment, quercetin-3,4′-O-diglucoside and quercetin-4′-O-monoglucoside represent approximately 88–89% of the total flavonol content and are relatively heat-stable [27]. Makris and Rossiter [4] reported a QDG:QMG ratio in boiled tissue and in water, and concluded that leaching of QDG was favored during boiling. Price et al [22] similarly demonstrated that decreases in QDG could be quantitatively explained by increases in QMG and quercetin content [22]. Different processing methods such as peeling and trimming of onions result in a great loss of flavonoids, and samples cooked in a microwave oven have a smaller loss, compared to samples cooked in water [26]. The pilot plant peeling and blanching of onions reduces the flavonoid content to approximately one-half of the starting levels; this is probably because of the loss of the outer flavonoid-rich onion layer [26]. Our results indicated that heating at different temperatures resulted in fluctuation in the content of the individual flavonoids, but the overall flavonoid content was retained. These findings coincide with the report of Woo et al [28], who reported that microwave cooking without water retains flavonoids better and that flavonoid loss may depend on the preparation method used such as boiling, frying with oil and butter, or microwaving of onions.
Table 2.
Effect of heating on flavonoid content (μmol/g DW) of six different onion varieties.
| Onion type | Heat treatment | Q | QMG | QDG | IMG | Total |
|---|---|---|---|---|---|---|
| Colossal | Untreated (ambient) | 0.217 ± 0.05a | 3.615 ± 0.55c | 4.807 ± 0.47a | 0.700 ± 0.06b | 9.339 |
| Heated, 80°C, 30 min | 0.258 ± 0.06a | 3.779 ± 0.38a | 4.661 ± 0.07a | 0.729 ± 0.03a | 9.429 | |
| Heated, 100°C, 30 min | 0.194 ± 0.03a | 3.394 ± 0.39a | 4.665 ± 0.15a | 0.673 ± 0.03b | 8.927 | |
| Heated, 120°C, 30 min | 0.306 ± 0.02c | 3.729 ± 0.37a | 4.866 ± 0.30a | 0.795 ± 0.07a | 9.695 | |
| Heated, 150°C, 30 min | 0.212 ± 0.02a | 2.081 ± 0.03b | 2.666 ± 0.30c | 0.444 ± 0.03c | 5.404 | |
| Sunpower | Untreated (ambient) | 0.259 ± 0.02a | 4.767 ± 0.40a | 5.482 ± 0.43a | 0.453 ± 0.03b | 10.96 |
| Heated, 80°C, 30 min | 0.260 ± 0.02b | 4.770 ± 0.43c | 5.483 ± 0.41a | 0.455 ± 0.03a | 10.97 | |
| Heated, 100°C, 30 min | 0.260 ± 0.02a | 4.790 ± 0.39a | 5.486 ± 0.45c | 0.456 ± 0.03d | 10.99 | |
| Heated, 120°C, 30 min | 0.288 ± 0.02c | 4.888 ± 0.42a | 5.745 ± 0.03a | 0.474 ± 0.03a | 11.36 | |
| Heated, 150°C, 30 min | 0.194 ± 0.03a | 3.394 ± 0.39a | 4.665 ± 0.15c | 0.673 ± 0.03a | 6.075 | |
| 110455 | Untreated (ambient) | 0.253 ± 0.04c | 2.751 ± 0.08a | 6.377 ± 0.26a | 0.458 ± 0.00a | 9.840 |
| Heated, 80°C, 30 min | 0.290 ± 0.06a | 2.691 ± 0.04d | 6.616 ± 0.14b | 0.563 ± 0.17a | 10.90 | |
| Heated, 100°C, 30 min | 0.392 ± 0.08a | 3.105 ± 0.62a | 7.633 ± 0.43a | 0.557 ± 0.09a | 11.68 | |
| Heated, 120°C, 30 min | 0.392 ± 0.08a | 3.105 ± 0.62b | 7.633 ± 0.45c | 0.557 ± 0.09d | 11.68 | |
| Heated, 150°C, 30 min | 0.219 ± 0.06c | 1.714 ± 0.28a | 3.743 ± 0.15a | 0.338 ± 0.04b | 6.019 | |
| Chairman | Untreated (ambient) | 0.055 ± 0.00a | 2.432 ± 0.26c | 3.568 ± 0.38a | 0.282 ± 0.02a | 6.338 |
| Heated, 80°C, 30 min | 0.057 ± 0.00a | 2.435 ± 0.26a | 3.571 ± 0.39a | 0.283 ± 0.02d | 6.346 | |
| Heated, 100°C, 30 min | 0.056 ± 0.00c | 2.429 ± 0.27a | 3.571 ± 0.38a | 0.285 ± 0.02a | 6.338 | |
| Heated, 120°C, 30 min | 0.066 ± 0.00a | 2.696 ± 0.32d | 4.137 ± 0.43c | 0.376 ± 0.06c | 7.276 | |
| Heated, 150°C, 30 min | 0.055 ± 0.00d | 1.704 ± 0.39a | 2.544 ± 0.15a | 0.257 ± 0.03a | 4.561 | |
| 110444 | Untreated (ambient) | 0.037 ± 0.00b | 0.093 ± 0.00c | nd | 0.090 ± 0.00d | 0.118 |
| Heated, 80°C, 30 min | 0.038 ± 0.00a | 0.095 ± 0.00a | nd | 0.092 ± 0.00a | 0.121 | |
| Heated, 100°C, 30 min | 0.038 ± 0.00d | 0.096 ± 0.00a | nd | 0.093 ± 0.00b | 0.122 | |
| Heated, 120°C, 30 min | 0.039 ± 0.00a | 0.098 ± 0.00a | nd | 0.094 ± 0.00a | 0.127 | |
| Heated, 150°C, 30 min | 0.036 ± 0.00a | 0.095 ± 0.00a | nd | 0.091 ± 0.00a | 0.114 | |
| B-67 | Untreated (ambient) | 0.036 ± 0.00a | 0.090 ± 0.00a | nd | 0.090 ± 0.00a | 0.110 |
| Heated, 80°C, 30 min | 0.037 ± 0.00a | 0.092 ± 0.00a | nd | 0.091 ± 0.00a | 0.113 | |
| Heated, 100°C, 30 min | 0.038 ± 0.00c | 0.093 ± 0.00a | nd | 0.093 ± 0.00a | 0.114 | |
| Heated, 120°C, 30 min | 0.038 ± 0.00a | 0.095 ± 0.00d | nd | 0.093 ± 0.00d | 0.118 | |
| Heated, 150°C, 30 min | 0.038 ± 0.00d | 0.095 ± 0.00c | nd | 0.093 ± 0.00c | 0.118 |
Column-wise values with the same superscripts indicate no significant difference (p < 0.05).
All values are expressed as the mean ± SD.
DW = dry weight; IMG = isorhamnetin-3-glucoside; nd = not detected; Q = quercetin aglycone; QDG = quercetin-3,4′-O-diglucoside; QMG = quercetin-4′-O-monoglucoside; SD = standard deviation; Total = Q + QMG + QDG + IMG.
3.3. Effect of heating on total flavonoids
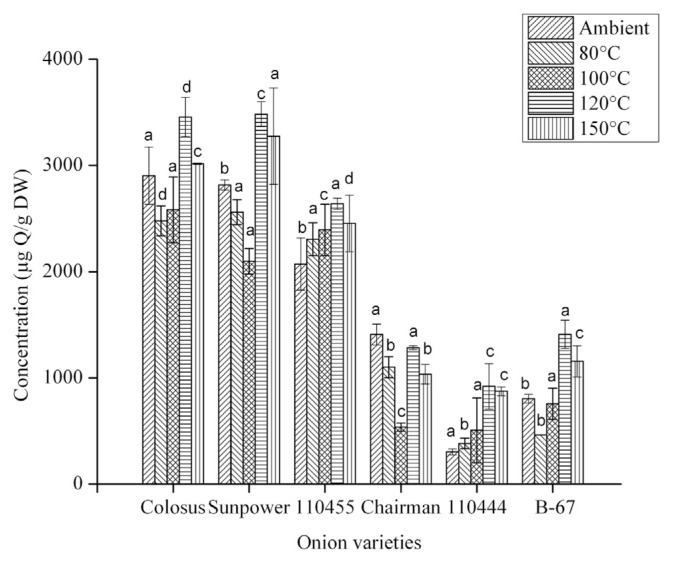
The total flavonoids in the six onion varieties examined varies in the range of 303.18 ± 28.63 μg Q/g DW to 2902.67 ± 269.71 μg Q/g DW. Fig. 1 shows the results. The total flavonoid content in decreasing order was red-skinned (Colossal), yellow-skinned (Sunpower), 110455, Chairman, white-skinned (B-67), and 110444. After heating at a certain temperature, there was a decrease in total flavonoids, which indicates that some flavonoids were probably destroyed. However, the total phenolics were increased. In most fruits and vegetables, flavonoids contain C–glycoside bonds and exist as dimers and oligomers, and the industrial processing such as heating or boiling results in the formation of monomers by the hydrolysis of C–glycosides bonds [29].
Fig. 1.
The effect of heating on total flavonoid content of six different onion varieties. The data are presented as the mean ± standard deviation. The bars depict the standard errors. μg Q/g DW = μg of quercetin equivalents/g dry weight. a–d Values with the same superscripts within the same treatment indicate no significant difference (p < 0.05).
Olsson et al [23] reported that neither storage nor heating causes significant differences in total flavonol content in sweet cultivars and in red onion cultivars. However, for minor quercetin and isorhamnetin glucosides, storage and heating both result in a significant change. Thermal instability of quercetin and kaempferol in vegetable tissue and in boiling water has been reported [30]. For instance, boiling for 60 minutes decreased quercetin 50–60% in yellow and red onions, compared to the original raw vegetables, and the total flavonoids in the green Welsh onion significantly decreased because of boiling for 15 minutes. Steaming had a positive effect on total phenolic content in most vegetables such as onion, garlic, and parsley. However, a negative effect has been reported for the total flavonoid content in parsley and onion [31]. Our results are consistent with the findings of Woo et al [28] who found that total flavonoids increased after heating at a certain temperature and magnitude of time, whereas heating for 3 hours at 150°C decreased the content of total flavonoids [28]. The reason for the decrease in the total flavonoid at higher temperature could be because of the degradation of flavonoids. It also depends on the structure of particular flavonoids.
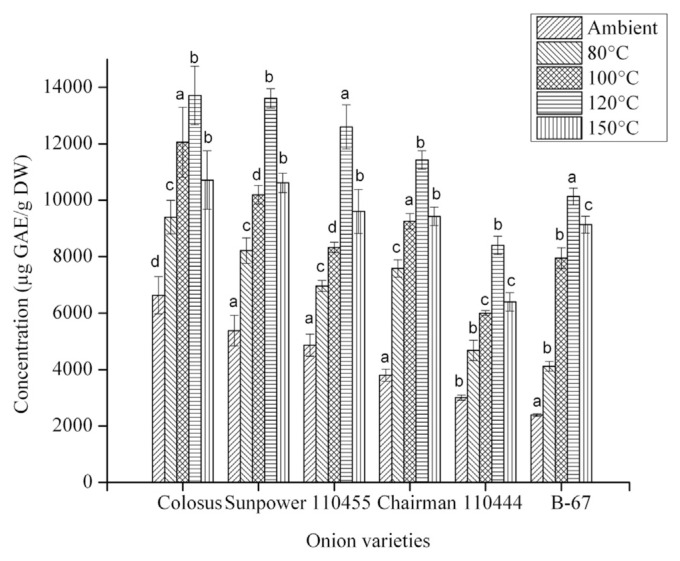
3.4. Effect of heating on total phenolics
Fig. 2 presents the effects of heating on the content of total phenolics for six onion varieties. At ambient temperature, the overall concentration of total phenolics varied from 2389.33 ± 46.46 μg GAE/g DW to 6631.33 ± 661.21 μg GAE/g DW. The total phenolic content was significantly increased after heating at 80°C, 100°C, and 120°C for 30 minutes each. For instance, on heating at 120°C for 30 minutes, the total phenolic content of the red variety (Colossal) increased from 6631.33 ± 661.21 μg GAE/g DW to 13712.67 ± 1034.85 μg GAE/g DW, the white variety (B-67) increased from 3009.33 ± 95.44 μg GAE/g DW to 8401.33 ± 324.42 μg GAE/g DW; and the yellow variety (Sunpower) increased from 5381.54 ± 542.24 μg GAE/g DW to 13611.83 ± 341.62 μg GAE/g DW. Heating at 150°C for 30 minutes decreased the total phenolic content for all of these onion varieties. Different processing steps such as boiling, sauteing, frying, and roasting can be used to liberate phenolic compounds from various plants. The result indicates that phenolic compounds in onion powder—either liberated by the cleaving of the esterified and glycosylated bond or by the formation of Maillard reaction products—are responsible for the increase in total phenolics after heating [32]. However, simple heating reportedly cannot cleave covalently bound phenolic compounds; however, far-infrared treatment can cleave the bond; the different bound status of the phenolic compound depends on the species [33].
Fig. 2.
Effect of heating on total phenolic content of six different onion varieties. The data are presented as the mean ± standard deviation. The bars depict the standard error. μg GAE/g DW = μg of gallic acid equivalents/g dry weight. a–d Values with same superscripts within the same treatment indicate no significant difference (p < 0.05).
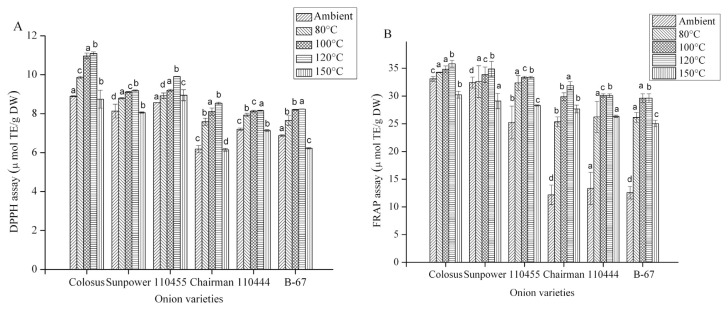
3.5. Effect of heating on antioxidant activities
The antioxidant activities of six onion varieties were evaluated by DPPH and FRAP assay methods. Fig. 3A and B present the results. The DPPH and FRAP values for all onion varieties were increased at higher temperatures, relative to the onion at ambient temperature. Red onion (Colossal) shows the highest antioxidant activity, followed by yellow onions (Sunpower, Chairman, 110455), and white onions (110444, B-67). The DPPH and FRAP values of red onion were approximately fourfold higher than those of white onion, and were slightly higher than yellow onion. The antioxidant activity of red onion increased from 8.90 ± 0.025 μmol TE/g DW to 11.09 ± 0.08 μmol TE/g DW, as measured by DPPH assay, and 33.18 ± 0.43 μmol TE/g DW to 35.20 ± 0.62 μmol TE/g DW as measured by FRAP assay, after heating at 120°C for 30 minutes. For white onions, the increase was similarly 6.87 ± 0.04 μmol TE/g DW to 8.23 ± 0.01 μmol TE/g DW, based on the DPPH assay, and 12.58 ± 1.14 μmol TE/g DW to 24.56 ± 0.79 μmol TE/g DW, based on the FRAP assay. The FRAP values for the white onion were consistent with the results for the Calcot and white onion [34]. The total antioxidant activity for all varieties was less at 150°C, compared to 120°C. After heating, the order of antioxidant activity measured with both DPPH and FRAP methods were (in decreasing order) redskinned (Colossal), yellow-skinned (Sunpower), 110455, white-skinned (110444), Chairman, and B-67. In both assays, the white-skinned onion showed the lowest antioxidant activity. The antioxidant activity may decrease with the loss of antioxidants or with the formation of compounds having pro-oxidant action. Alterations of the structure of the existing antioxidants and the formation of novel antioxidant components may enhance the initial antioxidant status [35,36]. This suggests that processing onions by heating does not cause a drastic loss in antioxidant values, which is in accordance with previous findings [37,38] that indicate that heating enhances antioxidant activity in fruits and vegetables because of the enhancement of the antioxidant properties of naturally occurring compounds or the formation of novel compounds such as Maillard reaction products that have antioxidant activity. However, a previous report suggests that, apart from allium species, the antioxidant activity of most foods is reduced after heating at 65°C or 100°C [39].
Fig. 3.
Effect of heating on antioxidant activity of six different onion varieties, as assessed by (A) DPPH; and (B) FRAP. Data are presented as the mean ± standard deviation. The bars depict the standard error. μmol TE/g DW =μmol of Trolox equivalent/g dry weight. a–d Values with same superscripts within the same treatment indicate no significant difference (p < 0.05).
3.6. Effect of heating on sugar content
Carbohydrates account for a major portion of the dry weight of onion bulbs. These carbohydrates include glucose, fructose, sucrose, and fructooligosaccharides (i.e., fructans) with a degree of polymerization of 3–12 [40,41]. In the present work, glucose was the predominant sugar component, followed by fructose and sucrose in all the studied varieties. Sucrose was nearly fourfold to fivefold less in molar concentration, compared to fructose and glucose. The sugar content decreased with increasing heating temperature, and the decrease was more or less the same in all studied varieties, except Colossal and 110455 (Table 3). In all varieties the decrease was minimal until the temperature rose to 100°C. In a few varieties such as Colossal and 110455, the content of fructose was almost constant with a minute drop in the glucose and sucrose content. Higher loss of glucose up to 74% and fructose up to 64% was observed for the Colossal (red) variety with the rise in temperature from 120°C to 150°C. The 110455 (yellow) variety similarly showed a loss of 50% and 63% for glucose and fructose, respectively. Other varieties such as Sunpower, Chairman, 110444, and B-67 showed a 10–12% loss for glucose and fructose after a temperature rise of 120°C to 150°C. Reyes et al [42] reported that, during heating, the fructose concentration decreases faster than glucose initially, but later the loss of glucose overtakes the fructose [42]. The decrease in sugar content may occur because of the formation of Maillard reaction products, which are formed by the reaction between sugars, amino acids, and proteins [43]. Heating and roasting other edible foodstuffs such as cocoa beans results in the complete loss of glucose and the loss was predicted because of the interaction of glucose with an amino acid to form other compounds [44]. This finding suggests that to maintain the sweetness of onion powder during the processing of onion, heating at high temperatures above 120°C should be completely prohibited.
Table 3.
Effect of heating on sugar content (mmol/g DW) of six different onion varieties.
| Onion type | Heat treatment | Fructose | Glucose | Sucrose |
|---|---|---|---|---|
| Colossal | Untreated (ambient) | 1.042 ± 0.06a | 1.281 ± 0.08a | 0.277 ± 0.02a |
| Heated, 80°C, 30 min | 1.071 ± 0.05a | 1.019 ± 0.08d | 0.264 ± 0.02a | |
| Heated, 100°C, 30 min | 1.042 ± 0.06b | 0.789 ± 0.08a | 0.267 ± 0.00c | |
| Heated, 120°C, 30 min | 0.966 ± 0.03a | 0.579 ± 0.07a | 0.217 ± 0.02a | |
| Heated, 150°C, 30 min | 0.341 ± 0.02b | 0.143 ± 0.03b | 0.118 ± 0.01a | |
| Sunpower | Untreated (ambient) | 1.079 ± 0.09a | 1.334 ± 0.11b | 0.248 ± 0.03a |
| Heated, 80°C, 30 min | 0.998 ± 0.03c | 1.019 ± 0.09a | 0.205 ± 0.03a | |
| Heated, 100°C, 30 min | 0.912 ± 0.06a | 1.157 ± 0.08a | 0.178 ± 0.02a | |
| Heated, 120°C, 30 min | 0.795 ± 0.04a | 1.013 ± 0.06a | 0.152 ± 0.02a | |
| Heated, 150°C, 30 min | 0.656 ± 0.06a | 0.889 ± 0.08a | 0.137 ± 0.02c | |
| 110455 | Untreated (ambient) | 1.104 ± 0.09a | 1.273 ± 0.09a | 0.316 ± 0.03c |
| Heated, 80°C, 30 min | 1.171 ± 0.03a | 1.160 ± 0.03a | 0.323 ± 0.01a | |
| Heated, 100°C, 30 min | 1.108 ± 0.03a | 0.932 ± 0.03a | 0.316 ± 0.02a | |
| Heated, 120°C, 30 min | 0.862 ± 0.03a | 0.456 ± 0.02b | 0.256 ± 0.03a | |
| Heated, 150°C, 30 min | 0.322 ± 0.06d | 0.255 ± 0.01a | 0.142 ± 0.00a | |
| Chairman | Untreated (ambient) | 0.917 ± 0.04a | 1.227 ± 0.07a | 0.207 ± 0.01a |
| Heated, 80°C, 30 min | 0.864 ± 0.03a | 1.143 ± 0.05a | 0.180 ± 0.00a | |
| Heated, 100°C, 30 min | 0.752 ± 0.02b | 1.007 ± 0.06c | 0.154 ± 0.00a | |
| Heated, 120°C, 30 min | 0.638 ± 0.02a | 0.891 ± 0.07a | 0.138 ± 0.00c | |
| Heated, 150°C, 30 min | 0.532 ± 0.06a | 0.704 ± 0.08a | 0.112 ± 0.00a | |
| 110444 | Untreated (ambient) | 0.966 ± 0.03b | 1.369 ± 0.11a | 0.348 ± 0.00d |
| Heated, 80°C, 30 min | 0.957 ± 0.03a | 1.074 ± 0.03d | 0.343 ± 0.02a | |
| Heated, 100°C, 30 min | 0.875 ± 0.02a | 0.897 ± 0.03a | 0.328 ± 0.01a | |
| Heated, 120°C, 30 min | 0.708 ± 0.02b | 0.683 ± 0.02c | 0.282 ± 0.04a | |
| Heated, 150°C, 30 min | 0.611 ± 0.02a | 0.581 ± 0.08a | 0.222 ± 0.05d | |
| B-67 | Untreated | 0.942 ± 0.03a | 0.783 ± 0.03a | 0.303 ± 0.01a |
| Heated, 80°C, 30 min | 0.928 ± 0.02c | 0.541 ± 0.03c | 0.304 ± 0.01b | |
| Heated, 100°C, 30 min | 0.862 ± 0.02a | 0.313 ± 0.01a | 0.287 ± 0.00a | |
| Heated, 120°C, 30 min | 0.754 ± 0.02a | 0.288 ± 0.02a | 0.240 ± 0.02b | |
| Heated, 150°C, 30 min | 0.635 ± 0.06b | 0.265 ± 0.02a | 0.196 ± 0.02b |
Column wise values with same superscripts indicate no significant difference (p < 0.05).
All values are expressed as the mean ± SD.
DW = dry weight; SD = standard deviation.
4. Conclusion
In this study, we have studied six onion varieties—red-skinned (Colossal), yellow-skinned (Sunpower, Chairman, 110455), and white-skinned (110444, B-67)—with the intention of analyzing the content of total flavonoids, phenolics, and antioxidants with respect to temperature. We found that the total phenolic and antioxidant activities were increased at 80°C, 100°C, and 120°C with respect to the ambient temperature in all varieties, whereas the total flavonoid content did not show any regular trend. A decrease in sugar content was observed with the rise in heating temperature in all varieties. In all varieties the increase in individual flavonoids such as quercetin and its glucoside was not significant, compared to the total phenolic content. The results suggest that it is improper to expose onion powder to temperatures higher than 120°C.
Acknowledgments
This work was supported by Konkuk University research program.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Day AJ, Williamson G. Biomarkers for exposure to dietary flavonoids: a review of the current evidence for identification of quercetin glycosides in plasma. Brit J Nutri. 2001;86:105–10. doi: 10.1079/bjn2001342. [DOI] [PubMed] [Google Scholar]
- 2. Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:R11–9. [Google Scholar]
- 3. Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–8. [Google Scholar]
- 4. Makris DP, Rossiter JT. Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): effect on flavonol content and antioxidant status. J Agric Food Chem. 2001;49:3216–22. doi: 10.1021/jf001497z. [DOI] [PubMed] [Google Scholar]
- 5. Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–80. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- 6. Patil BS, Pike LM, Yoo KS. Variation in the quercetin content in different colored onions (Allium cepa L.) J Am Society of Hortic Sci. 1995;120:909–13. [Google Scholar]
- 7. Shrefler J, Perkins Veazie P, Taylor M, Goodson T. Short-term storage options for fresh-market onions. Hort Sci. 2007;42:1020. [Google Scholar]
- 8. Maw BW, Butts CL, Purvis AC, Seebold K, Mullinix BG. High-temperature continuous-flow curing of sweet onions. Appl Eng Agric. 2004;20:657–63. [Google Scholar]
- 9. Debnath S, Hemavathy J, Bhat KK. Moisture sorption studies on onion powder. Food Chem. 2002;78:479–82. [Google Scholar]
- 10.Pokorny J, Schmidt S. The impact of food processing in phytochemicals: the case of antioxidants. In: Johnson I, Williamson G, editors. Phytochemical functional foods. 1st ed. Abington, Cambridge, England, UK: CRC Press Woodhead Publishing; 2003. p. 298. [Google Scholar]
- 11. Zhang D, Hamauzu Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004;88:503–9. [Google Scholar]
- 12. Ruiz-Rodriguez A, Marín FR, Ocaña A, Soler-Rivas C. Effect of domestic processing on bioactive compounds. Phytochemistry Rev. 2008;7:345–84. [Google Scholar]
- 13. Faller ALK, Fialho E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res Int. 2009;42:210–5. [Google Scholar]
- 14. Gazzani G, Papetti A, Massolini G, Daglia M. Anti and prooxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J Agric Food Chem. 1998;46:4118–22. [Google Scholar]
- 15. Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.) J Agric Food Chem. 2005;53:2733–40. doi: 10.1021/jf048152r. [DOI] [PubMed] [Google Scholar]
- 16. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoids content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 17. Dalamu Kaur C, Singh M, Walia S, Joshi S, Munshi AD. Variations in phenolics and antioxidants in Indian onions (Allium cepa L.) Genotype selection for breeding. Nutr Food Sci. 2010;40:6–19. [Google Scholar]
- 18. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 20. Kahane R, Vialle-Guérin E, Boukema I, Tzanoudakis D, Bellamy C, Chamaux C, Kik C. Changes in non-structural carbohydrate composition during bulbing in sweet and high-solid onions in field experiments. EnViron Exp Bot. 2001;45:73–83. doi: 10.1016/s0098-8472(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 21. Fu HY. Free radical scavenging and leukemia cell growth inhibitory properties of onion powders treated by different heating processes. J Food Sci. 2004;69:SNQ50–4. [Google Scholar]
- 22. Price KR, Bacon JR, Rhodes MJC. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa) J Agric Food Chem. 1997;45:938–42. [Google Scholar]
- 23. Olsson ME, Gustavsson KE, Vagen IM. Quercetin and isorhamnetin in sweet and red cultivars of onion (Allium cepa L.) at harvest, after field curing, heat treatment, and storage. J Agric Food Chem. 2010;58:2323–30. doi: 10.1021/jf9027014. [DOI] [PubMed] [Google Scholar]
- 24. Hirota S, Shimoda T, Takahama U. Tissue and spatial distribution of flavonol and peroxidase in onion bulbs and stability of flavonol glucosides during boiling of the scales. J Agric Food Chem. 1998;46:3497–502. [Google Scholar]
- 25. Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–5. [Google Scholar]
- 26. Edwald C, Fjelkiner-Modig S, Johansson K, Sjöholm I, Åkesson B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. 1999;64:231–5. [Google Scholar]
- 27. Lombard K, Peffley E, Geoffriau E, Thompson L, Herring A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J Food Comp Anal. 2005;18:571–81. [Google Scholar]
- 28. Ioku K, Aoyama Y, Tokuno A, Terao J, Nakatani N, Takei Y. Various cooking methods and the flavonoid content in onion. J Nutr Sci Vitaminol. 2001;47:78–83. doi: 10.3177/jnsv.47.78. [DOI] [PubMed] [Google Scholar]
- 29. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 30. Aoyama S, Yamamoto Y. Antioxidant activity and flavonoid content of Welsh onion (Allium fistulosum) and the effect of thermal treatment. Food Sci Technol Res. 2007;13:67–72. [Google Scholar]
- 31.Priecina L, Karlina D.Total polyphenol, flavonoid content and antiradical activity of celery, dill, parsley. Onion and garlic dried in convective and microwave-vacuum dryers; 2013 2nd International Conference on Nutrition and Food Sciences IPCBEE; Singapore: IACSIT Press; 2013. [Google Scholar]
- 32. Maillard MN, Soum MH, Boivia P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. Lebensmittel-Wissenschaft Und-Technoogie. 1996;3:238–44. [Google Scholar]
- 33. Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam KC, Ahn DU. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem. 2003;51:4400–3. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- 34. Santas J, Carbó R, Gordon MH, Almajanoc MP. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–6. [Google Scholar]
- 35. Jiménez-Monreal AM, García-Diz L, Martínez-Tomé M, Mariscal M, Murcia MA. Influence of cooking methods on antioxidant activity of vegetables. J Food Sci. 2009;74:H97–103. doi: 10.1111/j.1750-3841.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 36. Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetable. Trends Food Sci Technol. 1999;10:94–100. [Google Scholar]
- 37. Gorinstein S, Leontowicz H, Leontowicz M, Namiesnik J, Najman K, Drzewiecki J, Cvikrová M, Martincová O, Katrich E, Trakhtenberg S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J Agric Food Chem. 2008;56:4418–26. doi: 10.1021/jf800038h. [DOI] [PubMed] [Google Scholar]
- 38. Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2001;11:340–6. [Google Scholar]
- 39. Yin MC, Cheng WS. Antioxidant activity of several allium members. J Agric Food Chem. 1998;46:4097–101. [Google Scholar]
- 40. O’Donoghue EM, Somerfield SD, Shaw M, Bendall M, Hedderly D, Eason J, Sims I. Evaluation of carbohydrates in Pukekohe Longkeeper and Grano cultivars of Allium cepa. J Agric Food Chem. 2004;52:5383–90. doi: 10.1021/jf030832r. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki M, Cutcliffe JA. Fructans in onion bulbs in relation to storage life. Can J Plant Sci. 1989;69:1327–33. [Google Scholar]
- 42. Reyes FGR, Poocharoen B, Wrolstad RE. Maillard browning reaction of sugar–glycine model systems—changes in sugar concentration, color and appearance. J Food Sci. 1982;47:1376–7. [Google Scholar]
- 43. Martins SIFS, Jongen WMF, Van Boekel MAJS. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci Technol. 2000;11:364–73. [Google Scholar]
- 44. Redgwell RJ, Trovato V, Curti D. Cocoa bean carbohydrates: roasting-induced changes and polymer interactions. Food Chem. 2003;80:511–6. [Google Scholar]